Streamlining Analysis of RR Interval Variability in Elite Soccer Players: Preliminary Experience with a Composite Indicator of Cardiac Autonomic Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Protocol
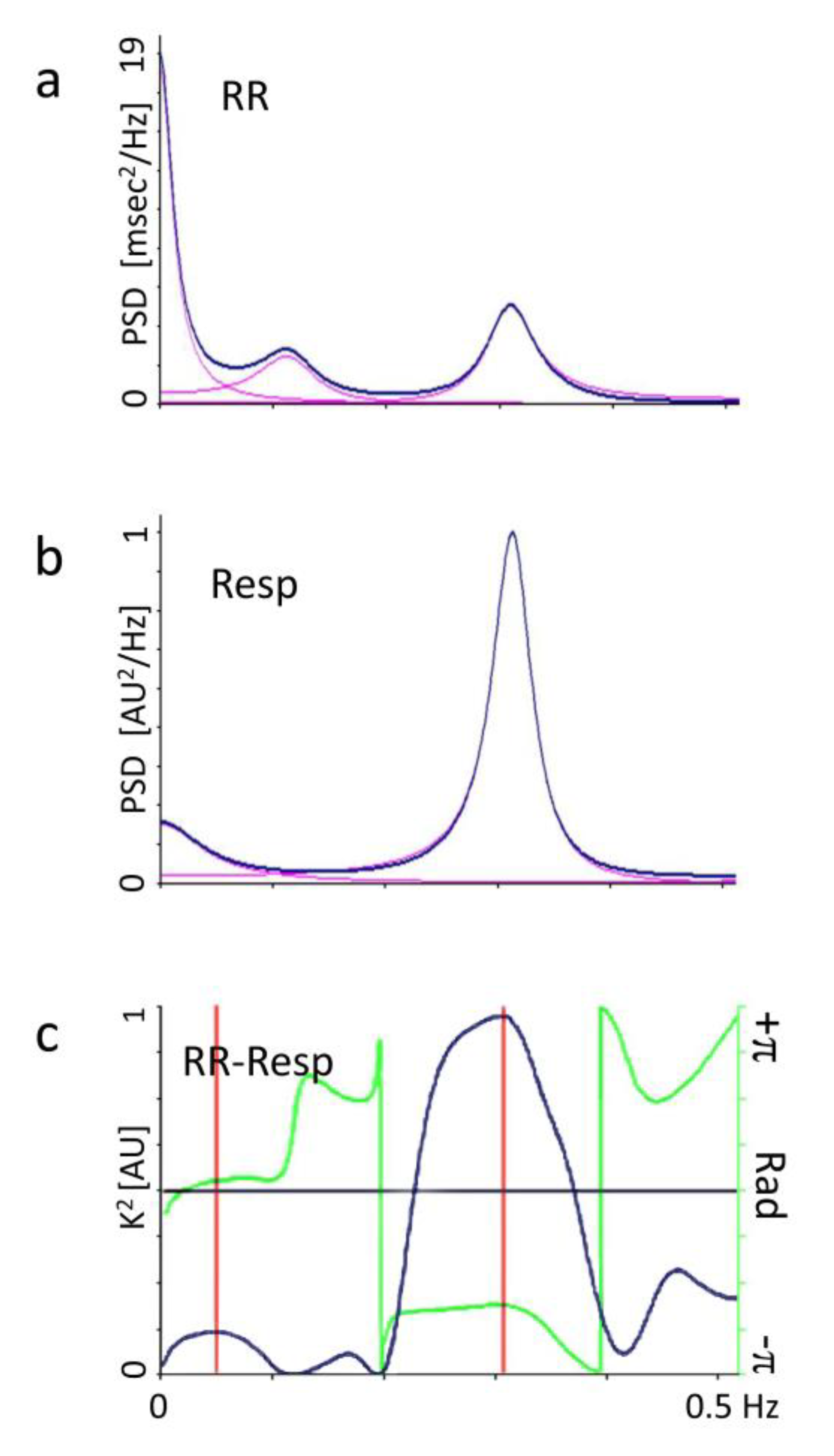
2.2. Autonomic Evaluation
2.3. ANSI, a Proxy of Cardiac Autonomic Regulation (CAR)
2.4. Statistics
3. Results
4. Discussion
4.1. General Findings
4.2. Non-Invasive Assessment of Cardiac Autonomic Regulation (CAR)
4.3. Limitations and Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kunz, M. 265 million playing football. FIFA Mag. 2007, 7, 11–15. [Google Scholar]
- Wragg, M. World Football Report 2018. Nielsen Sport 2018. Available online: https://nielsensports.com/wp-content/uploads/2014/12/Nielsen_World-Football-2018-6.11.18.pdf (accessed on 17 January 2020).
- HUFFPOST. Available online: https://www.huffpost.com/entry/soccer-worlds-best-sport_n_5248061 (accessed on 17 January 2020).
- TEACHER. Available online: https://www.teachermagazine.com.au/articles/motivating-students-to-participate-in-sport (accessed on 17 January 2020).
- Bangsbo, J.; Hansen, P.R.; Dvorak, J.; Krustrup, P. Recreational football for disease prevention and treatment in untrained men: A narrative review examining cardiovascular health, lipid profile, body composition, muscle strength and functional capacity. Br. J. Sports Med. 2015, 49, 568–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visek, A.; Achrati, S.M.; Mannix, H.; McDonnell, K.; Harris, B.S.; DiPietro, L.; Manning, H. The fun integration theory: Toward sustaining children and adolescents sport participation. J. Phys. Act. Heal. 2014, 12, 424–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjønniksen, L.; Anderssen, N.; Wold, B. Organized youth sport as a predictor of physical activity in adulthood. Scand. J. Med. Sci. Sports 2009, 19, 646–654. [Google Scholar] [CrossRef]
- Telama, R.; Yang, X. Decline of physical activity from youth to young adulthood in Finland. Med. Sci. Sports Exerc. 2000, 32, 1617–1622. [Google Scholar] [CrossRef]
- Koozehchian, M.S.; Nazem, F.; Kreider, R.B.; Roberts, W.J.; Best, T.M.; Rong, Y.; Zuo, L. The role of exercise training on lipoprotein profiles in adolescent males. Lipids Heal. Dis. 2014, 13, 95. [Google Scholar] [CrossRef] [Green Version]
- Levine, B.D.; Baggish, A.L.; Kovacs, R.J.; Link, M.S.; Maron, M.S.; Mitchell, J.H.; Chair, F.F. Facc Eligibility and Disqualification Recommendations for Competitive Athletes With Cardiovascular Abnormalities: Task Force 1: Classification of Sports: Dynamic, Static, and Impact. Circulation 2015, 132, e262–e266. [Google Scholar] [CrossRef]
- Sala, R.; Malacarne, M.; Tosi, F.; Benzi, M.; Solaro, N.; Tamorri, S.; Spataro, A.; Pagani, M.; Lucini, D. May a unitary autonomic index help assess autonomic cardiac regulation in elite athletes? Preliminary observations on the national Italian Olympic committee team. J. Sports Med. Phys. Fit. 2017, 57, 1702–1710. [Google Scholar]
- ACSM’s. Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincot Williams & Wilkins: Philadelphia, PA, USA, 2017. [Google Scholar]
- Buchheit, M. Sensitivity of Monthly Heart Rate and Psychometric Measures for Monitoring Physical Performance in Highly Trained Young Handball Players. Int. J. Sports Med. 2014, 36, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Pagani, M.; Lombardi, F.; Guzzetti, S.; Rimoldi, O.; Furlan, R.; Pizzinelli, P.; Sandrone, G.; Malfatto, G.; Dell’Orto, S.; Piccaluga, E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 1986, 59, 178–193. [Google Scholar] [CrossRef] [Green Version]
- La Rovere, M.T.; Bigger, J.T.; Marcus, F.I.; Mortara, A.; Schwartz, P.J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998, 351, 478–484. [Google Scholar] [CrossRef]
- Joyner, M.J.; Green, D.J. Exercise protects the cardiovascular system: Effects beyond traditional risk factors. J. Physiol. 2009, 587, 5551–5558. [Google Scholar] [CrossRef] [PubMed]
- Sala, R.; Malacarne, M.; Solaro, N.; Pagani, M.; Lucini, D. A composite autonomic index as unitary metric for heart rate variability: A proof of concept. Eur. J. Clin. Investig. 2017, 47, 241–249. [Google Scholar] [CrossRef]
- Lucini, D.; Marchetti, I.; Spataro, A.; Malacarne, M.; Benzi, M.; Tamorri, S.; Sala, R.; Pagani, M. Heart rate variability to monitor performance in elite athletes: Criticalities and avoidable pitfalls. Int. J. Cardiol. 2017, 240, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Solaro, N.; Malacarne, M.; Pagani, M.; Lucini, D. Cardiac Baroreflex, HRV, and Statistics: An Interdisciplinary Approach in Hypertension. Front. Physiol. 2019, 10, 478. [Google Scholar] [CrossRef]
- Oggionni, G.; Spataro, A.; Pelliccia, A.; Malacarne, M.; Pagani, M.; Lucini, D. Left ventricular hypertrophy in world class elite athletes is associated with signs of improved cardiac autonomic regulation. Eur. J. Prev. Cardiol. 2019. [Google Scholar] [CrossRef]
- Joint Research Centre-European Commission. Handbook on Constructing Composite Indicators: Methodology and User Guide; OECD publishing: Paris, France, 2008; ISBN 978-92-64-04345-9. [Google Scholar]
- Lucini, D.; Solaro, N.; Pagani, M. Autonomic Differentiation Map: A Novel Statistical Tool for Interpretation of Heart Rate Variability. Front. Physiol. 2018, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Voss, A.; Schroeder, R.; Heitmann, A.; Peters, A.; Perz, S. Short-Term Heart Rate Variability—Influence of Gender and Age in Healthy Subjects. PLoS ONE 2015, 10, e0118308. [Google Scholar] [CrossRef] [Green Version]
- Pagani, M.; Sala, R.; Malacarne, M.; Lucini, D. Benchmarking Heart Rate Variability to Overcome Sex-Related Bias. In Advances in Experimental Medicine and Biology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018; pp. 191–205. [Google Scholar]
- Sala, R.; Malacarne, M.; Pagani, M.; Lucini, D. Evidence of increased cardiac parasympathetic drive in subjects meeting current physical activity recommendations. Clin. Auton. Res. 2015, 25, 285–291. [Google Scholar] [CrossRef]
- Mallo, J.; Mena, E.; Nevado, F.; Paredes, V. Physical Demands of Top-Class Soccer Friendly Matches in Relation to a Playing Position Using Global Positioning System Technology. J. Hum. Kinet. 2015, 47, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Badilini, F.; Pagani, M.; Porta, A. Heartscope: A software tool adressing autonomic nervous system regulation. Comput. Cardiol. 2005, 32, 259–262. [Google Scholar]
- Akselrod, S.; Gordon, D.; Ubel, F.; Shannon, D.; Berger, A.; Cohen, R. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science 1981, 213, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.F.; Epstein, S.E.; Beiser, G.D.; Braunwald, E. Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ. Res. 1966, 19, 400–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, G. Assessment of Sympathetic Cardiovascular Drive in Human Hypertension. Hypertension 2009, 54, 690–697. [Google Scholar] [CrossRef] [Green Version]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology Heart-rate variability: Standards of measurements, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [CrossRef] [Green Version]
- Shaffer, F.; Ginsberg, J. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Heal. 2017, 5, 258. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Moneghetti, K.J.; Christle, J.W.; Hadley, D.; Plews, D.; Froelicher, V. Heart Rate Variability: An Old Metric with New Meaning in the Era of using mHealth Technologies for Health and Exercise Training Guidance. Part One: Physiology and Methods. Arrhythm. Electrophysiol. Rev. 2018, 7, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Billman, G.E. Heart Rate Variability—A Historical Perspective. Front. Physiol. 2011, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Hayano, J.; Yuda, E. Pitfalls of assessment of autonomic function by heart rate variability. J. Physiol. Anthr. 2019, 38, 3. [Google Scholar] [CrossRef] [Green Version]
- Langley, J.N. The Autonomic Nervous System (Pt. I); W. Heffer & Sons: Cambridge, UK, 1921. [Google Scholar]
- Hess, W.R. Nobel Lecture: The Central Control of the Activity of Internal Organs. Nobelprize.org.Nobel Media AB 2014. Available online: http://www.nobelprize.org/nobel_prizes/medicine/laureates/1949/hess-lecture.html (accessed on 29 September 2016).
