Metabolic Functional Community Diversity of Associated Bacteria during the Degradation of Phytoplankton from a Drinking Water Reservoir
Abstract
:1. Introduction
2. Experimental Section
2.1. Sampling Description
2.2. Water Quality Determination
2.3. Phytoplankton Community Composition Analysis
2.4. Associated Bacterial Community Metabolic Determination
2.5. Redundancy Analysis (RDA) of Phytoplankton Community Composition and Associated Bacterial Community Metabolic Fingerprints
2.6. Statistical Analysis
3. Results and Discussion
3.1. Water Quality
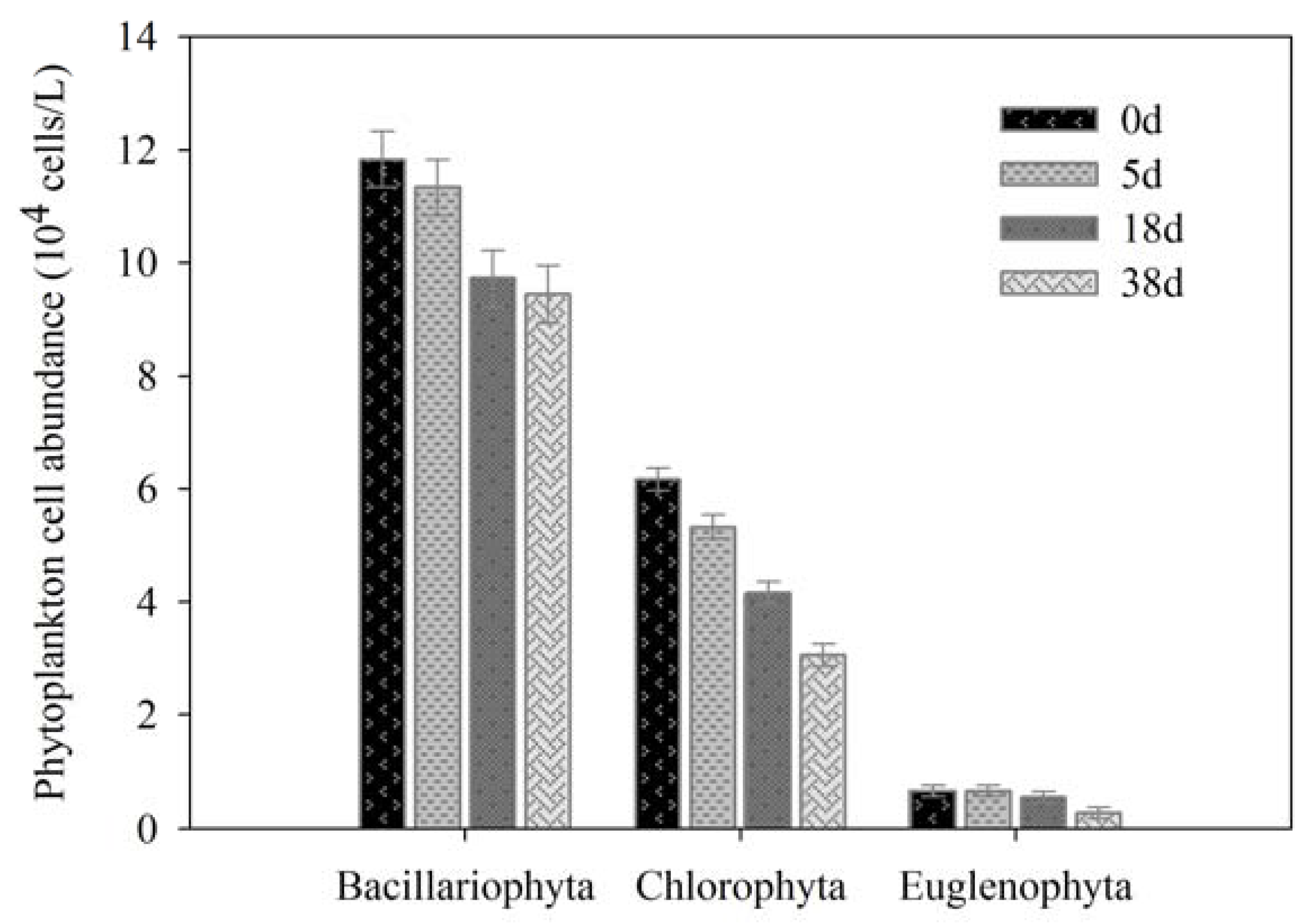
3.2. Phytoplankton Cell Concentration and Composition
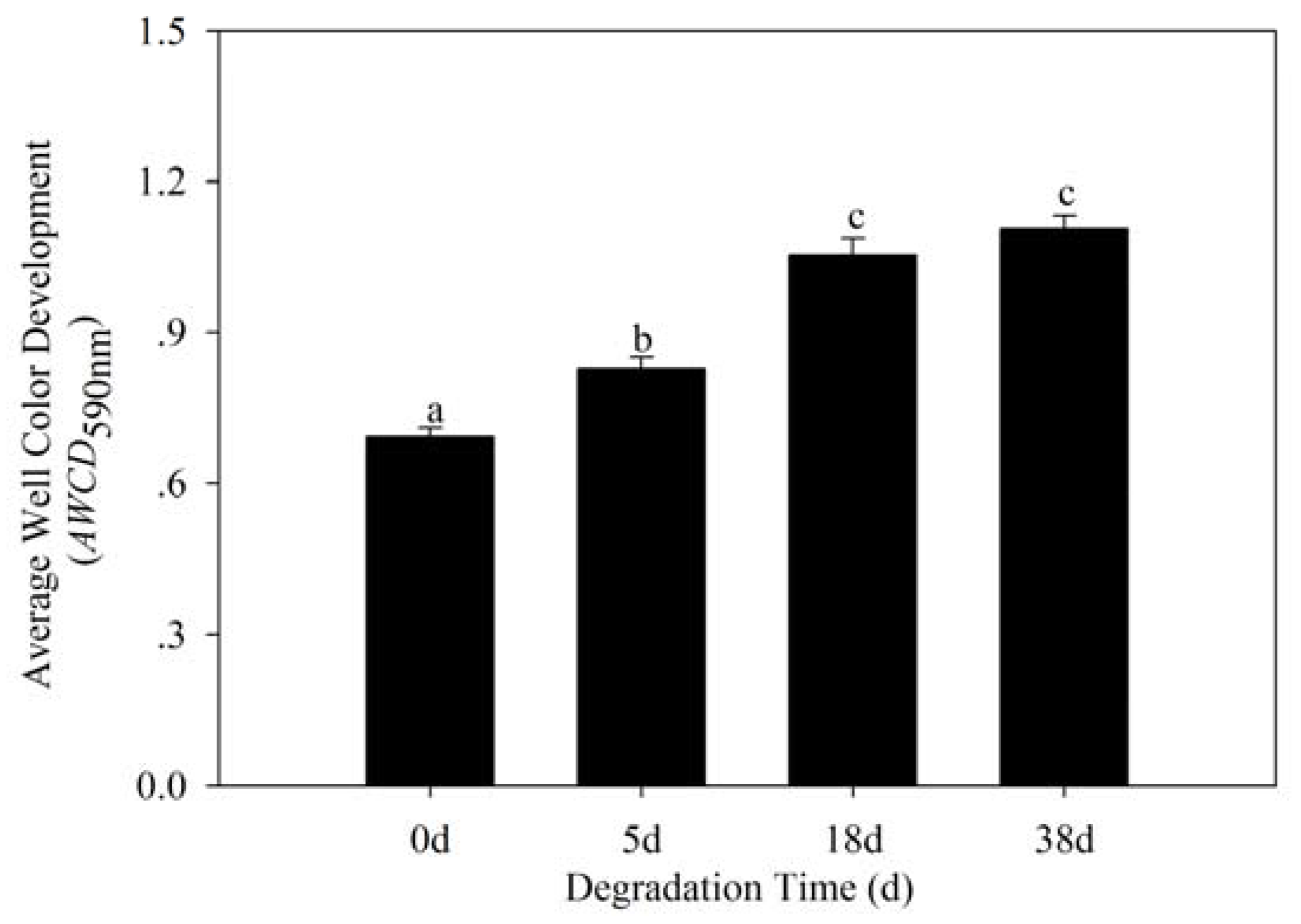
3.3. Bacterial Community Metabolic Profiles
3.4. Correlation between the Phytoplankton Community and Associated Bacterial Community Metabolic Fingerprints (RDA)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dickerson, T.L.; Williams, H.N. Functional diversity of bacterioplankton in three North Florida Freshwater Lakes over an annual cycle. Microb. Ecol. 2014, 67, 34–44. [Google Scholar] [CrossRef]
- Liu, L.; Yang, J.; Lv, H.; Yu, Z. Synchronous dynamics and correlations between bacteria and phytoplankton in a subtropical drinking water reservoir. FEMS Microbiol. Ecol. 2014, 90, 126–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.J. Interactions between bacteria and algae in aquatic ecosystems. Ann. Rev. Ecol. Syst. 2003, 13, 291–314. [Google Scholar] [CrossRef]
- Amin, S.A.; Parker, M.S.; Armbrust, E.V. Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 2012, 76, 667–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, S.; Harder, T.; Burke, C.; Steinberg, P.; Kjelleberg, S.; Thomas, T. The seaweed holobiont: Understanding seaweed-bacteria interactions. FEMS Microbiol. Rev. 2013, 37, 462–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aota, Y.; Nakajima, H. Mutualistic relationships between phytoplankton and bacteria caused by carbon excretion from phytoplankton. Ecol. Res. 2001, 16, 289–299. [Google Scholar] [CrossRef]
- Bickel, S.L.; Tang, K.W. Carbon substrate usage by zooplankton-associated bacteria, phytoplankton-associated bacteria, and free-living bacteria under aerobic and anaerobic conditions. Mar. Biol. 2014, 161, 2233–2242. [Google Scholar] [CrossRef] [Green Version]
- Fouilland, E.; Tolosa, I.; Bonnet, D.; Bouvier, C.; Bouvier, T.; Bouvy, M.; Got, P.; Le Floch, E.; Mostajir, B.; Roques, C.; et al. Bacterial carbon dependence on freshly produced phytoplankton exudates under different nutrient availability and grazing pressure conditions in coastal marine waters. FEMS Microbiol. Ecol. 2014, 87, 757–769. [Google Scholar] [CrossRef] [Green Version]
