Effects of an Acute Pilates Program under Hypoxic Conditions on Vascular Endothelial Function in Pilates Participants: A Randomized Crossover Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
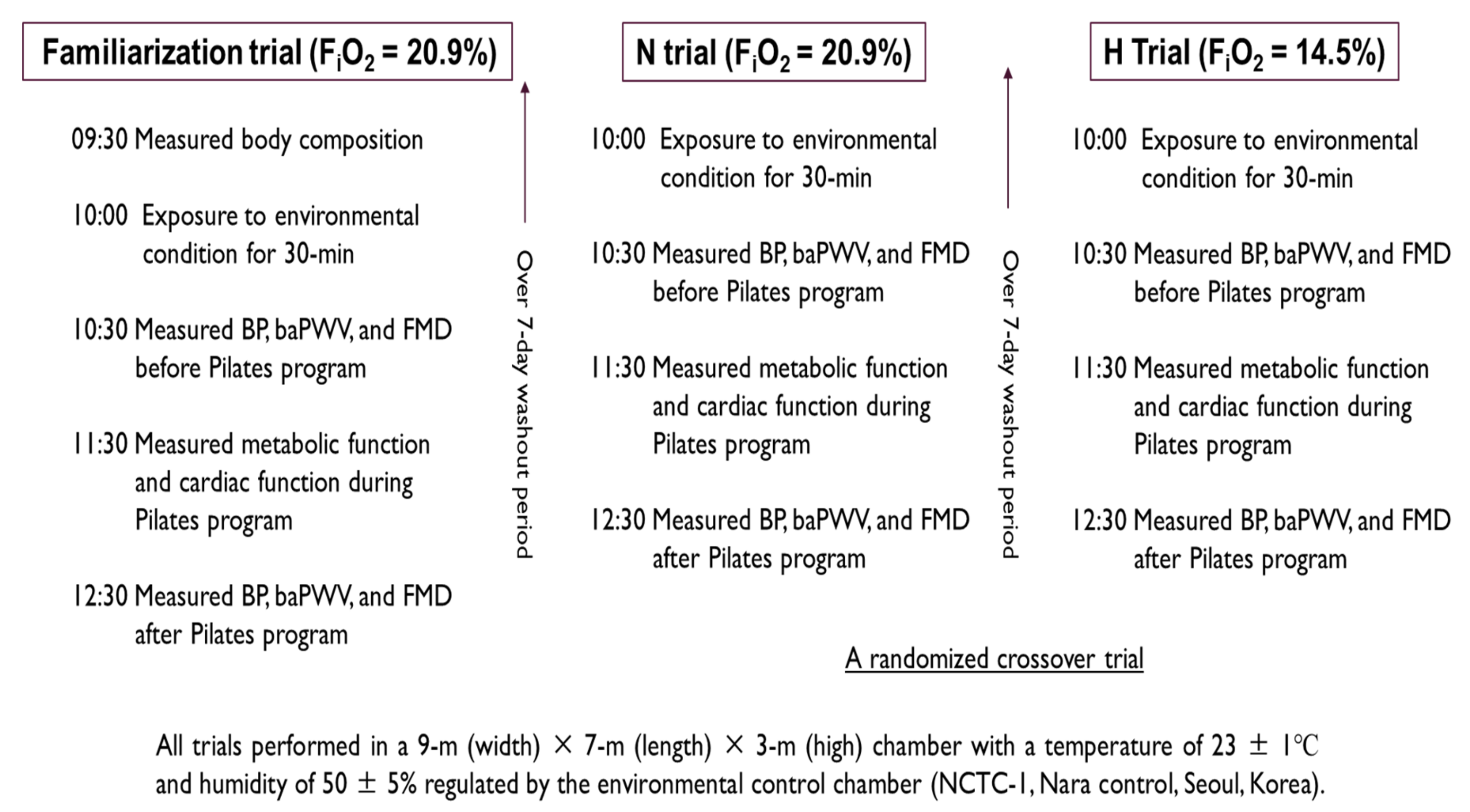
2.2. Study Design
2.3. Body Composition
2.4. Metabolic Function
2.5. Cardiac Function
2.6. Vascular Function
2.7. Statistical Analysis
3. Results
3.1. Metabolic Function
3.2. Cardiac Function
3.3. Vascular Function
4. Discussion
5. Limitation of the Study
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hwang, Y.; Park, J.; Lim, K. Effects of Pilates Exercise on Salivary Secretory Immunoglobulin A Levels in Older Women. J. Aging Phys. Act. 2016, 24, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.M.; Stegink Jansen, C.W.; Hogan, H.A.; Nassif, M.D. Material properties of Thera-Band Tubing. Phys. Ther. 2001, 81, 1437–1445. [Google Scholar] [CrossRef] [PubMed]
- Savkin, R.; Aslan, U.B. The effect of Pilates exercise on body composition in sedentary overweight and obese women. J. Sports Med. Phys. Fit. 2017, 57, 1464–1470. [Google Scholar] [CrossRef]
- Rayes, A.B.R.; de Lira, C.A.B.; Viana, R.B.; Benedito-Silva, A.A.; Vancini, R.L.; Mascarin, N.; Andrade, M.S. The effects of Pilates vs. aerobic training on cardiorespiratory fitness, isokinetic muscular strength, body composition, and functional tasks outcomes for individuals who are overweight/obese: A clinical trial. PeerJ 2019, 7, e6022. [Google Scholar] [CrossRef] [Green Version]
- Jago, R.; Jonker, M.L.; Missaghian, M.; Baranowski, T. Effect of 4 weeks of Pilates on the body composition of young girls. Prev. Med. 2006, 42, 177–180. [Google Scholar] [CrossRef]
- Cancela, J.M.; de Oliveira, I.M.; Rodríguez-Fuentes, G. Effects of Pilates method in physical fitness on older adults. A systematic review. Eur. Rev. Aging Phys. Act. 2014, 11, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Ferreira, A.; Fernandes, J.; Laranjo, L.; Bernardo, L.M.; Silva, A. A systematic review of the effects of pilates method of exercise in healthy people. Arch. Phys. Med. Rehabil. 2011, 92, 2071–2081. [Google Scholar] [CrossRef]
- Irez, G.B.; Ozdemir, R.A.; Evin, R.; Irez, S.G.; Korkusuz, F. Integrating pilates exercise into an exercise program for 65+ year-old women to reduce falls. J. Sports Sci. Med. 2011, 10, 105–111. [Google Scholar]
- Kovách, M.V.; Plachy, J.K.; Bognár, J.; Balogh, Z.O.; Barthalos, I. Effects of Pilates and aqua fitness training on older adults’ physical functioning and quality of life. Biomed. Hum. Kinet. 2013, 5, 22–27. [Google Scholar] [CrossRef]
- Ruiz-Montero, P.J.; Castillo-Rodriguez, A.; Mikalacki, M.; Nebojsa, C.; Korovljev, D. 24-weeks Pilates-aerobic and educative training to improve body fat mass in elderly Serbian women. Clin. Interv. Aging 2014, 9, 243–248. [Google Scholar] [CrossRef] [Green Version]
- Wolkodoff, N.; Andrick, R.; Lazarus, E.; Braunstein, B.; Patch, T. The physiological and health effects of a Pilates program combined with nutritional intervention on subjects with metabolic syndrome. J. Fit. Res. 2013, 2, 17–29. [Google Scholar]
- Hun-Young, P.; Won-Sang, J.; Jisu, K.; Hwang, H.; Kiwon, L. Changes in the Paradigm of Traditional Exercise in Obesity Therapy and Application of a New Exercise Modality: A Narrative Review Article. Iran. J. Public Health 2019, 48, 1395–1404. [Google Scholar]
- Millet, G.P.; Debevec, T.; Brocherie, F.; Malatesta, D.; Girard, O. Therapeutic Use of Exercising in Hypoxia: Promises and Limitations. Front. Physiol. 2016, 7, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girard, O.; Malatesta, D.; Millet, G.P. Walking in Hypoxia: An Efficient Treatment to Lessen Mechanical Constraints and Improve Health in Obese Individuals? Front. Physiol. 2017, 8, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, Z.; Zang, Y.; Hu, Y. Normobaric hypoxia training causes more weight loss than normoxia training after a 4-week residential camp for obese young adults. Sleep Breath. Schlaf Atm. 2014, 18, 591–597. [Google Scholar] [CrossRef]
- Netzer, N.C.; Chytra, R.; Kupper, T. Low intense physical exercise in normobaric hypoxia leads to more weight loss in obese people than low intense physical exercise in normobaric sham hypoxia. Sleep Breath. Schlaf Atm. 2008, 12, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Haufe, S.; Wiesner, S.; Engeli, S.; Luft, F.C.; Jordan, J. Influences of normobaric hypoxia training on metabolic risk markers in human subjects. Med. Sci. Sports Exerc. 2008, 40, 1939–1944. [Google Scholar] [CrossRef]
- Vedam, H.; Phillips, C.L.; Wang, D.; Barnes, D.J.; Hedner, J.A.; Unger, G.; Grunstein, R.R. Short-term hypoxia reduces arterial stiffness in healthy men. Eur. J. Appl. Physiol. 2009, 105, 19–25. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, J.; Park, M.Y.; Chung, N.; Hwang, H.; Nam, S.S.; Lim, K. Exposure and Exercise Training in Hypoxic Conditions as a New Obesity Therapeutic Modality: A Mini Review. J. Obes. Metab. Syndr. 2018, 27, 93–101. [Google Scholar] [CrossRef]
- Bernardi, L.; Passino, C.; Serebrovskaya, Z.; Serebrovskaya, T.; Appenzeller, O. Respiratory and cardiovascular adaptations to progressive hypoxia; effect of interval hypoxic training. Eur. Heart J. 2001, 22, 879–886. [Google Scholar] [CrossRef]
- Serebrovskaya, T.V.; Manukhina, E.B.; Smith, M.L.; Downey, H.F.; Mallet, R.T. Intermittent hypoxia: Cause of or therapy for systemic hypertension? Exp. Biol. Med. (Maywood) 2008, 233, 627–650. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, U.A.; Gray, K.; Herr, M.D. Adenosine contributes to hypoxia-induced forearm vasodilation in humans. J. Appl. Physiol. 1999, 87, 2218–2224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, E.J.; Sun, D.; Koller, A.; Wolin, M.S.; Kaley, G. Role of endothelium-derived prostaglandins in hypoxia-elicited arteriolar dilation in rat skeletal muscle. Circ. Res. 1992, 71, 790–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.-Y.; Lim, K. The effects of aerobic exercise at hypoxic condition during 6 weeks on body composition, blood pressure, arterial stiffness, and blood lipid level in obese women. Int. J. Sports Sci. Med. 2017, 1, 1–5. [Google Scholar]
- Kim, S.W.; Jung, W.S.; Park, W. Twelve Weeks of Combined Resistance and Aerobic Exercise Improves Cardiometabolic Biomarkers and Enhances Red Blood Cell Hemorheological Function in Obese Older Men: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2019, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.Y.; Jung, W.S.; Kim, J.; Lim, K. Twelve weeks of exercise modality in hypoxia enhances health-related function in obese older Korean men: A randomized controlled trial. Geriatr. Gerontol. Int. 2019, 19, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, S.; Haufe, S.; Engeli, S.; Mutschler, H.; Haas, U.; Luft, F.C.; Jordan, J. Influences of normobaric hypoxia training on physical fitness and metabolic risk markers in overweight to obese subjects. Obesity (Silver Spring) 2010, 18, 116–120. [Google Scholar] [CrossRef]
- Di Lorenzo, C.E. Pilates: What is it? Should it be used in rehabilitation? Sports Health 2011, 3, 352–361. [Google Scholar] [CrossRef] [Green Version]
- Kelly, L.P.; Basset, F.A. Acute Normobaric Hypoxia Increases Post-exercise Lipid Oxidation in Healthy Males. Front. Physiol. 2017, 8, 293. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.A.; Bourdon, P.C.; Schmidt, W.; Singh, B.; Cable, G.; Onus, K.; Woolford, S.; Stanef, T.; Gore, C.; Aughey, R. The effect of acute simulated moderate altitude on power, performance and pacing strategies in well-trained cyclists. Eur. J. Appl. Physiol. 2007, 102, 45–55. [Google Scholar] [CrossRef]
- Moon, H.W.; Sunoo, S.; Park, H.Y.; Lee, D.J.; Nam, S.S. Effects of various acute hypoxic conditions on metabolic parameters and cardiac function during exercise and recovery. SpringerPlus 2016, 5, 1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, H.W.; Shin, S.H.; Lee, C.H.; Park, H.Y.; Sunoo, S.; Nam, S.S. Effects of various acute hypoxic conditions on the hemorheological response during exercise and recovery. Clin. Hemorheol. Microcirc. 2016, 63, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Peronnet, F.; Massicotte, D.; Folch, N.; Melin, B.; Koulmann, N.; Jimenez, C.; Bourdon, L.; Launay, J.C.; Savourey, G. Substrate utilization during prolonged exercise with ingestion of (13)C-glucose in acute hypobaric hypoxia (4,300 m). Eur. J. Appl. Physiol. 2006, 97, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Park, W.; Lim, K. Living High-Training Low for 21 Days Enhances Exercise Economy, Hemodynamic Function, and Exercise Performance of Competitive Runners. J. Sports Sci. Med. 2019, 18, 427–437. [Google Scholar]
- Hill, N.E.; Stacey, M.J.; Woods, D.R. Energy at high altitude. J. R. Army Med. Corps 2011, 157, 43–48. [Google Scholar] [CrossRef]
- Mazzeo, R.S. Physiological responses to exercise at altitude: An update. Sports Med. 2008, 38, 1–8. [Google Scholar] [CrossRef]
- Park, H.Y.; Sunoo, S.; Nam, S.S. The Effect of 4 Weeks Fixed and Mixed Intermittent Hypoxic Training (IHT) on Respiratory Metabolic and Acid-base Response of Capillary Blood During Submaximal Bicycle Exercise in Male Elite Taekwondo Players. J. Exerc. Nutr. Biochem. 2016, 20, 35–43. [Google Scholar] [CrossRef]
- Ramos-Campo, D.J.; Rubio-Arias, J.A.; Dufour, S.; Chung, L.; Avila-Gandia, V.; Alcaraz, P.E. Biochemical responses and physical performance during high-intensity resistance circuit training in hypoxia and normoxia. Eur. J. Appl. Physiol. 2017, 117, 809–818. [Google Scholar] [CrossRef]
- Holloway, C.J.; Montgomery, H.E.; Murray, A.J.; Cochlin, L.E.; Codreanu, I.; Hopwood, N.; Johnson, A.W.; Rider, O.J.; Levett, D.Z.; Tyler, D.J.; et al. Cardiac response to hypobaric hypoxia: Persistent changes in cardiac mass, function, and energy metabolism after a trek to Mt. Everest Base Camp. FASEB J. 2011, 25, 792–796. [Google Scholar] [CrossRef]
- Hsu, A.R.; Barnholt, K.E.; Grundmann, N.K.; Lin, J.H.; McCallum, S.W.; Friedlander, A.L. Sildenafil improves cardiac output and exercise performance during acute hypoxia, but not normoxia. J. Appl. Physiol. 2006, 100, 2031–2040. [Google Scholar] [CrossRef]
- Mason, N. The physiology of high altitude: An introduction to the cardio-respiratory changes occurring on ascent to altitude. Curr. Anaesth. Crit. Care 2000, 11, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Naeije, R. Physiological adaptation of the cardiovascular system to high altitude. Prog. Cardiovasc. Dis. 2010, 52, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Tinken, T.M.; Thijssen, D.H.; Hopkins, N.; Black, M.A.; Dawson, E.A.; Minson, C.T.; Newcomer, S.C.; Laughlin, M.H.; Cable, N.T.; Green, D.J. Impact of shear rate modulation on vascular function in humans. Hypertension (Dallas Tex. 1979) 2009, 54, 278–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katayama, K.; Fujita, O.; Iemitsu, M.; Kawano, H.; Iwamoto, E.; Saito, M.; Ishida, K. The effect of acute exercise in hypoxia on flow-mediated vasodilation. Eur. J. Appl. Physiol. 2013, 113, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Gielen, S.; Schuler, G.; Hambrecht, R. Exercise training in coronary artery disease and coronary vasomotion. Circulation 2001, 103, E1–E6. [Google Scholar] [CrossRef] [PubMed]
- Cosio-Lima, L.M.; Thompson, P.D.; Reynolds, K.L.; Headley, S.A.; Winter, C.R.; Manos, T.; Lagasse, M.A.; Todorovich, J.R.; Germain, M. The acute effect of aerobic exercise on brachial artery endothelial function in renal transplant recipients. Prev. Cardiol. 2006, 9, 211–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rowell, L.B. Human Cardiovascular Control; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Casey, D.P.; Madery, B.D.; Curry, T.B.; Eisenach, J.H.; Wilkins, B.W.; Joyner, M.J. Nitric oxide contributes to the augmented vasodilatation during hypoxic exercise. J. Physiol. 2010, 588, 373–385. [Google Scholar] [CrossRef]
- Katayama, K.; Yoshitake, Y.; Watanabe, K.; Akima, H.; Ishida, K. Muscle deoxygenation during sustained and intermittent isometric exercise in hypoxia. Med. Sci. Sports Exerc. 2010, 42, 1269–1278. [Google Scholar] [CrossRef]
- Tschakovsky, M.E.; Joyner, M.J. Nitric oxide and muscle blood flow in exercise. Appl. Physiol. Nutr. Metab. 2008, 33, 151–161. [Google Scholar] [CrossRef]


| Variables | N Trial | H Trial | Cohen’s d (95% CI) | t-Value | p-Value |
|---|---|---|---|---|---|
| VE (L/50 min) | 1021.6 ± 196.7 | 1161.2 ± 188.7 | 0.7 (−0.2, 1.6) | −3.191 | 0.011 * |
| VO2 (mL/50 min) | 32,299.7 ± 5581.0 | 32,910.5 ± 5407.3 | 0.1 (−0.7, 0.9) | −0.747 | 0.474 |
| VCO2 (mL/50 min) | 25,967.4 ± 4403.5 | 29,983.5 ± 4946.7 | 0.8 (−0.1, 1.7) | −4.597 | 0.001 * |
| RER | 0.8 ± 0.0 | 0.9 ± 0.0 | 3.2 (1.8, 4.3) | −9.937 | 0.000 * |
| EE (kcal/50 min) | 160.3 ± 27.6 | 168.3 ± 25.3 | 0.3 (−0.6, 1.1) | −1.746 | 0.115 |
| CHO (g/50 min) | 14.0 ± 4.2 | 29.1 ± 6.2 | 2.8 (1.5, 3.8) | −8.494 | 0.000 * |
| FAO (g/50 min) | 10.6 ± 2.8 | 5.1 ± 2.0 | −2.1 (−3.1, −1.0) | 8.891 | 0.000 * |
| Variables | N Trial | H Trial | Cohen’s d (95% CI) | t-Value | p-Value |
|---|---|---|---|---|---|
| HR (bpm/50 min) | 5139.8 ± 656.2 | 5677.3 ± 553.9 | 0.9 (0.0, 1.7) | −3.204 | 0.011 * |
| SV (mL/50 min) | 3848.9 ± 530.2 | 3618.9 ± 224.0 | −0.5 (−1.3, 0.4) | 1.538 | 0.158 |
| CO (L/50 min) | 395.7 ± 56.1 | 416.0 ± 45.6 | 0.4 (−0.5, 1.2) | −1.147 | 0.281 |
| EDV (mL/50 min) | 4954.9 ± 657.1 | 4624.6 ± 355.7 | −0.6 (−1.4, 0.3) | 1.750 | 0.114 |
| ESV (mL/50 min) | 1106.0 ± 268.6 | 995.2 ± 281.4 | −0.4 (−1.2, 0.5) | 1.453 | 0.180 |
| EF | 77.7 ± 4.7 | 78.7 ± 5.0 | 0.2 (−0.6, 1.0) | −0.818 | 0.435 |
| Variables | N Trial | H Trial | F-Value (η2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before | After | Cohen’s d (95% CI) | p-Value | Before | After | Cohen’s d (95% CI) | p-Value | Time | Group | Interaction | |
| HR (bpm/min) | 76.7 ± 7.5 | 102.8 ± 13.1 | 2.4 (1.2, 3.4) | 0.000 | 86.9 ± 7.5 | 113.5 ± 11.1 | 2.8 (1.5, 3.8) | 0.000 | 13.738 (0.604) † | 51.278 (0.851) † | 0.061 (0.007) |
| SBP (mmHg) | 111.3 ± 8.4 | 109.1 ± 8.0 | −0.3 (−1.1, 0.6) | 0.343 | 108.4 ± 8.9 | 107.1 ± 7.3 | −0.1 (−1.0, 0.7) | 0.325 | 1.391 (0.134) | 1.945 (0.178) | 0.203 (0.022) |
| DBP (mmHg) | 66.2 ± 8.4 | 62.1 ± 10.2 | −0.4 (−1.3, 0.4) | 0.126 | 66.4 ± 8.5 | 63.3 ± 8.5 | −0.4 (−1.2, 0.5) | 0.115 | 4.872 (0.351) | 0.167 (0.018) | 0.133 (0.015) |
| MAP (mmHg) | 81.2 ± 7.5 | 77.3 ± 8.9 | −0.5 (−1.3, 0.4) | 0.089 | 80.7 ± 8.0 | 77.9 ± 7.3 | −0.4 (−1.2, 0.5) | 0.117 | 5.286 (0.370) † | 0.002 (0.000) | 0.232 (0.025) |
| PP (mmHg) | 45.1 ± 8.0 | 47.1 ± 5.6 | 0.3 (−0.6, 1.1) | 0.321 | 42.1 ± 7.7 | 43.8 ± 7.6 | 0.2 (−0.6, 1.1) | 0.309 | 1.683 (0.158) | 4.293 (0.323) | 0.022 (0.002) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, K.; Seo, J.; Jung, W.-S.; Kim, J.; Park, H.-Y.; Lim, K. Effects of an Acute Pilates Program under Hypoxic Conditions on Vascular Endothelial Function in Pilates Participants: A Randomized Crossover Trial. Int. J. Environ. Res. Public Health 2020, 17, 2584. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17072584
Jung K, Seo J, Jung W-S, Kim J, Park H-Y, Lim K. Effects of an Acute Pilates Program under Hypoxic Conditions on Vascular Endothelial Function in Pilates Participants: A Randomized Crossover Trial. International Journal of Environmental Research and Public Health. 2020; 17(7):2584. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17072584
Chicago/Turabian StyleJung, Kyounghwa, Jongbeom Seo, Won-Sang Jung, Jisu Kim, Hun-Young Park, and Kiwon Lim. 2020. "Effects of an Acute Pilates Program under Hypoxic Conditions on Vascular Endothelial Function in Pilates Participants: A Randomized Crossover Trial" International Journal of Environmental Research and Public Health 17, no. 7: 2584. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17072584