- Shen, T.; Tsao, Y. An investigation on autonomic effects by using PR intervals. In Proceedings of the 2007 Computers in Cardiology, Durham, NC, USA, 30 September–3 October 2007; pp. 481–484. [Google Scholar]
- Selvaraj, N.; Jaryal, A.; Santhosh, J.; Deepak, K.K.; Anand, S. Assessment of heart rate variability derived from finger-tip photoplethysmography as compared to electrocardiography. J. Med. Eng. Technol. 2008, 32, 479–484. [Google Scholar] [CrossRef]
- Pagani, M.; Malliani, A. Interpreting oscillations of muscle sympathetic nerve activity and heart rate variability. J. Hypertens. 2000, 18, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, J.J. Sympathovagal balance: How should we measure it? Am. J. Physiol. Content 1999, 276, H1273–H1280. [Google Scholar] [CrossRef] [PubMed]
- Eckberg, D.L. Sympathovagal Balance. Circulation 1997, 96, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Montano, N.; Porta, A.; Malliani, A.; Abboud, F.M.; Birkett, C.; Somers, V.K. Relationship Between Spectral Components of Cardiovascular Variabilities and Direct Measures of Muscle Sympathetic Nerve Activity in Humans. Circulation 1997, 95, 1441–1448. [Google Scholar] [CrossRef]
- Haken, H. Synergetics An Introduction; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Malliani, A.; Pagani, M.; Lombardi, F.; Cerutti, S. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991, 84, 482–492. [Google Scholar] [CrossRef] [Green Version]
- Miglis, M.; Muppidi, S. Is your autonomic function good enough to be an Olympian? And other updates on recent autonomic research. Clin. Auton. Res. 2018, 28, 177–179. [Google Scholar] [CrossRef] [Green Version]
| Variables | Definition | |
|---|---|---|
| HR | [beat/min] | Heart Rate |
| RR Mean | [msec] | Average of RR interval from tachogram sections |
| RR VAR | [msec 2] | RR variance from tachogram sections |
| RR LFa | [msec 2] | Absolute power(a) of Low Frequency (LF) component of RR variability (V) |
| RR HFa | [msec 2] | Absolute power(a) of High Frequency (HF) component of RRV |
| RR LFnu | [nu] | Normalized power (nu) of Low Frequency (LF) component of RRV |
| RR HFnu | [nu] | Normalized power (nu) of High Frequency (HF) component of RRV |
| RR LF/HF | . | Ratio between absolute values of LF and HF |
| ΔRRLFnu | [nu] | Difference of LF power in nu between stand and rest |
| SAP | [mmHg] | Systolic arterial pressure by sphygmomanometer |
| DAP | [mmHg] | Diastolic arterial pressure by sphygmomanometer |
| ANSI (b) | [%] | Composite index of Autonomic Nervous System regulation computed as a synthesis of RR Mean, RR TP, and ΔRRLFnu |
| Variable | Controls n = 56 | Soccer Players n = 56 | p | |
|---|---|---|---|---|
| Age | [years] | 22.2 ± 1.5 | 21.2 ± 4.2 | 0.115 |
| Weight | [kg] | 74.2 ± 9.7 | 76.9 ± 6.0 | 0.240 |
| Height | [cm] | 178.7 ± 7.2 | 182.6 ± 5.7 | < 0.001 |
| BMI | [kg/m2] | 23.2 ± 2.3 | 23.0 ± 1.0 | 0.443 |
| SAP | [mmHg] | 117.2 ± 10.5 | 130.2 ± 14.2 | < 0.001 |
| DAP | [mmHg] | 68.4 ± 9.7 | 71.9 ± 7.3 | 0.011 |
| HR | [beat/min] | 65.1 ± 10.7 | 50.7 ± 8.4 | < 0.001 |
| RR | [msec] | 946.3 ± 155.5 | 1213.2 ± 185.9 | 0.025 |
| RR VAR | [msec2] | 3970.4 ± 3504.2 | 10,729.2 ± 17,096.8 | < 0.001 |
| RR LFa | [msec2] | 1189.7 ± 966.0 | 2072.4 ± 2630.1 | 0.249 |
| RR HFa | [msec2] | 1540.0 ± 2290.6 | 5530.5 ± 11,374.6 | < 0.001 |
| RR LFnu | [nu] | 51.5 ± 21.8 | 32.3 ± 15.4 | < 0.001 |
| RR HFnu | [nu] | 42.3 ± 21.2 | 64.9 ± 16.8 | <0.001 |
| RR LF/HF | . | 2.43 ± 3.89 | 0.63 ± 0.57 | <0.001 |
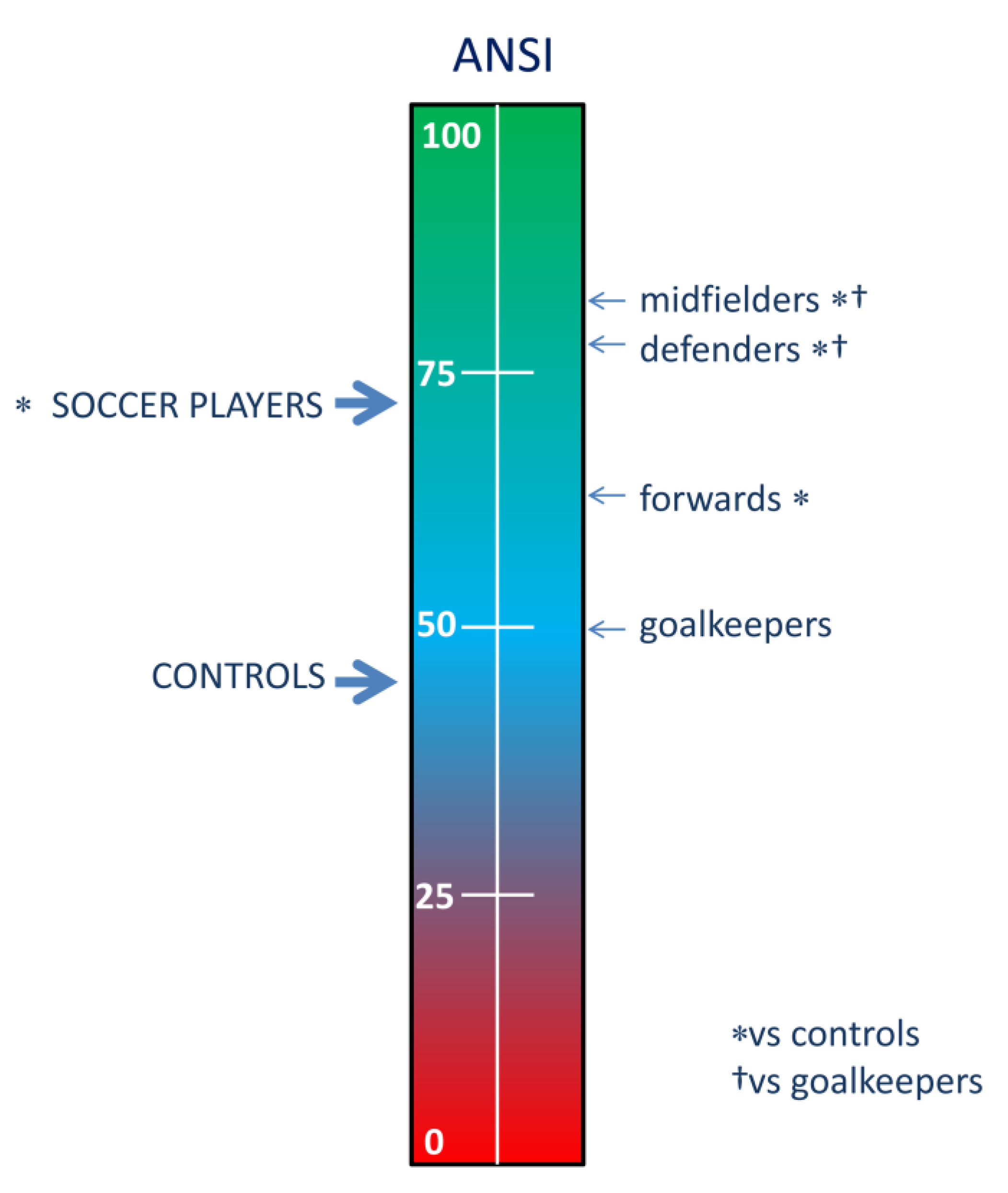
| ANSI | [%] | 45.5 ± 27.6 | 72.7 ± 23.7 | <0.001 |
| Variable | Goalkeepers n = 5 | Defenders n = 18 | Midfielders n = 21 | Forwards n = 12 | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | [years] | 23.8 ± 5.9 | 20.5 ± 3.9 | ‡ | 21.6 ± 4.3 | 20.6 ± 3.9 | ‡ | 0.030 | ||
| Weight | [kg] | 84.6 ± 4.3 | ‡ | 79.4 ± 5.5 | ‡ | 73.9 ± 4.1 | *+ | 75.2 ± 6.2 | * | 0.011 |
| Height | [cm] | 188.6 ± 2.1 | ‡ | 185.1 ± 4.8 | ‡ | 180.0 ± 4.5 | *+ | 180.8 ± 6.6 | * | < 0.001 |
| BMI | [kg/m2] | 23.8 ± 0.9 | 23.1 ± 0.9 | 22.8 ± 1.2 | 23.0 ± 1.1 | 0.719 | ||||
| SAP | [mmHg] | 127 ± 10 | 130 ± 12 | ‡ | 133 ± 17 | ‡ | 126 ± 14 | < 0.001 | ||
| DAP | [mmHg] | 71 ± 9 | 70 ± 8 | 73 ± 7 | 73 ± 7 | 0.091 | ||||
| HR | [beat/min] | 55.2 ± 9.9 | 51.0 ± 9.9 | ‡ | 48.1 ± 6.5 | ‡ | 52.9 ± 8.1 | ‡ | < 0.001 | |