- Kent, A.D.; Jones, S.E.; Lauster, G.H.; Graham, J.M.; Newton, R.J.; McMahon, K.D. Experimental manipulations of microbial food web interactions in a humic lake: Shifting biological drivers of bacterial community structure. Environ. Microbiol. 2006, 8, 1448–1459. [Google Scholar] [CrossRef]
- Xing, P.; Kong, F.X.; Cao, H.S.; Zhang, M. Relationship between bacterioplankton and phytoplankton community dynamics during late spring and early summer in Lake Taihu. Acta Ecol. Sin. 2007, 27, 1696–1702. [Google Scholar]
- Rooney-varga, J.N.; Giewat, M.W.; Savin, M.C.; Sood, S.; LeGresley, M.; Martin, J.L. Links between phytoplankton and bacterial community dynamics in a coastal marine environment. Microb. Ecol. 2005, 49, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Baiser, B.; Ardeshiri, R.S.; Ellison, A.M. Species richness and trophic diversity increase decomposition in a co-evolved food web. PLoS ONE 2011, 6, e20672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.H.; Huang, T.L.; Chen, S.N.; Liu, T.T. Spatial pattern of bacterial community functional diversity in a drinking water reservoir, Shaanxi Province, Northwest China. J. Pure Appl. Microbiol. 2013, 7, 1647–1654. [Google Scholar]
- Zhang, H.H.; Tang, M.; Chen, H.; Zheng, C.L. Inoculation with ectomycorrhizal fungi affects microbial biomass and bacterial functional diversity in the rhizosphere of Pinus tabulaeformis seedlings. Eur. J. Soil Biol. 2010, 46, 55–61. [Google Scholar] [CrossRef]
- Zhu, L. Correlation and Interaction Between Bloom-Forming Cyanobacteria and Their Associated Bacteria; University of Chinese Academy of Sciences: Beijing, China, 2014. [Google Scholar]
- Cong, H.B.; Huang, T.L.; Chai, B.B. A new mixing-oxygenating technology for water quality improvement of urban water source and its implication in a reservoir. Renew. Energy 2009, 34, 2054–2060. [Google Scholar] [CrossRef]
- Zhang, H.H.; Chen, S.N.; Huang, T.L.; Ma, W.X.; Xu, J.L.; Sun, X. Vertical distribution of bacterial community diversity and water quality during the reservoir thermal stratification. Int. J. Environ. Res. Public Health 2015, 12, 6933–6945. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y. Study on the Response Characteristics of Water Quality to the Seasonal Thermal Stratification and its In-Situ Pollution Control in a Deep Channel Reservoir of Northern China; Xi’an University of Architecture and Technology: Xi’an, China, 2012. [Google Scholar]
