Effects of Acute Exercise on Cutaneous Thermal Sensation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
2.3. Thermal Sensory Function Assessment
2.4. Cardiovascular and Local Thermoregulatory Assessment
2.5. Statistical Analysis
3. Results
3.1. Exercise Responses
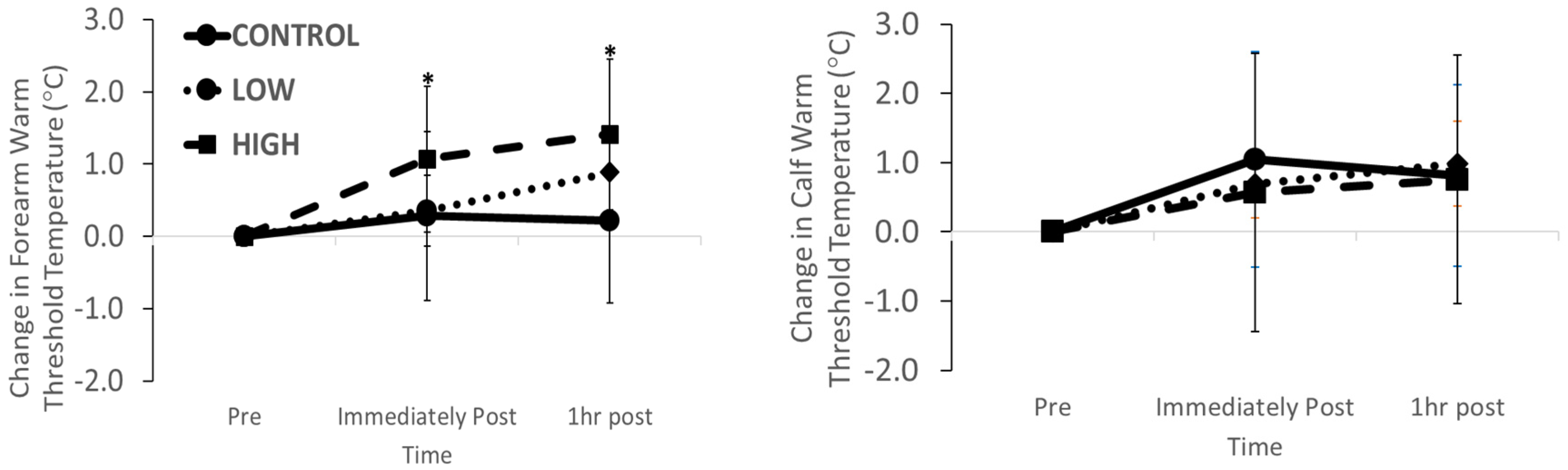
3.2. Thermal Sensation Function
3.3. Thermal Pain Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schlader, Z.J.; Stannard, S.R.; Mündel, T. Human thermoregulatory behavior during rest and exercise—A prospective review. Physiol. Behav. 2010, 99, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Flouris, A.D. Functional architecture of behavioural thermoregulation. Graefe’s Arch. Clin. Exp. Ophthalmol. 2010, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.J.; Johnson, J.M. Responses to hyperthermia. Optimizing heat dissipation by convection and evaporation: Neural control of skin blood flow and sweating in humans. Auton. Neurosci. 2016, 196, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Vargas, N.T.; Chapman, C.L.; Johnson, B.D.; Gathercole, R.; Cramer, M.; Schlader, Z.J. Thermal behavior alleviates thermal discomfort during steady-state exercise without affecting whole body heat loss. J. Appl. Physiol. 2019, 127, 984–994. [Google Scholar] [CrossRef]
- Kenny, G.P.; McGinn, R. Restoration of thermoregulation after exercise. J. Appl. Physiol. 2017, 122, 933–944. [Google Scholar] [CrossRef]
- Vargas, N.T.; Chapman, C.L.; Sackett, J.R.; Abdul-Rashed, J.; McBryde, M.; Johnson, B.D.; Gathercole, R.; Schlader, Z.J. Thermal behavior remains engaged following exercise despite autonomic thermoeffector withdrawal. Physiol. Behav. 2018, 188, 94–102. [Google Scholar] [CrossRef]
- Koltyn, K.F. Analgesia following exercise: A review. Sports Med. 2000, 29. [Google Scholar] [CrossRef]
- Kemppainen, P.; Pertovaara, A.; Huopaniemi, T.; Johansson, G.; Karonen, S.-L. Modification of dental pain and cutaneous thermal sensitivity by physical exercise in man. Brain Res. 1985, 360, 33–40. [Google Scholar] [CrossRef]
- Gerrett, N.; Ouzzahra, Y.; Redortier, B.; Voelcker, T.; Havenith, G. Female thermal sensitivity to hot and cold during rest and exercise. Physiol. Behav. 2015, 152, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Ouzzahra, Y.; Havenith, G.; Redortier, B. Regional distribution of thermal sensitivity to cold at rest and during mild exercise in males. J. Therm. Boil. 2012, 37, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Gerrett, N.; Ouzzahra, Y.; Coleby, S.; Hobbs, S.; Redortier, B.; Voelcker, T.; Havenith, G. Thermal sensitivity to warmth during rest and exercise: A sex comparison. Graefe’s Arch. Clin. Exp. Ophthalmol. 2014, 114, 1451–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, N.T.; Chapman, C.L.; Johnson, B.D.; Gathercole, R.; Schlader, Z.J. Exercise intensity independently modulates thermal behavior during exercise recovery but not during exercise. J. Appl. Physiol. 2019, 126, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Ruble, S.B.; Hoffman, M.D.; Shepanski, M.A.; Valic, Z.; Buckwalter, J.B.; Clifford, P. Thermal Pain Perception after Aerobic Exercise. Arch. Phys. Med. Rehabil. 2005, 86, 1019–1023. [Google Scholar] [CrossRef]
- Stevens, J.C.; Marks, L.E.; Simonson, D.C. Regional sensitivity and spatial summation in the warmth sense. Physiol. Behav. 1974, 13, 825–836. [Google Scholar] [CrossRef]
- Nadel, E.R.; Mitchell, J.W.; Stolwijk, J.A.J. Differential thermal sensitivity in the human skin. Pflügers Arch. Eur. J. Physiol. 1973, 340, 71–76. [Google Scholar] [CrossRef]
- Jones, H.; Green, D.J.; George, K.; Atkinson, G. Intermittent exercise abolishes the diurnal variation in endothelial-dependent flow-mediated dilation in humans. Am. J. Physiol. Integr. Comp. Physiol. 2010, 298, R427–R432. [Google Scholar] [CrossRef] [Green Version]
- Rolke, R.; Magerl, W.; Campbell, K.A.; Schalber, C.; Caspari, S.; Birklein, F.; Treede, R.-D. Quantitative sensory testing: A comprehensive protocol for clinical trials. Eur. J. Pain 2006, 10, 77. [Google Scholar] [CrossRef]
- Toner, M.M.; Drolet, L.L.; Pandolf, K.B. Perceptual and Physiological Responses during Exercise in Cool and Cold Water. Percept. Mot. Ski. 1986, 62, 211–220. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Beaumont, A.; Hughes, J. Biology of Opioid Peptides. Annu. Rev. Pharmacol. Toxicol. 1979, 19, 245–267. [Google Scholar] [CrossRef]
- Farrell, P.A.; Gustafson, A.B.; Morgan, W.P.; Pert, C.B. Enkephalins, catecholamines, and psychological mood alterations: Effects of prolonged exercise. Med. Sci. Sports Exerc. 1987, 19. [Google Scholar] [CrossRef]
- O’Connor, P.J.; Cook, D.B. Exercise and pain: The neurobiology, measurement, and laboratory study of pain in relation to exercise in humans. Exerc. Sport Sci. Rev. 1999, 27, 119–166. [Google Scholar] [PubMed]
- Bushnell, M.; Duncan, G.; Dubner, R.; Jones, R.; Maixner, W. Attentional influences on noxious and innocuous cutaneous heat detection in humans and monkeys. J. Neurosci. 1985, 5, 1103–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Control | Low | High | p Values | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | Ex | IMM | 1HR | PRE | Ex | IMM | 1HR | PRE | Ex | IMM | 1HR | Stage | Intensity | Stage * Intensity | |
| Heart rate (beats·min) | 64 ± 9 | 59 ± 10 | 59 ± 10 | 57 ± 8 | 61 ± 7 | 101 ± 4 | 65 ± 8 | 60 ± 9 | 58 ± 7 | 147 ± 7 | 86 ± 14 | 64 ± 11 | <0.001 | <0.001 | <0.001 |
| Skin temperature (°C) | |||||||||||||||
| Forearm | 32.3 ± 0.8 | 32.3 ± 1.0 | 32.4 ± 1.0 | 32.2 ± 1.0 | 32.3 ± 0.7 | 31.0 ± 0.9 | 31.3 ± 1.0 | 32.8 ± 1.1 | 32.2 ± 0.8 | 32.0 ± 0.8 | 31.9 ± 1.1 | 32.4 ± 1.1 | 0.006 | 0.050 | 0.014 |
| Calf | 31.0 ± 0.7 | 31.2 ± 1.0 | 31.2 ± 0.9 | 30.9 ± 0.9 | 31.1 ± 0.7 | 31.4 ± 1.5 | 31.8 ± 1.3 | 31.6 ± 1.0 | 31.5 ± 1.4 | 32.2 ± 0.9 | 32.0 ± 1.2 | 31.8 ± 0.9 | 0.174 | <0.001 | 0.778 |
| Blood pressure (mmHg) | |||||||||||||||
| Systolic | 122 ± 8 | 118 ± 9 | 119 ± 9 | 121 ± 8 | 118 ± 6 | 137 ± 11 | 124 ± 5 | 116 ± 9 | 121 ± 8 | 142 ± 17 | 124 ± 8 | 116 ± 8 | <0.001 | 0.006 | <0.001 |
| Diastolic | 67 ± 7 | 69 ± 7 | 68 ± 8 | 69 ± 8 | 65 ± 8 | 80 ± 12 | 67 ± 9 | 68 ± 9 | 66 ± 8 | 78 ± 11 | 67 ± 7 | 63 ± 6 | <0.001 | 0.633 | 0.026 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.D.; Carter, H.H.; Jones, H.; Thijssen, D.H.J.; Low, D.A. Effects of Acute Exercise on Cutaneous Thermal Sensation. Int. J. Environ. Res. Public Health 2020, 17, 2491. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17072491
Thomas SD, Carter HH, Jones H, Thijssen DHJ, Low DA. Effects of Acute Exercise on Cutaneous Thermal Sensation. International Journal of Environmental Research and Public Health. 2020; 17(7):2491. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17072491
Chicago/Turabian StyleThomas, Samuel D., Howard H. Carter, Helen Jones, Dick H.J. Thijssen, and David A. Low. 2020. "Effects of Acute Exercise on Cutaneous Thermal Sensation" International Journal of Environmental Research and Public Health 17, no. 7: 2491. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17072491






