How Has the Hazard to Humans of Microorganisms Found in Atmospheric Aerosol in the South of Western Siberia Changed over 10 Years?
Abstract
:1. Introduction
- an index that evaluates the pathogenicity or hazard of individual isolates of microorganisms to humans;
- an index that evaluates the resistance of microorganisms to adverse environmental factors;
- an index that evaluates the resistance of microorganisms to antibiotics or other drugs.
2. Materials and Methods
2.1. Atmospheric Air Sampling
2.2. Culturable Microorganisms’ Concentration
2.3. Microorganisms’ Biochemical and Morphological Characteristics
2.3.1. Pathogenic Properties of the Isolates
- plasma-coagulase activity was determined by placing a loop of the test culture in a test tube with 0.5 mL of rabbit citrate plasma diluted with 0.9% sterile sodium chloride solution and incubating the suspension at 37 °C for up to 24 hours; a positive reaction was determined by plasma coagulation [28];
- hemolytic activity was determined by seeding cultures on nutrient agar containing 5% defibrinated blood and the seeding was incubated at 37 °C for 24–48 hours; a positive result was the presence of hemolysis zones around the grown colonies [59];
- fibrinolytic activity was determined by sowing 0.25 mL of an 18–20-hour broth culture of the test strain in test tubes containing 0.1 mL of citrate plasma, 0.4 mL of 0.9% sodium chloride, 0.25 mL of 0.25% CaCl2 solution; tubes with suspension were kept in an incubator at a temperature of 37 °C for 15–20 minutes; a clot formed in a test tube with a positive reaction (the presence of fibrinolysin) liquefies after the next two hours of incubation in a thermostat; the test culture does not have fibrinolytic properties if the clot persists, as in the control tube, where the culture was not added;
- gelatinase activity was determined by sowing microorganisms by injection in test tubes with meat–peptone broth containing 12% gelatin, kept in an incubator for up to 20 days; a liquefaction of the nutrient medium is noted in the presence of the gelatinase enzyme [57].
2.3.2. Growth Characteristics of Bacteria at Increased Salt Concentration
2.3.3. The Determination of Enzymatic Activity of Isolated Bacteria
- proteolytic activity was determined by plating the studied microorganisms on an agar medium containing milk casein (milk agar); to prepare agar with casein, two components were prepared: 3% “hungry” agar (distilled water + 3% agar), sterilized at 1 atm for 30 minutes, and 12% milk sterilized at 1 atm for 20 minutes; then, agar was cooled to 50–55 °C, mixed with milk heated to the same temperature under aseptic conditions in a 1: 1 ratio, and poured into Petri dishes; after solidification of the medium, cultures were streaked and incubated under optimal conditions; the formation of transparent hydrolysis zones around crops in casein-containing agar indicated protease production [57];
- the amylolytic activity of cultures of microorganisms was determined when they were streaked on starch–ammonia agar; after incubation in a thermostat for 24–48 hours, the grown cultures in the dishes were poured into 5 mL of Lugol’s solution; the appearance of bright areas around crops within 3–5 minutes is a positive result;
- determination of lecithinase and lipase activity was carried out by two methods:
- seeding cultures with a stroke on yolk nutrient agar, for the preparation of which, under aseptic conditions, in yolk of molten and cooled to 50–55 °C fish-peptone agar (FPA) medium, yolk from a chicken egg is introduced; then medium is thoroughly mixed and poured into Petri dishes; the studied culture is streaked, incubated at the required temperature for 24–48 hours and the result is taken into account; when observed in an oblique light, lipase production was judged by the formation of a pearly shiny hydrolysis zone on agar around grown colonies; lecithinase (phospholipase) hydrolyzes the yolk lecithin; as a result of the precipitation reaction, a turbid whitish zone forms around the lecithin-fermenting colonies [57];
- plating cultures on a complete LB or RPA medium with 1% Tween-20 or Tween-40 and 0.01% CaCl2 as a substrate; sown cultures were incubated in a thermostat for 3–4 days and the result of the presence or absence of hydrolysis zones was determined [57];
- testing of cultures for the production of alkaline phosphatase was carried out using a reaction mixture of the composition: 0.3 mL of 0.85% NaCl added to 0.3 mL of substrate solution containing 0.04 M glycine buffer pH 10.5 and 0.01 M disodium-n-nitrophenyl phosphate (Sigma); the reaction mixture was incubated at 37 °C for 3 hours; Positive reaction manifested itself as yellow staining of the reaction mixture [59]; enzyme activity was determined within 3 hours of incubation by absorption on Uniplan apparatus (Russia) with a color filter at the wavelength of 450 nm.
- nuclease activity was determined by streaking cultures on RPA medium with the addition of an aqueous DNA solution to a final concentration of 2 mg/mL; before filling the medium, a sterile solution of CaCl2 (0.8 mg/mL) and 0.01% toluidine blue were added to the Petri dishes; in the case of the formation of DNase, a pink colored zone arose around the bacterial culture [59];
- the concentration of plasmid DNA in the strains was determined with screening method using a standard procedure; cells from a solid medium were suspended with a loop in 100 μl of buffer (50 mM Tris pH 8.0, 50 mM Na2-EDTA, 15% sucrose), 200 μl of alkaline solution (0.2 N NaOH, 1% SDS) and 150 μl of 3 M sodium acetate pH 5.0 were added, and centrifugation was performed for 5 minutes on a desktop centrifuge, then 1 mL of 96% ethanol was added to the sediment. The obtained DNA was analyzed in 0.8 % agarose in Tris-borate buffer pH 8.0 [48];
- when screening the strains for the presence of restriction endonucleases, individual colonies collected from a solid culture were suspended in 100–200 μl of TEN-buffer (0.1 M Tris, pH 7.5, 0.01 M EDTA, 0.05 M NaCl), lysocime and triton X-100 were used to destroy the cell wall of bacteria; the obtained cell extract was used for analysis for the presence of restriction endonucleases. DNAs of phages λcI857 and T7 were used as substrates for hydrolysis; electrophoresis of DNA after restriction was performed in 1% agarose (Sigma) [63]; the presence of restriction endonucleases in microorganism strains was revealed by the appearance of discrete fragments of substrate DNA in electrophoregram in UV light.
2.3.4. Microorganisms’ Antibiotic Resistance
2.4. Changes in the Methods Used in the 2006–2008 and 2012–2016 Studies
- A Tupolev-134 aircraft (Figure 3) was used for sounding of the atmosphere since 2011 [64]. The speed of this aircraft was maintained at the level of the Antonov-30 aircraft to maintain stable operation of the air intake. For sampling air containing microorganisms, the same impingers were used on both airplanes and in on-ground sampling; Figure 4. As noted above, the sampling time for airborne atmospheric sounding was 10 minutes for 2006–2008 and 5 minutes for 2012–2016.
- When analyzing samples by cultural methods in 2012–2016, in addition to the cultivation temperature of 28–30 °C, some isolates were additionally incubated at the temperature of 6–10 °C.
- And the last difference between the methods of samples analysis in the 2006–2008 and 2012–2016 investigations-the lists of antibiotics the sensitivity of isolates to which was determined are slightly different. For 2012–2016, the sensitivity of isolates was determined for a larger number of antibiotics, but this was done for not all identified isolates.
2.5. Data Analysis and Statistics
3. Results
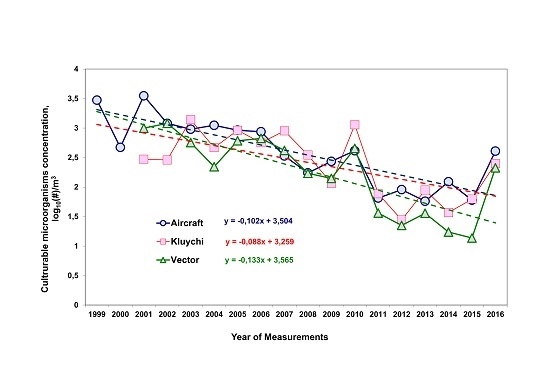
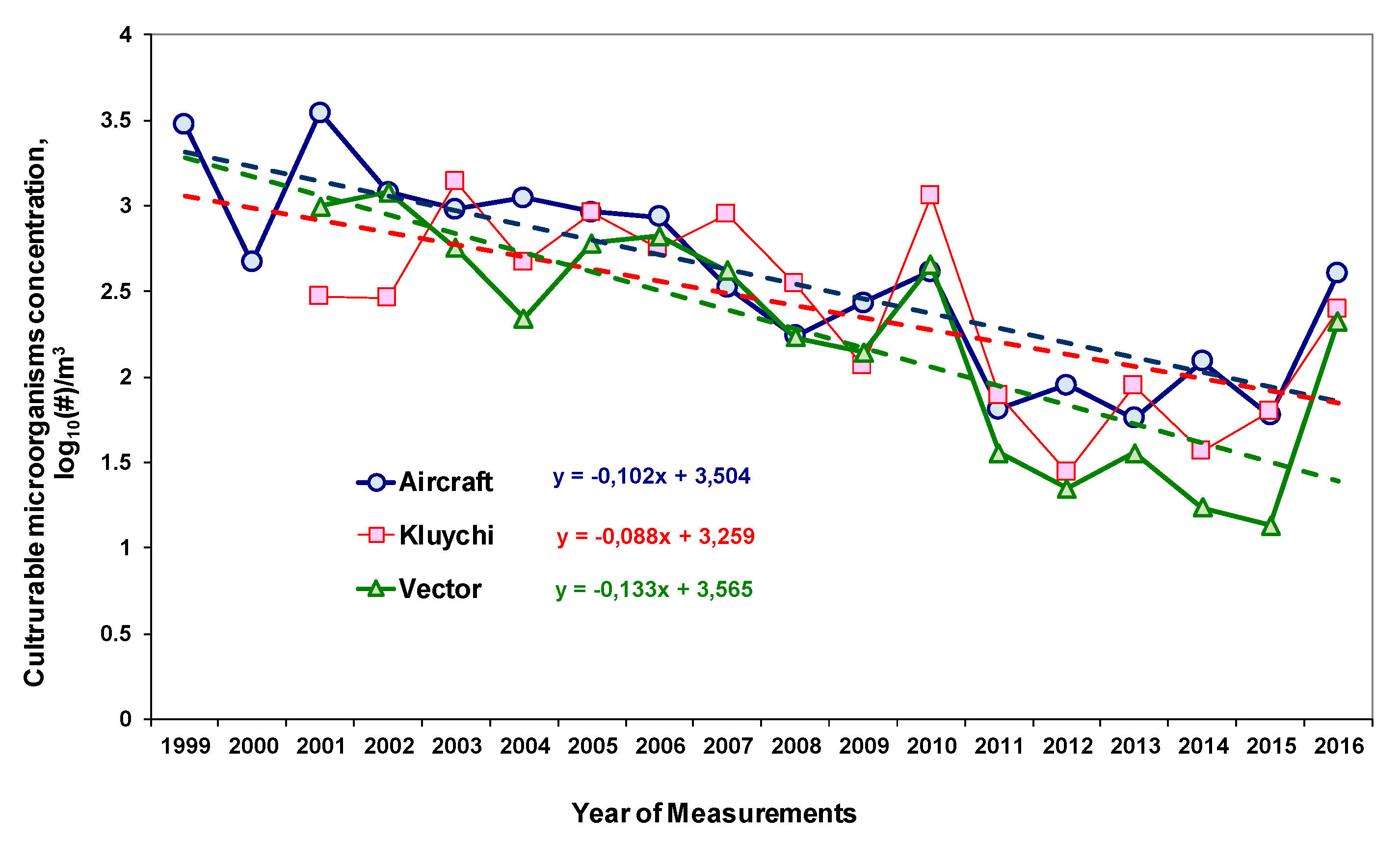
3.1. Long-Term Trends of Average Annual Concentrations of Culturable Microorganisms in the Atmospheric Aerosol of the South of Western Siberia
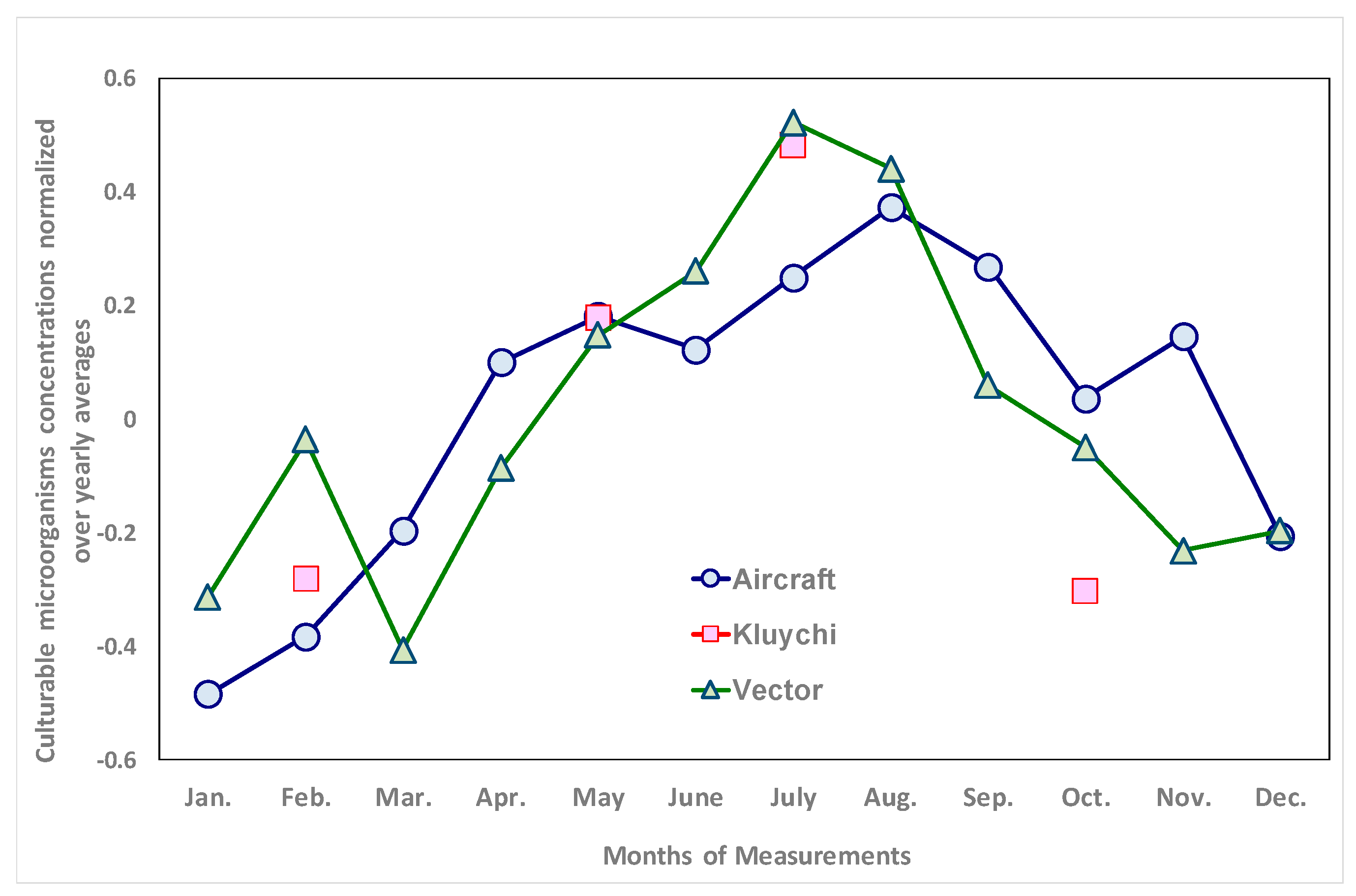
3.2. Sustainability of Culturable Microorganisms in the Environment
3.3. Potential Pathogenicity of Culturable Microorganisms in the Atmospheric Aerosol of the South of Western Siberia
3.4. Culturable Microorganisms’ Resistance to Antibiotics
3.5. Integral Index
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Artaxo, P.; Storms, H.; Bruynseels, F.; Van Grieken, R.; Maenhaut, W. Composition and sources of aerosols from the Amazon basin. J. Geophys. Res. 1988, 93, 1605–1615. [Google Scholar] [CrossRef]
- Artaxo, P.; Maenhaut, W.; Storms, H.; Van Grieken, R. Aerosol characteristics and sources for the Amazon basin during the wet season. Geophys. Res. 1990, 95, 16971–16985. [Google Scholar] [CrossRef]
- Matthias-Maser, S.; Jaenicke, R. Examination of atmospheric bioaerosol particles with radii >0.2 µm. J. Aerosol Sci. 1994, 25, 1605–1613. [Google Scholar] [CrossRef]
- Zhang, Q.; Jimenez, J.L.; Canagaratna, M.R.; Allan, J.D.; Coe, H.; Ulbrich, I.; Alfarra, M.R.; Takami, A.; Middlebrook, A.M.; Sun, Y.L.; et al. Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes. Geophys. Res. Lett. 2007, 34, L13801:1–L13801:6. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich-Nowoisky, J.; Kampf, C.J.; Weber, B.; Huffman, J.A.; Pöhlker, C.; Andreae, M.O.; Lang-Yona, N.; Burrows, S.M.; Gunthe, S.S.; Elbert, W.; et al. Bioaerosols in the Earth system: Climate, health, and ecosystem interactions. Atmos. Res. 2016, 182, 346–376. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.E.; Sands, D.C.; Bardin, M.; Jaenicke, R.; Vogel, B.; Leyronas, C.; Ariya, P.A.; Psenner, R. Microbiology and atmospheric processes: Research challenges concerning the impact of airborne micro-organisms on the atmosphere and climate. Biogeosciences 2011, 8, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Sahyoun, M.; Wex, H.; Gosewinkel, U.; Santl-Temkiv, T.; Nielsen, N.W.; Finster, K.; Sørensen, J.H.; Stratmann, F.; Korsholm, U.S. On the usage of classical nucleation theory in quantification of the impact of bacterial INP on weather and climate. Atmos. Environ. 2016, 139, 230–240. [Google Scholar] [CrossRef]
