Selected Physicochemical Properties of Saliva in Menopausal Women—A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients’ Population
2.2. Saliva Collection
- 0.26–0.30 mL/min—proper unstimulated salivation
- 0.25–0.1 mL/min—reduced unstimulated salivation (oligosialy)
- <0.1 mL/min—impaired salivation–kserostomy
2.3. Analysis of Saliva
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aryeh, H.B.; Gottlieb, I.; Ish-Shalom, S.; David, A.; Szargel, H.; Laufer, D. Oral complaints related to menopause. Maturitas 1996, 24, 185–189. [Google Scholar] [CrossRef]
- Meurman, J.H.; Tarkkila, L.; Tiitinen, A. Themenopauseand oral health. Maturitas 2009, 63, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Suri, V.; Suri, V. Menopauseand oral health. J. Midlife Health 2014, 5, 115–120. [Google Scholar] [PubMed]
- Petkowicz, B.; Piotrkowicz, J.; Szeszko, Ł.; Banakiewicz, K.; Zieliński, P. Selected aspects of oral cavity diseases in menopausal women. PrzeglądMenopauzalny 2013, 4, 352–357. [Google Scholar] [CrossRef] [Green Version]
- Srebrzyńska-Witek, A.; Koszowski, R. Effect of menopause on salivary glands and oral mucosa. Przegląd Menopauzalny 2013, 5, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Leimola-Virtanen, R.; Pennanen, R.; Syrjänen, K.; Syrjänen, S. Estrogen response in buccal mucosa—Acytological and immunohistological assay. Maturitas 1997, 27, 41–45. [Google Scholar] [CrossRef]
- Leimola-Virtanen, R.; Salo, T.; Toikkanen, S.; Pulkkinen, J.; Syrjänen, S. Expression of estrogen receptor (ER) in oral mucosa and salivary glands. Maturitas 2000, 36, 131–137. [Google Scholar] [CrossRef]
- Välimaa, H.; Savolainen, S.; Soukka, T.; Silvoniemi, P.; Mäkelä, S.; Kujari, H.; Gustafsson, J.-A.; Laine, M. Estrogen receptor-beta is the predominant estrogen receptor subtype in human oral epithelium and salivary glands. J. Endocrinol. 2004, 180, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Asplund, R.; Aberg, H.E. Oral dryness, nocturiaand the menopause. Maturitas 2005, 50, 86–90. [Google Scholar] [CrossRef]
- Pink, R.; Simek, J.; Vondrakova, J. Saliva as a diagnostic medium. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 2009, 153, 103–110. [Google Scholar] [CrossRef] [Green Version]
- LyngePedersen, A.M.; Belstrøm, D. The role ofnatural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 68, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Fábián, T.K.; Hermann, P.; Beck, A.; Fejérdy, P.; Fábián, G. Salivary defense proteins: Their network and rolein innate and acquired oral immunity. Int. J. Mol. Sci. 2012, 13, 4295–4320. [Google Scholar] [CrossRef] [PubMed]
- Sewón, L.; Laine, M.; Karjalainen, S.; Leimola-Virtanen, R.; Hiidenkari, T.; Helenius, H. The effect of hormone replacement therapy on salivary calcium concentrations in menopausal women. Arch. Oral Biol. 2000, 45, 201–206. [Google Scholar] [CrossRef]
- Agha-Hosseini, F.; Mirzaii-Dizgah, I.; Moghaddam, P.P.; Akrad, Z.T. Stimulated whole salivary flow rate and composition in menopausal women with oral dryness feeling. Oral Dis. 2007, 13, 320–323. [Google Scholar] [CrossRef]
- Rajesh, K.S.; Zareena Hegde, S.; Arun Kumar, M.S. Assessment of salivary calcium, phosphate, magnesium, pH, and flow rate in healthy subjects, periodontitis, and dental caries. Contemp. Clin. Dent. 2015, 6, 461–465. [Google Scholar]
- Fiyaz, M.; Ramesh, A.; Ramalingam, K.; Thomas, B.; Shetty, S.; Prakash, P. Association of salivary calcium, phosphate, pH and flow rate on oral health: A study on 90 subjects. J. Indian Soc. Periodontol. 2013, 17, 454–460. [Google Scholar] [CrossRef]
- Sewón, L.A.; Karjalainen, S.M.; Söderling, E.; Lapinleimu, H.; Simell, O. Associations between salivary calcium and oral health. J. Clin. Periodontol. 1998, 25, 915–919. [Google Scholar] [CrossRef]
- Jannssen, J.W.; Helbing, A.R. Arsenazzo III: An improvement of the toutine calcium determination in serum. Eur. J. Clin. Chem. Clin. Biochem. 1991, 29, 197–201. [Google Scholar]
- Mahesh, D.R.; Komali, G.; Jayanthi, K.; Dinesh, D.; Saikavitha, T.V. PreetiDinesh.Evaluation of Salivary Flow Rate, pH and Buffer in Pre, Post &Post Menopausal Women on HRT. J. Clin. Diagn. Res. 2014, 8, 233–236. [Google Scholar]
- Foglio-Bonda, P.L.; Rocchetti, V.; Nardella, A.; Fantinato, M.; Sandhu, K.; Foglio-Bonda, A. Salivary pH and flow rate inmenopausalwomen. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 918–922. [Google Scholar]
- Rukmini, J.N.; Sachan, R.; Sibi, N.; Meghana, A.; Malar, C.I. Effect ofMenopauseon Saliva and DentalHealth. J. Int. