Effects of 8-Week Whole-Body Vibration Training on the HbA1c, Quality of Life, Physical Fitness, Body Composition and Foot Health Status in People with T2DM: A Double-Blinded Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
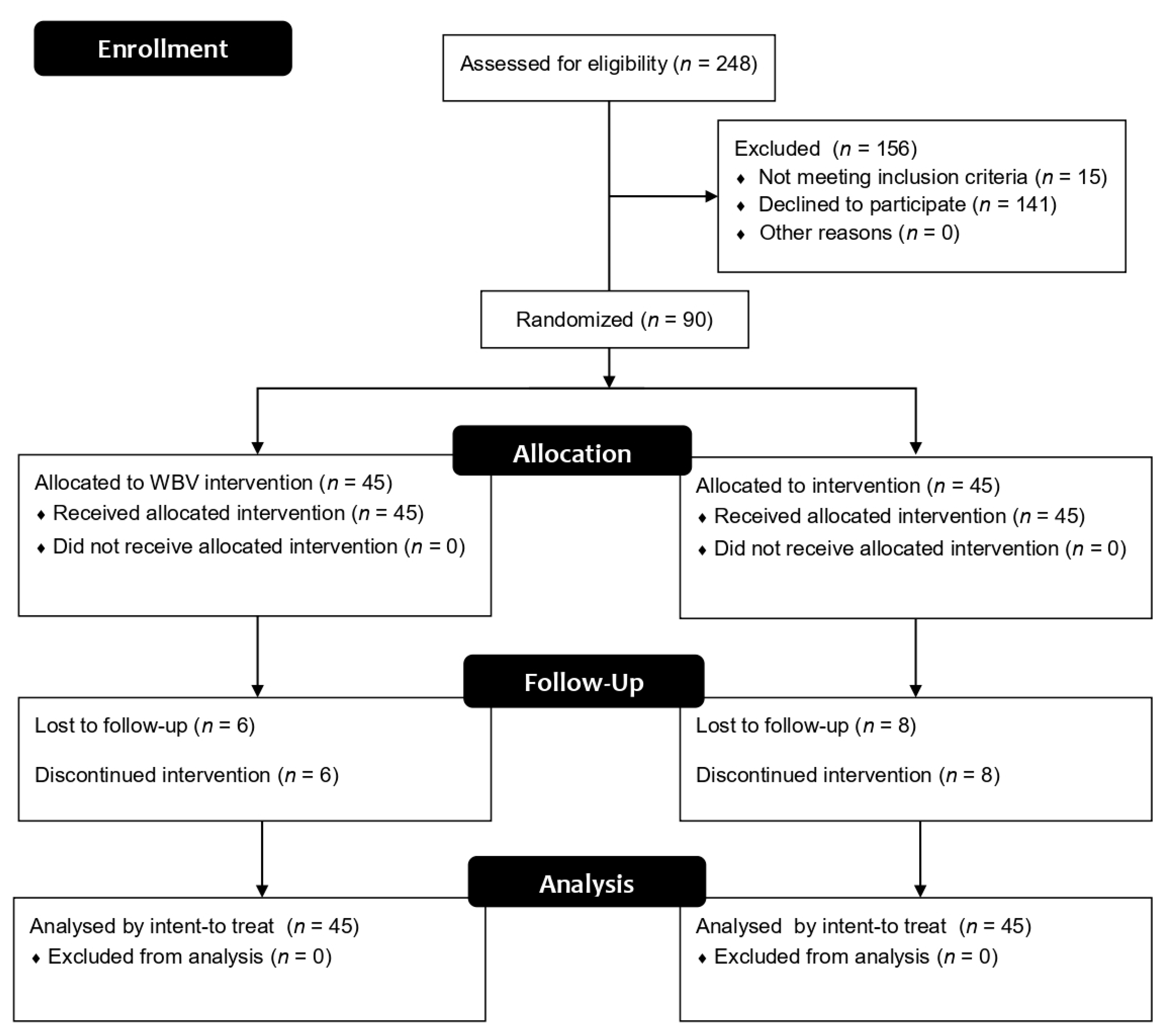
2.2. Participants
- (a)
- Men or women with T2DM diagnosed between 40 and 85 years old. T2DM is characterized by hyperglycemia caused by insulin resistance [3].
- (b)
- Have read, accepted and signed the written informed consent.
- (a)
- have Type I diabetes mellitus diagnose, since the aetiology completely differs from T2DM.
- (b)
- have a condition that may make the high intensity exercises contraindicated, such as retinopathy, musculoskeletal injuries, major balance problems or high risk of thrombosis.
- (c)
- Be under psychotropic or neurotoxic treatment.
- (d)
- Be exposed to neurotoxins (industrial accidents or be in contact with toxic residues).
- (e)
- Receive radiation therapy.
- (f)
- High risk of non-diabetic neuropathy (such as HIV, alcoholism or uraemia).
- (g)
- Have or had a job with high exposure to mechanical whole-body vibrations.
- (h)
- Have performed whole-body vibration exercises prior to this intervention.
2.3. Intervention
2.4. Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Available online: https://care.diabetesjournals.org/content/diacare/37/Supplement_1/S81.full.pdf (accessed on 11 November 2019).
- Kerner, W.; Bruckel, J. Definition, classification and diagnosis of diabetes mellitus. Exp. Clin. Endocrinol. Diabetes 2014, 122, 384–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyton, A.C.; Hall, J.E. Text Book of Medical Physiology, 12th ed.; Saunders: Philadelphia, PA, USA, 2010. [Google Scholar]
- Whiting, D.R.; Guariguata, L.; Weil, C.; Shaw, J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011, 94, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.; Dunstan, D.W.; Davies, M.J.; Yates, T. Sedentary behaviour as a new behavioural target in the prevention and treatment of type 2 diabetes. Diabetes Metab. Res. Rev. 2016, 32, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Goblan, A.S.; Al-Alfi, M.A.; Khan, M.Z. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587. [Google Scholar] [CrossRef] [Green Version]
- Maser, R.E.; Mitchell, B.D.; Vinik, A.I.; Freeman, R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes—A meta-analysis. Diabetes Care 2003, 26, 1895–1901. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.K. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes 1997, 46, S54–S57. [Google Scholar] [CrossRef]
- Singleton, J.R.; Smith, A.G.; Marcus, R.L. Exercise as therapy for diabetic and prediabetic neuropathy. Curr. Diabetes Rep. 2015, 15, 120. [Google Scholar] [CrossRef]
- Balducci, S.; Iacobellis, G.; Parisi, L.; Di Biase, N.; Calandriello, E.; Leonetti, F.; Fallucca, F. Exercise training can modify the natural history of diabetic peripheral neuropathy. J. Diabetes Its Complicat. 2006, 20, 216–223. [Google Scholar] [CrossRef]
- Allet, L.; Armand, S.; de Bie, R.A.; Golay, A.; Monnin, D.; Aminian, K.; Staal, J.B.; de Bruin, E.D. The gait and balance of patients with diabetes can be improved: A randomised controlled trial. Diabetologia 2010, 53, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, M.M.P.; van Dooren, F.E.P.; Pop, V.J.M.; Pouwer, F. Effects of exercise training on quality of life, symptoms of depression, symptoms of anxiety and emotional well-being in type 2 diabetes mellitus: A systematic review. Diabetologia 2013, 56, 1210–1225. [Google Scholar] [CrossRef] [Green Version]
- Villafaina, S.; Collado-Mateo, D.; Fuentes, J.P.; Merellano-Navarro, E.; Gusi, N. Physical exercise improves heart rate variability in patients with type 2 diabetes: A systematic review. Curr. Diabetes Rep. 2017, 17, 110. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Beedie, C.; Balducci, S.; Zanuso, S.; Allgrove, J.; Bertiato, F.; Jimenez, A. Changes in insulin sensitivity in response to different modalities of exercise: A review of the evidence. Diabetes Metab. Res. Rev. 2014, 30, 257–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karstoft, K.; Pedersen, B.K. Exercise and type 2 diabetes: Focus on metabolism and inflammation. Immunol. Cell Biol. 2016, 94, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Kluding, P.M.; Pasnoor, M.; Singh, R.; Jernigan, S.; Farmer, K.; Rucker, J.; Sharma, N.K.; Wright, D.E. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J. Diabetes Its Complicat. 2012, 26, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Singleton, J.R.; Marcus, R.L.; Lessard, M.K.; Jackson, J.E.; Smith, A.G. Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann. Neurol. 2015, 77, 146–153. [Google Scholar] [CrossRef]