- Douwes, J.; Thorne, P.; Pearce, N.; Heederik, D. Bioaerosols health effects and exposure assessment: Progress and prospects. Ann. Occup. Hyg. 2003, 47, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Airborne bioaerosols and their impact on human health. J. Environ. Sci. 2018, 67, 23–35. [Google Scholar] [CrossRef]
- Walser, S.M.; Gerstner, D.G.; Brenner, B.; Bünger, J.; Eikmann, T.; Janssen, B.; Kolb, S.; Kolk, A.; Nowak, D.; Raulf, M.; et al. Evaluation of exposure–response relationships for health effects of microbial bio-aerosols—A systematic review. Int. J. Hyg. Environ. Health 2015, 218, 577–589. [Google Scholar] [CrossRef]
- Fernstrom, A.; Goldblatt, M. Aerobiology and Its Role in the Transmission of Infectious Diseases. J. Pathogens 2013, 2013, 493960:1–493960:14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ypma, R.J.F.; Jonges, M.; Bataille, A.; Stegeman, A.; Koch, G.; van Boven, M.; Koopmans, M.; van Ballegooijen, W.M.; Wallinga, J. Genetic Data Provide Evidence for Wind-Mediated Transmission of Highly Pathogenic Avian Influenza. J. Infect. Dis. 2013, 207, 730–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, H.R.; Mainelis, G.; White, L. Development and calibration of real-time PCR for quantification of airborne microorganisms in air samples. Atmos. Environ. 2006, 40, 7924–7939. [Google Scholar] [CrossRef]
- Belgrader, P.; Elkin, C.J.; Brown, S.B.; Nasarabadi, S.N.; Langlois, R.G.; Milanovich, F.P.; Colston, B.W., Jr.; Marshall, G.D. A reusable flow-through polymerase chain reaction instrument for the continuous monitoring of infectious biological agents. Anal. Chem. 2003, 75, 3446–3450. [Google Scholar] [CrossRef]
- Jiang, W.; Liang, P.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T.F. Optimized DNA extraction and metagenomics sequencing of airborne microbial communities. Nature Protocols 2015, 10, 768–779. [Google Scholar] [CrossRef]
- Maron, P.A.; Lejon, D.P.H.; Carvalho, E.; Bizet, K.; Lemanceau, P.; Ranjard, L.; Mougel, C. Assessing genetic structure and diversity of airborne bacterial communities by DNA fingerprinting and 16S rDNA clone libraries. Atmos. Environ. 2005, 39, 3687–3695. [Google Scholar] [CrossRef]
- Mbareche, H.; Brisebois, E.; Veillette, M.; Duchaine, C. Bioaerosol sampling and detection methods based on molecular approaches: No pain no gain. Sci. Total Environ. 2017, 599–600, 2095–2104. [Google Scholar] [CrossRef]
- Rinsoz, T.; Duquenne, P.; Greff-Mirguet, G.; Oppliger, A. Application of real-time PCR for total airborne bacterial assessment: Comparison with epifluorescence microscopy and culture-dependent methods. Atmos. Environ. 2008, 42, 6767–6774. [Google Scholar] [CrossRef]
- Xu, S.; Yao, M. NanoPCR detection of bacterial aerosols. J. Aerosol Sci. 2013, 65, 1–9. [Google Scholar] [CrossRef]
- Zhang, K.; Martiny, A.C.; Reppas, N.B.; Barry, K.W.; Malek, J.; Chisholm, S.W.; Church, G.M. Sequencing genomes from single cells by polymerase cloning. Nature Biotechnol. 2006, 24, 680–686. [Google Scholar] [CrossRef]
- Després, V.R.; Nowoisky, J.F.; Klose, M.; Conrad, R.; Andreae, M.O.; Pöschl, U. Characterisation of primary biogenic aerosol particles in urban, rural, and high-alpine air by DNA sequence and restriction fragment analysis of ribosomal RNA genes. Biogeosciences 2007, 4, 1127–1141. [Google Scholar] [CrossRef] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, S.; Srinivasan, S.; Jang, J.H.; Lee, D.; Lim, S.; Kim, K.S.; Jheong, W.; Lee, D.-W.; Park, E.-R.; Chung, H.-M.; et al. Metagenomic Analysis of Airborne Bacterial Community and Diversity in Seoul, Korea, during December 2014, Asian Dust Event. PLoS ONE 2017, 12, e0170693:1–e0170693:12. [Google Scholar] [CrossRef] [PubMed]