- Wang, G.F.; Li, X.N.; Fang, Y.; Huang, R.; Lv, X.W. DMTS production and water quality variation during decomposition of algal mats. J. Jiang Su Univ. 2013, 34, 361–366. [Google Scholar]
- Huang, T.L.; Li, X.; Rijnaarts, H.; Grotenhuis, T.; Ma, W.X.; Sun, X.; Xu, J. Effects of storm runoff on the thermal regime and water quality of a deep, stratified reservoir in a temperate monsoon zone, in Northwest China. Sci. Total Environ. 2014, 485, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.M.; Chen, S.N.; Huang, T.L.; Li, B.Q.; Li, N.; Liu, K.W.; Zong, R.R.; Miao, Y.T.; Huang, X. Community compositions of phytoplankton and eukaryotes during the mixing periods of a drinking water reservoir: Dynamics and interactions. Int. J. Environ. Res. Public Health 2020, 17, 1128. [Google Scholar] [CrossRef] [Green Version]
- Classen, A.T.; Boyle, S.I.; Haskins, K.E.; Overby, S.T.; Hart, S.C. Community-level physiological profiles of bacteria and fungi: Plate type and incubation temperature influences on contrasting soils. FEMS Microbiol. Ecol. 2003, 44, 319–328. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.H.; Chen, S.N.; Huang, T.L.; Shang, P.L.; Yang, X.; Ma, W.X. Indoor heating drives water bacterial growth and community metabolic profile changes in building tap pipes during the winter season. Int. J. Environ. Res. Public Health 2015, 12, 13649–13661. [Google Scholar] [CrossRef] [PubMed]
- Garland, J.L. Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol. Biochem. 1996, 28, 223–231. [Google Scholar] [CrossRef]
- Fisk, M.C.; Ruether, K.F.; Yavitt, J.B. Microbial activity and functional composition among northern peatland ecosystems. Soil Biol. Biochem. 2003, 35, 591–602. [Google Scholar] [CrossRef]
- Lan, G.Y.; Zhu, H.; Cao, M. Scale effects of three diversity in a tropical rain forest in Xishuangbanna. Acta Bot. Boreal. 2012, 32, 1454–1458. [Google Scholar]
- Davis, T.W.; Berry, D.L.; Boyer, G.L.; Gobler, C.J. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis, during cyanobacteria blooms. Harmful Algae 2009, 8, 715–725. [Google Scholar] [CrossRef]
- Ishaq, F.; Khan, A. Aquatic Biodiversity as an ecological indicators for water quality criteria of River Yamuna in Doon Valley, Uttarakhand, India. Word J. Fish. Mar. Sci. 2013, 5, 322–334. [Google Scholar]
- Sipaúba-Tavares, L.H.; Donadon, A.R.V.; Milan, R.N. Water quality and plankton populations in an earthen polyculture pond. Braz. J. Biol. 2011, 71, 845–855. [Google Scholar] [CrossRef]
- Kent, A.D.; Jones, S.E.; Yannarell, A.C.; Graham, J.M.; Lauster, G.H.; Kratz, T.K.; Triplett, E.W. Annual patterns in bacterioplankton community variability in a humic lake. Microb. Ecol. 2004, 48, 550–560. [Google Scholar] [CrossRef]
- Paver, S.F.; Youngblut, N.D.; Whitaker, R.J.; Kent, A.D. Phytoplankton succession affects the composition of Polynucleobacter subtypes in humic lakes. Environ. Microbiol. 2015, 17, 816–828. [Google Scholar] [CrossRef]
- Van Hannen, E.J.; Mooij, W.; van Agterveld, M.P.; Gons, H.J.; Laanbroek, H.J. Detritus-dependent development of the microbial community in an experimental system: Qualitative analysis by denaturing gradient gel electrophoresis. Appl. Environ. Microb. 1999, 65, 2478–2484. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.J.; Qin, B.Q.; Zhu, G.W. Release of colloidal phosphorus, nitrogen and organic carbon in the course of dying and decomposing of canobacteria. China Environ. Sci. 2007, 27, 341–345. [Google Scholar]
- Tranvik, L. Effects of colloidal organic matter on the growth of bacteria and protists in lake water. Limnol. Oceanogr. 1994, 39, 1276–1285. [Google Scholar] [CrossRef]
- Sarmento, H.; Gasol, J.M. Use of phytoplankton-derived dissolved organic carbon by different types of bacterioplankton. Environ. Microbiol. 2012, 14, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Hmelo, L.R.; Tol, H.M.V.; Durham, B.P.; Carlson, L.T.; Heal, K.R.; Morales, R.L.; Bethiaume, C.T.; Parker, M.S.; Djunaedi, B.; et al. Interaction and signaling between a cosmopolitan phytoplankton and associated bacteria. Nature 2015, 522, 98–101. [Google Scholar] [CrossRef]
- Dang, H.; Lovell, C.R. Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes. Appl. Environ. Microb. 2000, 66, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Hold, G.L.; Smith, E.A.; Rappe’, M.S.; Maas, E.W.; Moore, E.R.B.; Stroempl, C.; Stephen, J.R.; Prosser, J.I.; Birkbeck, T.H.; Gallacher, S. Characterisation of bacterial communities associated with toxic and non-toxic dinoflagellates: Alexandrium spp. and Sprippsiellatrochoidea. FEMS Microbiol. Ecol. 2001, 37, 161–173. [Google Scholar] [CrossRef]
- Sapp, M.; Gerdts, G.; Wellinger, M.; Wichels, A. Consuming algal products: Trophic interactions of bacteria and a diatom species determined by RNA stable isotope probing. Helgoland. Mar. Res. 2008, 62, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Wawrik, B.; Boling, W.B.; Nostrand, J.D.V.; Xie, J.; Zhou, J.; Bronk, D.A. Assimilatory nitrate utilization by bacteria on the West Florida Shelf as determined by stable isotope probing and functional microarray analysis. FEMS Microbiol. Ecol. 2012, 79, 400–411. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Cupples, A.M. Diversity of five anaerobic toluene-degrading microbial communities investigated using stable isotope probing. Appl. Environ. Microb. 2012, 78, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Sun, X.; Cupples, A.M. Anaerobic methyl tert-butyl ether-degrading microorganisms identified in wastewater treatment plant samples by stable isotope probing. Appl. Environ. Microb. 2012, 78, 2973–2980. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Sharp, C.E.; Jones, G.M.; Grasby, S.E.; Brady, A.L.; Dunfield, P.F. Stable-isotope probing identifies uncultured planctomycetes as primary degraders of a complex Heteropolysaccharide in soil. Appl. Environ. Microb. 2015, 81, 4607–4615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.H.; Feng, J.; Chen, S.N.; Zhao, Z.F.; Li, B.Q.; Wang, Y.; Jia, J.Y.; Li, S.L.; Wang, Y.; Yan, M.M.; et al. Geographical patterns of nirS gene abundance and nirS-Type denitrifying bacterial community associated with activated sludge from different wastewater treatment plants. Microb. Ecol. 2019, 77, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; He, H.Y.; Zong, R.R.; Liu, K.W.; Miao, Y.T.; Yan, M.M.; Xu, L. Geographical patterns of algal communities associated with different urban lakes in China. Int. J. Environ. Res. Public Health 2020, 17, 1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Carbohydrates | Carboxylic Acids | Amino Acids | Polymers | Phenolic Compounds | Amines |
|---|---|---|---|---|---|
| D,L-a-Glycerol a-D-lactose β-Methyl-D-glucoside I-Erythritol D-Cellobiose D-Mannitol D-Xylose Glucose-1-phosphate N-Acetyl-D-glucosamine D-Galactonic acid y-lactone | Pyruvic acid Methyl ester y-Hydroxy butyric acid D-Galacturonic acid a-Ketobutyric acid D-Glucosaminic acid D-Malic acid Itaconic acid | L-Arginine L-Threonine L-Serine L-Phenylalanine L-Asparagine Glycyl-L-glutamic acid | a-Cyclodextrin Glycogen Tween40 Tween80 | 4-Hydroxy benzoic acid 2-Hydroxy benzoic acid | Phenylethyl-amine Putrescine |
| Dep (m) | T (°C) | DO (mg/L) | pH | ORP (mV) | Con (µS/cm) | Tur (NTU) | Chl-a (µg/L) | TN (mg/L) | TP (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| 0.5 | 12.06 | 9.91 | 7.98 | 347 | 174 | 9 | 1.54 | 0.98 | 0.012 |
| Degradation Time | Shannon’s Diversity (Hp) | Relative Abundance of Melosira (M) |
|---|---|---|
| 0 d | 1.479 | 0.518 |
| 5 d | 1.432 | 0.538 |