| RR | [msec] | 1118 ± 214.8 | ‡ | 1210.8 ± 196.5 | ‡ | 1268.2 ± 167.9 | ‡ | 1160.2 ± 179.4 | ‡ | < 0.001 |
| RR VAR | [msec2] | 4192.4 ± 2027.2 | 10,625 ± 9932.3 | ‡ | 10,003.9 ± 10,352.8 | ‡ | 14,878.4 ± 32,763.6 | 0.003 | ||
| RR LFa | [msec2] | 1180.1 ± 618.5 | 1849.1 ± 2049.7 | 2019 ± 1908.2 | 2872.7 ± 4493.1 | 0.848 | ||||
| RR HFa | [msec2] | 1554.6 ± 1042.9 | 5573.2 ± 6845.3 | ‡ | 5083.9 ± 7379.6 | ‡ | 7904.5 ± 21,479.4 | < 0.001 | ||
| RR LFnu | [nu] | 43 ± 16.2 | 25.1 ± 13.4 | ‡* | 31.1 ± 14.9 | ‡ | 40.8 ± 13.6 | + | < 0.001 | |
| RR HFnu | [nu] | 55.1 ± 16.9 | 71.1 ± 16 | ‡* | 67.8 ± 15 | ‡ | 54.8 ± 16.3 | ‡+ | < 0.001 | |
| RR LF/HF | . | 1.02 ± 1.00 | 0.43 ± 0.43 | ‡* | 0.53 ± 0.38 | ‡ | 0.92 ± 0.69 | + | < 0.001 | |
| ANSI | [%] | 50.1 ± 27.7 | 77 ± 22.9 | ‡* | 79.6 ± 17.8 | ‡* | 63.4 ± 26.4 | (‡) | < 0.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucini, D.; Fallanca, A.; Malacarne, M.; Casasco, M.; Galiuto, L.; Pigozzi, F.; Galanti, G.; Pagani, M. Streamlining Analysis of RR Interval Variability in Elite Soccer Players: Preliminary Experience with a Composite Indicator of Cardiac Autonomic Regulation. Int. J. Environ. Res. Public Health 2020, 17, 1844. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17061844
Lucini D, Fallanca A, Malacarne M, Casasco M, Galiuto L, Pigozzi F, Galanti G, Pagani M. Streamlining Analysis of RR Interval Variability in Elite Soccer Players: Preliminary Experience with a Composite Indicator of Cardiac Autonomic Regulation. International Journal of Environmental Research and Public Health. 2020; 17(6):1844. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17061844
Chicago/Turabian StyleLucini, Daniela, Angelo Fallanca, Mara Malacarne, Maurizio Casasco, Leonarda Galiuto, Fabio Pigozzi, Giorgio Galanti, and Massimo Pagani. 2020. "Streamlining Analysis of RR Interval Variability in Elite Soccer Players: Preliminary Experience with a Composite Indicator of Cardiac Autonomic Regulation" International Journal of Environmental Research and Public Health 17, no. 6: 1844. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17061844