- Rees, S.S.; Murphy, A.J.; Watsford, M.L. Effects of whole-body vibration exercise on lower-extremity muscle strength and power in an older population: A randomized clinical trial. Phys. Ther. 2008, 88, 462–470. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Lee, S.; Song, C. Whole-body vibration training improves balance, muscle strength and glycosylated hemoglobin in elderly patients with diabetic neuropathy. Tohoku J. Exp. Med. 2013, 231, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Weng, C.; Liu, M.; Wang, Q.; Liu, L.; He, Y. Effect of whole-body vibration exercise on mobility, balance ability and general health status in frail elderly patients: A pilot randomized controlled trial. Clin. Rehabil. 2014, 28, 59–68. [Google Scholar] [CrossRef]
- Bogaerts, A.C.; Delecluse, C.; Claessens, A.L.; Troosters, T.; Boonen, S.; Verschueren, S.M. Effects of whole body vibration training on cardiorespiratory fitness and muscle strength in older individuals (a 1-year randomised controlled trial). Age Ageing 2009, 38, 448–454. [Google Scholar] [CrossRef] [Green Version]
- Gusi, N.; Raimundo, A.; Leal, A. Low-frequency vibratory exercise reduces the risk of bone fracture more than walking: A randomized controlled trial. BMC Musculoskelet. Disord. 2006, 7, 92. [Google Scholar] [CrossRef]
- Del Pozo-Cruz, B.; Hernandez Mocholi, M.A.; Adsuar, J.C.; Parraca, J.A.; Muro, I.; Gusi, N. Effects of whole body vibration therapy on main outcome measures for chronic non-specific low back pain: A single-blind randomized controlled trial. J. Rehabil. Med. 2011, 43, 689–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes-Neto, M.; de Sa-Caputo, D.D.C.; Paineiras-Domingos, L.L.; Brandao, A.A.; Neves, M.F.; Marin, P.J.; Sanudo, B.; Bernardo-Filho, M. Effects of whole-body vibration in older adult patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Can. J. Diabetes 2019, 43, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Manimmanakorn, N.; Manimmanakorn, A.; Phuttharak, W.; Hamlin, M.J. Effects of whole body vibration on glycemic indices and peripheral blood flow in type II diabetic patients. Malays. J. Med Sci. MJMS 2017, 24, 55. [Google Scholar] [CrossRef] [PubMed]
- Baum, K.; Votteler, T.; Schiab, J. Efficiency of vibration exercise for glycemic control in type 2 diabetes patients. Int. J. Med Sci. 2007, 4, 159. [Google Scholar] [CrossRef] [Green Version]
- Behboudi, L.; Azarbayjani, M.-A.; Aghaalinejad, H.; Salavati, M. Effects of aerobic exercise and whole body vibration on glycaemia control in type 2 diabetic males. Asian J. Sports Med. 2011, 2, 83. [Google Scholar] [CrossRef] [Green Version]
- Del Pozo-Cruz, B.; Alfonso-Rosa, R.M.; del Pozo-Cruz, J.; Sanudo, B.; Rogers, M.E. Effects of a 12-wk whole-body vibration based intervention to improve type 2 diabetes. Maturitas 2014, 77, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Sanudo, B.; Alfonso-Rosa, R.; Del Pozo-Cruz, B.; Del Pozo-Cruz, J.; Galiano, D.; Figueroa, A. Whole body vibration training improves leg blood flow and adiposity in patients with type 2 diabetes mellitus. Eur. J. Appl. Physiol. 2013, 113, 2245–2252. [Google Scholar] [CrossRef] [Green Version]
- Zago, M.; Capodaglio, P.; Ferrario, C.; Tarabini, M.; Galli, M. Whole-body vibration training in obese subjects: A systematic review. PLoS ONE 2018, 13, e0202866. [Google Scholar] [CrossRef] [Green Version]
- Umpierre, D.; Ribeiro, P.A.; Kramer, C.K.; Leitao, C.B.; Zucatti, A.T.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef] [Green Version]
- Church, T.S. Recent purchase of development rights agreements protecting forest land. JAMA 2002, 304, 2253–2262. [Google Scholar] [CrossRef] [Green Version]
- Gassner, H.; Janzen, A.; Schwirtz, A.; Jansen, P. Random Whole body vibration over 5 weeks leads to effects similar to placebo: A controlled study in parkinson’s disease. Parkinson’s Dis. 2014, 2014, 386495. [Google Scholar] [CrossRef]
- Hernandez-Mocholi, M.A.; Dominguez-Munoz, F.J.; Corzo, H.; Silva, S.C.; Adsuar, J.C.; Gusi, N. Whole body vibration training improves vibration perception threshold in healthy young adults: A randomized clinical trial pilot study. J. Musculoskelet. Neuronal Interact. 2016, 16, 12–17. [Google Scholar] [PubMed]
- Sirera-Vercher, M.; Sáez-Zamora, P.; Sanz-Amaro, M. Traducción y adaptación transcultural al castellano y al valenciano del Foot Health Status Questionnaire. Revista Española de Cirugía Ortopédica y Traumatología 2010, 54, 211–219. [Google Scholar] [CrossRef]
- Sintonen, H. The 15D instrument of health-related quality of life: Properties and applications. Ann. Med. 2001, 33, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Rikli, R.E.; Jones, C.J. Development and validation of a functional fitness test for community-residing older adults. J. Aging Phys. Act. 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Alfonso-Rosa, R.M.; Del Pozo-Cruz, B.; Del Pozo-Cruz, J.; Sanudo, B.; Rogers, M.E. Test-retest reliability and minimal detectable change scores for fitness assessment in older adults with type 2 diabetes. Rehabil. Nurs. Off. J. Assoc. Rehabil. Nurses 2014, 39, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Muñoz, F.J.; Hernández-Mocholi, M.A.; Manso, L.J.; Collado-Mateo, D.; Villafaina, S.; Adsuar, J.C.; Gusi, N. Test-retest reliability of kinematic parameters of timed up and go in people with type 2 diabetes. Appl. Sci. 2019, 9, 4709. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2012, 141, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Coolican, H. Research Methods and Statistics in Psychology; Psychology Press: New York, NY, USA, 2017. [Google Scholar]
- Fjeldstad, C.; Palmer, I.J.; Bemben, M.G.; Bemben, D.A. Whole-body vibration augments resistance training effects on body composition in postmenopausal women. Maturitas 2009, 63, 79–83. [Google Scholar] [CrossRef]
- Iannucci, C.V.; Capoccia, D.; Calabria, M.; Leonetti, F. Metabolic syndrome and adipose tissue: New clinical aspects and therapeutic targets. Curr. Pharm. Des. 2007, 13, 2148–2168. [Google Scholar] [CrossRef] [PubMed]
- Santin-Medeiros, F.; Santos-Lozano, A.; Cristi-Montero, C.; Garatachea Vallejo, N. Effect of 8 months of whole-body vibration training on quality of life in elderly women. Res. Sports Med. 2017, 25, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, G.; Wang, Y.; Wang, X.; Zhou, H.; Li, H.; Chen, L. The effect of whole body vibration on health-related quality of life in patients with chronic conditions: A systematic review. Qual. Life Res. 2019, 28, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Lima, R.P.; Sá-Caputo, D.C.; Moreira-Marconi, E.; Dionello, C.; Paineiras-Domingos, L.L.; Sousa-Gonçalves, C.R.; Morel, D.S.; Frederico, E.H.; Neves, M.F.; Oliveira, R. Quality of life of patients with metabolic syndrome is improved after whole body vibration exercises. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, H.; Kan, L.; Zhang, C.; Wang, P. The effect of whole body vibration therapy on the physical function of people with type II diabetes mellitus: A systematic review. J. Phys. Ther. Sci. 2016, 28, 2675–2680. [Google Scholar] [CrossRef] [Green Version]
- Yoosefinejad, A.K.; Shadmehr, A.; Olyaei, G.; Talebian, S.; Bagheri, H.; Mohajeri-Tehrani, M.R. Short-term effects of the whole-body vibration on the balance and muscle strength of type 2 diabetic patients with peripheral neuropathy: A quasi-randomized-controlled trial study. J. Diabetes Metab. Disord. 2015, 14, 45. [Google Scholar] [CrossRef] [Green Version]
- Trans, T.; Aaboe, J.; Henriksen, M.; Christensen, R.; Bliddal, H.; Lund, H. Effect of whole body vibration exercise on muscle strength and proprioception in females with knee osteoarthritis. Knee 2009, 16, 256–261. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- Jamal, A.; Ahmad, I.; Ahamed, N.; Azharuddin, M.; Alam, F.; Hussain, M.E. Whole Body Vibration Showed Beneficial Effect on Pain, Balance Measures and Quality of Life in Painful Diabetic Peripheral Neuropathy: A Randomized Controlled Trial. Available online: https://0-link-springer-com.brum.beds.ac.uk/article/10.1007/s40200-019-00476-1#citeas (accessed on 11 November 2019).
- Álvarez-Barbosa, F.; del Pozo-Cruz, J.; del Pozo-Cruz, B.; Alfonso-Rosa, R.M.; Rogers, M.E.; Zhang, Y. Effects of supervised whole body vibration exercise on fall risk factors, functional dependence and health-related quality of life in nursing home residents aged 80+. Maturitas 2014, 79, 456–463. [Google Scholar] [CrossRef]
| Week | Number of Series per Session | Duration of the Serie (seconds) | Vibration Frequency (Hertzios) | Rest between Series (seconds) | Intervention Duration per Week (seconds) |
|---|---|---|---|---|---|
| 1 | 8 | 30 | 12.5 | 30 | 720 |
| 2 | 5 | 60 | 13.5 | 30 | 900 |
| 3 | 6 | 60 | 14.5 | 30 | 1080 |
| 4 | 7 | 60 | 15.5 | 30 | 1260 |
| 5 | 8 | 60 | 16.5 | 30 | 1440 |
| 6 | 9 | 60 | 17.5 | 30 | 1620 |
| 7 | 9 | 60 | 18.5 | 30 | 1620 |
| 8 | 9 | 60 | 18.5 | 30 | 1620 |
| Variables | All (n = 90) | WBV Group (n = 45) | Placebo Group (n = 45) | p-Value |
|---|---|---|---|---|
| Weight (kg) | 80.63 (6.19) | 81.78 (18.04) | 79.48 (14.22) | 0.926 |
| BMI (kg/m2) | 29.65 (4.39) | 30.37 (5.28) | 28.93 (3.17) | 0.298 |
| Fat Free Mass (%) | 32.98 (7.49) | 33.50 (7.73) | 32.45 (7.30) | 0.583 |
| HbA1c (%) | 6.78 (1.02) | 6.78 (1.15) | 6.79 (0.89) | 0.572 |
| Total cholesterol | 172.74 (35.59) | 168.37 (34.66) | 177.12 (36.34) | 0.081 |
| Systolic Blood Pressure | 140.28 (22.52) | 142.63 (21.41) | 137.93 (23.57) | 0.165 |
| Blood Glucose | 131.29 (30.62) | 132.50 (33.54) | 130.07 (27.71) | 0.870 |
| GFH of the FHSQ | 57.96 (25.08) | 57.72 (25.43) | 58.20 (25.01) | 0.760 |
| Timed-Up and Go (s) | 8.12 (2.01) | 8.29 (2.28) | 7.96 (1.71) | 0.696 |
| Chair Stand Test | 11.51 (2.24) | 11.91 (2.24) | 11.11 (2.21) | 0.063 |
| 15D HRQoL Questionnaire | 0.89 (0.09) | 0.90 (0.09) | 0.89 (0.10) | 0.777 |
| Body Composition Variables | Within Group Comparison | Between Group Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Groups | Pre | Post | Z | p-Value * | Effect Size | Z | p-Value * | Effect Size | |
| Weight (kg) | WBV | 81.78 (18.04) | 81.35 (17.90) | −2.25 | 0.056 | −0.33 | −0.92 | 0.404 | −0.10 |
| Placebo | 79.48 (14.22) | 78.43 (14.00) | −2.99 | 0.004 * | −0.45 | ||||
| BMI (kg/m2) | WBV | 30.37 (5.28) | 28.43 (2.95) | −2.98 | 0.005 * | −0.44 | −0.77 | 0.445 | −0.08 |
| Placebo | 28.93 (3.17) | 30.21 (5.24) | −2.32 | 0.025 * | −0.34 | ||||
| Free fat mass (%) | WBV | 66.11 (8.81) | 67.27 (7.62) | −2.98 | 0.006 * | −0.44 | −1.79 | 0.104 | −0.19 |
| Placebo | 67.57 (7.30) | 67.53 (7.24) | −0.32 | 0.757 | −0.48 | ||||
| Fat mass (%) | WBV | 33.50 (7.73) | 32.83 (7.77) | −3.04 | 0.003 * | −0.45 | −2.132 | 0.049 * | −0.22 |
| Placebo | 32.45 (7.30) | 32.60 (7.20) | −0.36 | 0.740 | −0.05 | ||||
| Total body water (%) | WBV | 48.70 (5.64) | 49.25 (5.73) | −2.91 | 0.009 * | −0.43 | −2.039 | 0.070 | −0.21 |
| Placebo | 49.47 (5.33) | 49.37 (5.31) | −0.24 | 0.811 | −0.03 | ||||
| Basal metabolic rate (kcal) | WBV | 1606.23 (339.80) | 1610.21 (339.50) | −0.85 | 0.418 | −0.13 | −1.597 | 0.133 | −0.17 |
| Placebo | 1589.96 (319.06) | 1578.59 (325.11) | −1.49 | 0.148 | −0.22 | ||||
| HbA1c (%) | WBV | 6.78 (1.16) | 6.54 (0.97) | −3.19 | 0.001 * | −0.47 | −0.307 | 0.765 | −0.03 |
| Placebo | 6.79 (0.89) | 6.60 (0.91) | −2.94 | 0.004 * | −0.44 | ||||
| Total cholesterol (mg/dL) | WBV | 168.37 (34.66) | 158.01 (25.97) | −3.00 | 0.005 * | −0.44 | −0.697 | 0.554 | −0.07 |
| Placebo | 177.12 (36.34) | 170.17 (28.05) | −2.08 | 0.080 | −0.31 | ||||
| HDL (mg/dL) | WBV | 49.22 (12.18) | 50.18 (10.44) | −2.38 | 0.036 * | −0.35 | −0.659 | 0.548 | −0.07 |
| Placebo | 52.45 (15.00) | 54.15 (14.52) | −1.75 | 0.133 | −0.26 | ||||
| LDL (mg/dL) | WBV | 91.47 (31.14) | 83.32 (23.58) | −2.54 | 0.018 * | −0.38 | −0.463 | 0.652 | 0.05 |
| Placebo | 99.93 (32.47) | 98.17 (42.76) | −1.77 | 0.164 | −0.26 | ||||
| Systolic blood pressure (mmHg) | WBV | 142.63 (21.41) | 127.57 (27.58) | −3.87 | 0.002 * | −0.58 | −0.289 | 0.800 | 0.03 |
| Placebo | 137.93 (23.57) | 125.44 (25.09) | −3.51 | <0.001 * | −0.52 | ||||
| Fasting blood glucose (mg/dL) | WBV | 132.50 (33.54) | 122.15 (31.67) | −2.41 | 0.022 * | −0.36 | −1.514 | 0.176 | 0.16 |
| Placebo | 130.07 (27.71) | 115.27 (26.72) | −4.35 | <0.001 * | −0.65 | ||||
| Variables | Within Group Comparison | Between Group Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Groups | Pre | Post | Z | p-Value * | Effect Size | Z | p-Value * | Effect Size | |
| Foot Pain | WBV | 87.99 (21.94) | 93.64 (16.02) | −1.78 | 0.118 | −0.26 | −0.661 | 0.551 | −0.07 |
| Placebo | 89.50 (19.85) | 94.00 (14.94) | −1.63 | 0.131 | −0.24 | ||||
| Foot function | WBV | 94.86 (15.38) | 97.03 (10.27) | −1.02 | 0.353 | −0.15 | −0.856 | 0.457 | −0.09 |
| Placebo | 93.64 (17.30) | 95.74 (12.52) | −1.50 | 0.317 | −0.22 | ||||
| Shoe | WBV | 79.44 (36.26) | 90.75 (25.22) | −2.61 | 0.015* | −0.39 | −0.815 | 0.485 | −0.08 |
| Placebo | 71.39 (39.98) | 79.79 (35.25) | −1.79 | 0.074 | −0.27 | ||||
| General foot health | WBV | 57.72 (25.43) | 62.97 (30.20) | −1.45 | 0.187 | −0.22 | −0.408 | 0.703 | −0.04 |
| Placebo | 58.20 (25.01) | 62.19 (21.54) | −1.21 | 0.233 | −0.18 | ||||
| General Health | WBV | 69.11 (22.85) | 71.68 (24.15) | −1.13 | 0.285 | −0.17 | −0.981 | 0.331 | −0.10 |
| Placebo | 64.36 (23.86) | 69.92 (22.32) | −1.69 | 0.107 | −0.25 | ||||
| Physical Activity | WBV | 85.43 (16.59) | 85.64 (14.75) | −0.45 | 0.658 | −0.07 | −1.205 | 0.258 | −0.13 |