- Duquenne, P. On the Identification of Culturable Microorganisms for the Assessment of Biodiversity in Bioaerosols. Ann. Work Expo. Health. 2018, 62, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.-F.; Fan, X.-Y.; Li, H.-Y.; Pan, K.-L. Airborne Bacterial Communities of PM2.5 in Beijing-Tianjin-Hebei Megalopolis, China as Revealed by Illumina MiSeq Sequencing: A Case Study. Aerosol Air Qual. Res. 2017, 17, 788–798. [Google Scholar] [CrossRef]
- Serrano-Silva, N.; Calderón-Ezquerro, M.C. Metagenomic survey of bacterial diversity in the atmosphere of Mexico City using different sampling methods. Environ. Pollut. 2018, 235, 20–29. [Google Scholar] [CrossRef]
- Yoo, K.; Lee, T.K.; Choi, E.J.; Yang, J.; Shukla, S.K.; Hwang, S.-I.; Park, J. Molecular approaches for the detection and monitoring of microbial communities in bio-aerosols: A review. J. Environ. Sci. 2017, 51, 234–247. [Google Scholar] [CrossRef]
- Safatov, A.S.; Andreeva, I.S.; Belan, B.D.; Buryak, G.A.; Emel’yanova, E.K.; Jaenicke, R.; Panchenko, M.V.; Pechurkina, N.I.; Puchkova, L.I.; Repin, V.E.; et al. To what extent can viable bacteria in atmospheric aerosols be dangerous for humans? Clean 2008, 36, 564–571. [Google Scholar] [CrossRef]
- Peñuelas, J.; Fernández-Martínez, M.; Ciais, P.; Jou, D.; Piao, S.; Obersteiner, M.; Vicca, S.; Janssens, I.A.; Sardans, J. The bioelements, the elementome, and the biogeochemical niche. Ecology 2019, 100, e02652:1–e02652:15. [Google Scholar] [CrossRef]
- Jenhani, A.B.R.; Fathalli, A.; Djemali, I.; Changeux, T.; Romdhane, M.S. Tunisian reservoirs: Diagnosis and biological potentialities. Aquat. Living Resour. 2019, 32, 17:1–17:17. [Google Scholar] [CrossRef]
- Huang, Y.; Guenet, B.; Ciais, P.; Janssens, I.A.; Soong, J.L.; Wang, Y.; Goll, D.; Blagodatskaya, E.; Huang, Y. ORCHIMIC (v1.0), a microbe-mediated model for soil organic matter decomposition. Geosci. Model Dev. 2018, 11, 2111–2138. [Google Scholar] [CrossRef] [Green Version]
- Zouidi, M.; Borsali, A.H.; Allam, A.; Gros, R. Characterization of coniferous forest soils in the arid zone. Environ. Sci. 2018, 68, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Boyd, P.W.; Collins, S.; Dupont, S.; Fabricius, K.; Gattuso, J.-P.; Havenhand, J.; Hutchins, D.A.; Riebesell, U.; Rintoul, M.S.; Vichi, M.; et al. Experimental strategies to assess the biological ramifications of multiple drivers of global ocean change—A review. Glob Change Biol. 2018, 24, 2239–2261. [Google Scholar] [CrossRef]
- Błażejczyk, K.; Baranowski, J.; Błażejczyk, A. Climate related diseases. Current regional variability and projections to the year 2100. Quaestiones Geographicae 2018, 37, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Masyagina, O.V.; Menyailo, O.V. The impact of permafrost on carbon dioxide and methane fluxes in Siberia: A meta-analysis. Environ. Res. 2020, 182, 109096. [Google Scholar] [CrossRef] [PubMed]
- Zhang-Turpeinen, H.; Kivimäenpää, M.; Aaltonen, H.; Berninger, F.; Köster, E.; Köster, K.; Menyailo, O.; Prokushkin, A.; Pumpanen, J. Wildfire effects on BVOC emissions from boreal forest floor on permafrost soil in Siberia. Sci. Total Environ. 2020, 711, 134851. [Google Scholar] [CrossRef]
- Revich, B.A.; Shaposhnikov, D.A. Extreme temperature episodes and mortality in Yakutsk, East Siberia. Rural Remote Health. 2010, 10, 1338. [Google Scholar]
- Konovalov, I.B.; Lvova, D.A.; Beekmann, M.; Jethva, H.; Mikhailov, E.F.; Paris, J.-D.; Belan, B.D.; Kozlov, V.S.; Ciais, P.; Andreae, M.O. Estimation of black carbon emissions from Siberian fires using satellite observations of absorption and extinction optical depths. Atmos. Chem. Phys. 2018, 18, 14889–14924. [Google Scholar] [CrossRef] [Green Version]
- Antokhina, O.Y.; Antokhin, P.N.; Martynova, Y.V. Methane emissions from wildfires in Siberia caused by the atmospheric blocking in the summertime. In Proceedings of the 25th International Symposium on Atmospheric and Ocean Optics: Atmospheric Physics, Novosibirsk, Russia, 1–5 June 2019. [Google Scholar]
- Korotkova, E.M.; Zuev, V.V.; Pavlinsky, A.V. Trend and correlation analysis of air temperature and NDVI in Western Siberia over the period 1982-2015. In Proceedings of the 25th International Symposium on Atmospheric and Ocean Optics: Atmospheric Physics, Novosibirsk, Russia, 1–5 June 2019. [Google Scholar]
- Babushkina, E.A.; Zhirnova, D.F.; Belokopytova, L.V.; Tychkov, I.I.; Vaganov, E.A.; Krutovsky, K.V. Response of Four Tree Species to Changing Climate in a Moisture-Limited Area of South Siberia. Forests 2019, 10, 999. [Google Scholar] [CrossRef] [Green Version]
- Koenigk, T.; Fuentes-Franco, R. Towards normal Siberian winter temperatures? Int. J. Climatol. 2019, 39, 4567–4574. [Google Scholar] [CrossRef]
- Song, L.; Wu, R. Intraseasonal snow cover variations over western Siberia and associated atmospheric processes. J. Geophys. Res. Atmospheres. 2019, 124, 8994–9010. [Google Scholar] [CrossRef]
- Gorbatenko, V.P.; Sevastyanov, V.V.; Konstantinova, D.A.; Nosyreva, O.V. Characteristic of the snow cover for the Western Siberia territory. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; Volume 232. [Google Scholar] [CrossRef]
- Podnebesnykh, N.V.; Loginov, S.V.; Kharyutkina, E.V.; Usova, E.I. Vortex circulation and anomalous meteorological phenomena over the Asian territory of Russia in the context of climate change. In Proceedings of the 25th International Symposium on Atmospheric and Ocean Optics: Atmospheric Physics, Novosibirsk, Russia, 1–5 June 2019. [Google Scholar]
- Costello, A.; Abbas, M.; Allen, A.; Ball, S.; Bell, S.; Bellamy, R.; Friel, S.; Groce, N.; Johnson, A.; Kett, M.; et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet. 2009, 373, 1693–1733. [Google Scholar] [CrossRef]
- Ryti, N.R.I.; Guo, Y.; Jaakkola, J.J.K. Global association of cold spells and adverse health effects: A systematic review and meta-analysis. Environ Health Perspect. 2016, 124, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Zuev, V.E.; Belan, B.D.; Kabanov, D.M.; Kovalevskii, V.K.; Luk’yanov, O.Y.; Meleshkin, V.E.; Mikushev, M.K.; Panchenko, M.V.; Penner, I.E.; Pokrovskii, E.D.; et al. The "OPTIK – E,’" AN – 30 aircraft – laboratory for ecological investigations. Atmos. Oceanic Optics 1992, 5, 658–663. [Google Scholar]
- Belan, B.D.; Zuev, V.E.; Panchenko, M.V. Main results of airborne sounding of aerosol conducted at the Institute of Atmospheric Optics from 1981 till 1991. Atmos. Oceanic Optics 1995, 8, 131–156. [Google Scholar]
- Belan, B.D.; Ligotskii, A.V.; Luk’yanov, O.Y.; Mikushev, M.K.; Plokhikh, I.N.; Podanev, A.V.; Tolmachev, G.N. Database on the results of ecological survey of air basins. Atmos. Oceanic Optics 1994, 7, 585–590. [Google Scholar]
- Belan, B.D. Airborne ecological sounding of the atmosphere. Atmos. Oceanic Optics 1993, 6, 205–222. [Google Scholar]
- Equipment for Remote Probing of Atmospheric Parameters; V.E. Zuev, TP SB USSR AS, USSR: Tomsk, Russia, 1987; p. 156.
- Nazarov, L.E. Isokinetic atmospheric aerosol sampling from an airplane. Tr. Inst. Exp. Meteor. 1985, 9, 76–81. (In Russian) [Google Scholar]
- Griffiths, W.D.; DeCosemo, G.A.L. The assessment of bioaerosols: A critical review. J. Aerosol Sci. 1994, 25, 1425–1458. [Google Scholar] [CrossRef]
- Miller, J.H. Experiments in Molecular Genetics; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1972; p. 468. [Google Scholar]
- Saggie, J. The Methods of Soil Microbiology; Kolos Publishers, USSR: Moscow, Russia, 1983; p. 295. [Google Scholar]
- Methods of General Bacteriology, 2nd ed.; Gerhardt, F.; Murray, R.G.E.; Wood, W.A.; Krieg, N.R. (Eds.) Publisher American Society for Microbiology: Washington, DC, USA, 1994; p. 791. [Google Scholar]
- The Prokaryotes. A Handbook on Habitats, Isolation, and Identification of Bacteria; Starr, M.P.; Stolp, H.; Truper, H.G.; Balows, A.; Schlegel, H.G. (Eds.) Springer: Berlin/Heidelberg, Germany, 1981; p. 2596. [Google Scholar]
- Lebedeva, M.N. A Guide for Practical Studies in Medical Microbiology; Medicine, USSR: Moscow, Russia, 1973; p. 312. [Google Scholar]
- Maniatis, T.; Fritsch, E.E.; Sambrook, J. Molecular Cloning. A laboratory Manual; Cold Spring Harbor Laboratory: New York, NY, USA, 1982; p. 545. [Google Scholar]
- Weisburg, W.G.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- Statistical Methods in Microbiological Studies; Ashmarin, I.P.; Vorobyov, A.A. (Eds.) Medgiz, USSR: Leningrad, Russia, 1962; p. 180. [Google Scholar]
- Repin, V.E.; Lebedev, L.R.; Andreeva, I.S.; Puchkova, L.I.; Zernov, Y.P.; Serov, G.D.; Tereshchenko, T.A.; Afinogenova, G.N.; Pustoshilova, N.M. The producers of restriction endonucleases from natural microbe isolates and the development on this basis of enzymes production technologies. Biotechnology 1998, 2, 18–27. (In Russian) [Google Scholar]
- Anokhin, G.G.; Antokhin, P.N.; Arshinov, M.Y.; Barsuk, V.E.; Belan, B.D.; Belan, S.B.; Davydov, D.K.; Ivlev, G.A.; Kozlov, A.V.; Kozlov, V.S.; et al. OPTIK Tu–134 Aircraft Laboratory. Atmos. Oceanic Optics 2011, 24, 805–816. [Google Scholar] [CrossRef]
- Safatov, A.S.; Buryak, G.A.; Andreeva, I.S.; Olkin, S.E.; Reznikova, I.K.; Sergeev, A.N.; Belan, B.D.; Panchenko, M.V. Atmospheric bioaerosols. In Aerosols – Science and Technology; Agranovski, I., Ed.; Wiley – VCH Verlag GmbH & Co. KGaA: Wienheim, Germany, 2010; pp. 407–454. [Google Scholar]
- Armstrong, J.L.; Shigeno, D.S.; Calomiris, J.J.; Seigler, R.J. Antibiotic-resistant bacteria in drinking water. Appl. Environ. Microbiol. 1981, 42, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baquero, F.; Martinez, J.-L.; Cantón, R. Antibiotics and antibiotic resistance in water environment. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Boczek, L.A.; Rice, E.W.; Johnston, B.; Johnston, J.R. Occurrence of antibiotic-resistant uropathogenic Escherichia coli clonal group A in a wastewater effluent. Appl. Environ. Microbiol. 2007, 73, 4180–4184. [Google Scholar] [CrossRef] [Green Version]
- Esiobu, N.; Armenta, L.; Ike, J. Antibiotic resistance in soil and water environments. Int. J. Environ. Health Res. 2002, 12, 133–144. [Google Scholar] [CrossRef]
- Hermansson, M.; Jones, G.W.; Kjelleberg, S. Frequency of antibiotic and heavy metal resistance, pigmentation, and plasmids in bacteria of the marine air-water interface. Appl. Environ. Microbiol. 1987, 53, 2338–2342. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, T.; Kohnen, W.; Jansen, B.; Obst, U. Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol. Ecol. 2003, 43, 325–335. [Google Scholar] [CrossRef]
- Sengeløv, G.; Agersø, Y.; Halling-Sørensen, B.; Baloda, S.B.; Andersen, J.S.; Jensen, L.B. Bacterial antibiotic resistance levels in Danish farmland as a result of treatment with pig manure slurry. Environ. Int. 2003, 28, 587–595. [Google Scholar] [CrossRef]
- Sarda-Estève, R.; Baisnée, D.; Guinot, B.; Sodeau, J.; O’Connor, D.; Belmonte, J.; Besancenot, J.-P.; Petit, J.-E.; Thibaudon, M.; Oliver, G.; et al. Variability and Geographical Origin of Five Years Airborne Fungal Spore Concentrations Measured at Saclay, France from 2014 to 2018. Remote Sens. 2019, 11, 1671. [Google Scholar] [CrossRef] [Green Version]
- Millington, W.M.; Corden, M. Long term trends in indoor Aspergillus/Penicillum spore in Derby, UK form 1970 to 2003 and comparative study in 1994 and 1996 with indoor air of two local houses. Aerobiologia 2005, 21, 105–113. [Google Scholar] [CrossRef]
- Burrows, S.M.; Elbert, W.; Lawrence, M.G.; Pöschl, U. Bacteria in the global atmosphere – Part 1: Review and synthesis of literature data for different ecosystems. Atmos. Chem. Phys. 2009, 9, 9263–9280. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Lighthart, B. The annual bacterial particle concentration and size distribution in the ambient atmosphere in a rural area of the Willamette Valley, Oregon. Aerosol Sci. Technol. 2000, 32, 393–403. [Google Scholar] [CrossRef]
- Negrin, M.M.; Del Panno, M.T.; Ronco, A.E. Study of bioaerosols and site influence in the La Plata area (Argentina) using conventional and DNA (fingerprint) based methods. Aerobiologia 2007, 23, 249–258. [Google Scholar] [CrossRef]
- Bovallius, Å.; Bucht, B.; Roffey, R.; Ånäs, P. Three-year investigation of the natural airborne bacterial flora at four localities in Sweden. Appl. Environ. Microbiol. 1978, 35, 847–852. [Google Scholar] [CrossRef] [Green Version]
- Di Giorgio, C.; Krempff, A.; Guiraud, H.; Binder, P.; Tiret, C.; Dumenil, G. Atmospheric pollution by airborne microorganisms in the city of Marseilles. Atmos. Environ. 1996, 30, 155–160. [Google Scholar] [CrossRef]
- Bowers, R.M.; Clements, N.; Emerson, J.B.; Wiedinmyer, C.; Hannigan, M.P.; Fierer, N. Seasonal Variability in Bacterial and Fungal Diversity of the Near-Surface Atmosphere. Environ. Sci. Technol. 2013, 47, 12097–12106. [Google Scholar] [CrossRef]
- Li, M.; Qi, J.; Zhang, H.; Huang, S.; Li, L.; Gao, D. Concentration and size distribution of bioaerosols in an outdoor environment in the Qingdao coastal region. Sci. Total Environ. 2011, 409, 3812–3819. [Google Scholar] [CrossRef]
- Bertolini, V.; Gandolfi, I.; Ambrosini, R.; Bestetti, G.; Innocente, E.; Rampazzo, G.; Franzetti, A. Temporal variability and effect of environmental variables on airborne bacterial communities in an urban area of Northern Italy. Appl. Microbiol. Biotechnol. 2013, 97, 6561–6570. [Google Scholar] [CrossRef] [Green Version]
- Brągoszewska, E.; Mainka, A.; Pastuszka, J.S. Concentration and Size Distribution of Culturable Bacteria in Ambient Air during Spring and Winter in Gliwice: A Typical Urban Area. Atmos. 2017, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Striluk, M.L.; Aho, K.; Weber, C.F. The effect of season and terrestrial biome on the abundance of bacteria with plant growth-promoting traits in the lower atmosphere. Aerobiologia 2017, 33, 137–149. [Google Scholar] [CrossRef]
- Tanaka, D.; Terada, Y.; Nakashima, T.; Sakatoku, A.; Nakamura, S. Seasonal variations in airborne bacterial community structures at a suburban site of central Japan over a 1-year time period using PCR-DGGE method. Aerobiologia 2015, 31, 143–157. [Google Scholar] [CrossRef]
- Ravva, S.V.; Hernlem, B.J.; Sarreal, C.Z.; Mandrell, R.E. Bacterial communities in urban aerosols collected with wetted-wall cyclonic samplers and seasonal fluctuations of live and culturable airborne bacteria. J. Environ. Monit. 2012, 14, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Mandal, P.; Majumdar, D.; Aggarwal, S.G.; Srivastava, A. Characterization of Bioaerosols and their Relation with OC, EC and Carbonyl VOCs at a Busy Roadside Restaurants-Cluster in New Delhi. Aerosol Air Qual. Res. 2016, 16, 3198–3211. [Google Scholar] [CrossRef] [Green Version]
- Rajput, P.; Anjum, M.H.; Gupta, T. One year record of bioaerosols and particles concentration in Indo-Gangetic Plain: Implications of biomass burning emissions to high-level of endotoxin exposure. Environ. Pollut. 2017, 224, 98–106. [Google Scholar] [CrossRef]
- Agarwal, S. Seasonal variability in size-segregated airborne bacterial particles and their characterization at different source-sites. Environ. Sci. Pollut. Res. 2017, 24, 13519–13527. [Google Scholar] [CrossRef]
- Park, J.; Ichijo, T.; Nasu, M.; Yamaguchi, N. Investigation of bacterial effects of Asian dust events through comparison with seasonal variability in outdoor airborne bacterial community. Sci. Rep 2016a, 6, 35706:1–35706:8. [Google Scholar] [CrossRef] [Green Version]
- Kallawicha, K.; Lung, S.-C.C.; Chuang, Y.-C.; Wu, C.-D.; Chen, T.-H.; Tsai, Y.-J.; Chao, H.J. Spatiotemporal Distributions and Land-Use Regression Models of Ambient Bacteria and Endotoxins in the Greater Taipei Area. Aerosol Air Qual. Res. 2015, 15, 1448–1459. [Google Scholar] [CrossRef] [Green Version]
- Barrett, T.C.; Mok, W.W.K.; Murawski, A.M.; Brynildsen, M.P. Enhanced antibiotic resistance development from fluoroquinolone persisters after a single exposure to antibiotic. Nat. Commun. 2019, 10, 117:1–117:11. [Google Scholar] [CrossRef] [Green Version]
- Knapp, C.W.; Dolfing, J.; Ehlert, P.A.; Graham, D.W. Evidence of increasing antibiotic resistance gene abundances in archived soils since 1940. Environ. Sci. Technol. 2009, 44, 580–587. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Wang, F.; Manaia, C.M.; Virta, M.; Sheng, H.J.; Ma, L.P.; Zhang, T.; Topp, E. Antibiotic resistance genes in the human-impacted environment: A One Health perspective. Pedosphere 2019, 29, 273–282. [Google Scholar] [CrossRef]
- Xu, R.; Yang, Z.-H.; Zheng, Y.; Wang, Q.-P.; Bai, Y.; Liu, J.-B.; Zhang, Y.-R.; Xiong, W.-P.; Lu, Y.; Fan, C.-Z. Metagenomic analysis reveals the effects of long-term antibiotic pressure on sludge anaerobic digestion and antimicrobial resistance risk. Bioresource Technol. 2019, 282, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Yasir, M.; Bibi, F.; Abujamel, T.S.; Hashem, A.M.; Sartaj Sohrab, S.; Al-Ansari, A.; Al-Sofyani, A.A.; Al-Ghamdi, A.K.; Al-sieni, A.; et al. Taxonomic diversity of antimicrobial-resistant bacteria and genes in the Red Sea coast. Sci. Total Environ. 2019, 677, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Houndt, T.; Ochman, H. Long-term shifts in patterns of antibiotic resistance in enteric bacteria. Appl. Environ. Microbiol. 2000, 66, 5406–5409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moellering, R.C., Jr.; Graybill, J.R.; McGowan, J.E.; Corey, L. Antimicrobial resistance prevention initiative—An update: Proceedings of an Expert Panel on Resistance. Am. J. Med. 2007, 120, S4–S25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zuo, J.; Yu, X.; Shi, X.; Chen, L.; Li, Z. Quantification of multi-antibiotic resistant opportunistic pathogenic bacteria in bioaerosols in and around a pharmaceutical wastewater treatment plant. J. Environ. Sci. 2018, 72, 53–63. [Google Scholar] [CrossRef]
- Cole, M.L.; Singh, O.V. Microbial occurrence and antibiotic resistance in ready-to-go food items. J. Food Sci. Technol. 2018, 55, 2600–2609. [Google Scholar] [CrossRef]
- Mishra, M.; Arukha, A.P.; Patel, A.K.; Behera, N.; Mohanta, T.K.; Yadav, D. Multi-Drug Resistant Coliform: Water Sanitary Standards and Health Hazards. Frontiers Pharmacol. 2018, 9, 311:1–311:8. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Ekka, R.; Mishra, M.; Mohapatra, H. Association study of multiple antibiotic resistance and virulence: A strategy to assess the extent of risk posed by bacterial population in aquatic environment. Environ. Monit. Assess. 2017, 189, 320:1–320:12. [Google Scholar] [CrossRef]
- Safatov, A.S.; Andreeva, I.S.; Buryak, G.A.; Vechkanov, V.A.; Vorobyeva, I.G.; Ol’kin, S.E.; Reznikova, I.K.; Solovyanova, N.A.; Teplyakova, T.V.; Arshinov, M.Y.; et al. The results of long-term monitoring of atmospheric aerosols biogenic components at altitudes 500–7000 m in the South of Western Siberia. In Proceedings of the Abstract European Aerosol Conference 2016, Tours, France, 4–9 September 2016; p. O2-AAS-AAP-08. [Google Scholar]
- Blais Lecours, P.; Duchaine, C.; Thibaudon, M.; Marsolais, D. Health Impacts of Bioaerosol Exposure. In Microbiology of Aerosols; Delort, A.-M., Amato, P., Eds.; Wiley-Blackwell Published: Hoboken, NJ, USA, 2018; Chapter 4.1; pp. 251–268. [Google Scholar]




| Sampling Site | Values | 2006–2007 | 2007–2008 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|---|
| Vector | Average | 0.359 | 0.372 | 0.324 | 0.247 | 0.244 | 0.135 | 0.252 |
| Standard deviation | 0.223 | 0.206 | 0.256 | 0.243 | 0.229 | 0.132 | 0.209 | |
| Klyuchi | Average | 0.315 | 0.335 | 0.452 | 0.399 | 0.381 | 0.354 | 0.354 |
| Standard deviation | 0.216 | 0.182 | 0.246 | 0.222 | 0.215 | 0.108 | 0.149 | |
| Aircraft | Average | 0.307 | 0.399 | 0.567 | 0.380 | 0.415 | 0.405 | 0.431 |
| Standard deviation | 0.212 | 0.222 | 0.333 | 0.191 | 0.137 | 0.125 | 0.176 |
| Sampling Site | Values | 2006–2007 | 2007–2008 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|---|
| Vector | Average | 0.239 | 0.239 | 0.315 | 0.202 | 0.213 | 0.374 | 0.292 |
| Standard deviation | 0.148 | 0.121 | 0.177 | 0.117 | 0.146 | 0.177 | 0.175 | |
| Klyuchi | Average | 0.209 | 0.275 | 0.232 | 0.205 | 0.210 | 0.371 | 0.175 |
| Standard deviation | 0.132 | 0.139 | 0.187 | 0.138 | 0.134 | 0.197 | 0.175 | |
| Aircraft | Average | 0.235 | 0.270 | 0.306 | 0.247 | 0.203 | 0.409 | 0.303 |
| Standard deviation | 0.139 | 0.150 | 0.196 | 0.130 | 0.116 | 0.182 | 0.177 |
| Sampling Site | Values | 2006–2007 | 2007–2008 | 2012 | 2013 | 2014 |
|---|---|---|---|---|---|---|
| Vector | Average | 0.207 | 0.206 | 0.170 | 0.195 | 0.152 |
| Standard deviation | 0.181 | 0.129 | 0.173 | 0.208 | 0.107 | |
| Klyuchi | Average | 0.262 | 0.245 | 0.265 | 0.239 | 0.144 |
| Standard deviation | 0.211 | 0.146 | 0.106 | 0.231 | 0.107 | |
| Aircraft | Average | 0.232 | 0.206 | 0.200 | 0.314 | 0.278 |
| Standard deviation | 0.184 | 0.149 | 0.188 | 0.319 | 0.207 |
| Sampling Site | Values | 2006–2007 | 2007–2008 | 2012 | 2013 | 2014 | 2015 | 2016 |
|---|---|---|---|---|---|---|---|---|
| Vector | Average | 0.0035 | 0.0007 | 0.0001 | 0.0011 | 0.0003 | 0.0001 | 0.0018 |
| Standard deviation | 0.0110 | 0.0017 | 0.0002 | 0.0026 | 0.0008 | 0.00015 | 0.0061 | |
| Klyuchi | Average | 0.0010 | 0.0047 | 0.0002 | 0.0005 | 0.0001 | 0.0007 | 0.0032 |
| Standard deviation | 0.0045 | 0.0109 | 0.0003 | 0.0009 | 0.0002 | 0.0012 | 0.0057 | |
| Aircraft | Average | 0.0016 | 0.0019 | 0.0000 | 0.0000 | 0.0037 | 0.0004 | 0.0018 |
| Standard deviation | 0.0034 | 0.0058 | 0.0001 | 0.0001 | 0.0059 | 0.0004 | 0.0024 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safatov, A.; Andreeva, I.; Buryak, G.; Ohlopkova, O.; Olkin, S.; Puchkova, L.; Reznikova, I.; Solovyanova, N.; Belan, B.; Panchenko, M.; et al. How Has the Hazard to Humans of Microorganisms Found in Atmospheric Aerosol in the South of Western Siberia Changed over 10 Years? Int. J. Environ. Res. Public Health 2020, 17, 1651. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17051651
Safatov A, Andreeva I, Buryak G, Ohlopkova O, Olkin S, Puchkova L, Reznikova I, Solovyanova N, Belan B, Panchenko M, et al. How Has the Hazard to Humans of Microorganisms Found in Atmospheric Aerosol in the South of Western Siberia Changed over 10 Years? International Journal of Environmental Research and Public Health. 2020; 17(5):1651. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17051651
Chicago/Turabian StyleSafatov, Alexandr, Irina Andreeva, Galina Buryak, Olesia Ohlopkova, Sergei Olkin, Larisa Puchkova, Irina Reznikova, Nadezda Solovyanova, Boris Belan, Mikhail Panchenko, and et al. 2020. "How Has the Hazard to Humans of Microorganisms Found in Atmospheric Aerosol in the South of Western Siberia Changed over 10 Years?" International Journal of Environmental Research and Public Health 17, no. 5: 1651. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17051651