| 18 d | 1.453 | 0.519 |
| 38 d | 1.299 | 0.543 |
| Degradation Time | Species Richness (S) | Simpson’s Diversity (D) | Shannon’s Diversity (H) |
|---|---|---|---|
| 0 d | 23.667 ± 1.155b | 0.949 ± 0.002b | 3.130 ± 0.037b |
| 5 d | 27.667 ± 0.577a | 0.957 ± 0.001a | 3.257 ± 0.015a |
| 18 d | 28.333 ± 0.577a | 0.960 ± 0.0004a | 3.297 ± 0.007a |
| 38 d | 27.667 ± 1.528a | 0.958 ± 0.002a | 3.261 ± 0.040a |
| Degradation Time | Amino Acids | Amines | Polymers | Phenolic Compounds | Carbohydrates | Carboxylic Acids |
|---|---|---|---|---|---|---|
| 0 d | 0.62 ± 0.07b | 0.74 ± 0.25a | 0.95 ± 0.20a | 0.30 ± 0.09a | 0.59 ± 0.07b | 0.86 ± 0.02b |
| 5 d | 0.84 ± 0.04b | 0.39 ± 0.09a | 0.93 ± 0.05a | 0.78 ± 0.24b | 0.65 ± 0.02b | 1.16 ± 0.05a |
| 18 d | 1.36 ± 0.12a | 0.67 ± 0.06a | 1.00 ± 0.07a | 1.62 ± 0.06c | 0.96 ± 0.06a | 0.90 ± 0.03b |
| 38 d | 1.18 ± 0.15a | 0.92 ± 0.45a | 1.38 ± 0.12b | 1.00 ± 0.04b | 1.02 ± 0.08a | 1.09 ± 0.06a |
| Carbon Substrates Located in BIOLOG ECO Plate | PC1 Score | PC2 Score |
|---|---|---|
| Tween80 | −0.703 | 0.521 |
| Glycogen | 0.768 | −0.006 |
| D-Cellobiose | 0.880 | 0.342 |
| ß-Methyl-D-Glucoside | 0.806 | 0.428 |
| D-Mannitol | −0.868 | 0.437 |
| N-Acetyl-D-Glucosamine | 0.862 | 0.072 |
| D-Glucosaminic Acid | −0.848 | 0.425 |
| D,L-a-Glycerol | 0.939 | −0.222 |
| 2-Hydroxy Benzoic | 0.964 | −0.029 |
| y-Hydroxybutyric Acid | 0.949 | −0.255 |
| L-Phenylalanine | 0.743 | 0.579 |
| L-Threonine | 0.945 | 0.048 |
| Glycyl-L-Glutamic Acid | 0.938 | −0.285 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-N.; Shang, P.-L.; Kang, P.-L.; Du, M.-M. Metabolic Functional Community Diversity of Associated Bacteria during the Degradation of Phytoplankton from a Drinking Water Reservoir. Int. J. Environ. Res. Public Health 2020, 17, 1687. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17051687
Chen S-N, Shang P-L, Kang P-L, Du M-M. Metabolic Functional Community Diversity of Associated Bacteria during the Degradation of Phytoplankton from a Drinking Water Reservoir. International Journal of Environmental Research and Public Health. 2020; 17(5):1687. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17051687
Chicago/Turabian StyleChen, Sheng-Nan, Pan-Lu Shang, Peng-Liang Kang, and Man-Man Du. 2020. "Metabolic Functional Community Diversity of Associated Bacteria during the Degradation of Phytoplankton from a Drinking Water Reservoir" International Journal of Environmental Research and Public Health 17, no. 5: 1687. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17051687