| Placebo | 75.34 (25.80) | 79.94 (20.81) | −1.42 | 0.190 | −0.21 | ||||
| Social capacity | WBV | 94.17 (16.12) | 96.31 (16.66) | −0.76 | 0.469 | −0.11 | −0.894 | 0.394 | −0.09 |
| Placebo | 87.99 (24.18) | 93.66 (20.07) | −1.98 | 0.053 | −0.29 | ||||
| Vigour | WBV | 72.78 (19.86) | 74.65 (17.71) | −0.94 | 0.367 | −0.14 | −1.297 | 0.220 | −0.14 |
| Placebo | 61.84 (26.26) | 69.11 (23.06) | −2.28 | 0.034 * | −0.34 | ||||
| Physical Fitness and Quality of Life | Within Group Comparison | Between Group Comparison | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Groups | Pre | Post | Z | p-Value * | Effect Size | Z | p-Value * | Effect Size | |
| TUG (s) | WBV | 8.29 (2.28) | 7.42 (1.72) | −4.24 | <0.001 * | −0.63 | −1.718 | 0.095 | −0.18 |
| Placebo | 7.96 (1.71) | 7.49 (1.41) | −2.82 | 0.006 * | −0.42 | ||||
| Chair-stand test (rep) | WBV | 11.91 (2.24) | 12.96 (2.24) | −4.03 | <0.001 * | −0.60 | −1.316 | 0.256 | −0.14 |
| Placebo | 11.11 (2.21) | 11.70 (2.31) | −2.96 | 0.005 * | −0.44 | ||||
| 15D HRQoL Questionnaire | WBV | 0.90 (0.09) | 0.94 (0.07) | −4.28 | <0.001 * | −0.64 | −0.723 | 0.526 | −0.08 |
| Placebo | 0.89 (0.10) | 0.93 (0.06) | −4.52 | <0.001 * | −0.67 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Muñoz, F.J.; Villafaina, S.; García-Gordillo, M.A.; Hernández-Mocholi, M.Á.; Collado-Mateo, D.; Adsuar, J.C.; Gusi, N. Effects of 8-Week Whole-Body Vibration Training on the HbA1c, Quality of Life, Physical Fitness, Body Composition and Foot Health Status in People with T2DM: A Double-Blinded Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 1317. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17041317
Domínguez-Muñoz FJ, Villafaina S, García-Gordillo MA, Hernández-Mocholi MÁ, Collado-Mateo D, Adsuar JC, Gusi N. Effects of 8-Week Whole-Body Vibration Training on the HbA1c, Quality of Life, Physical Fitness, Body Composition and Foot Health Status in People with T2DM: A Double-Blinded Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2020; 17(4):1317. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17041317
Chicago/Turabian StyleDomínguez-Muñoz, Francisco Javier, Santos Villafaina, Miguel A. García-Gordillo, Miguel Ángel Hernández-Mocholi, Daniel Collado-Mateo, José C. Adsuar, and Narcis Gusi. 2020. "Effects of 8-Week Whole-Body Vibration Training on the HbA1c, Quality of Life, Physical Fitness, Body Composition and Foot Health Status in People with T2DM: A Double-Blinded Randomized Controlled Trial" International Journal of Environmental Research and Public Health 17, no. 4: 1317. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17041317





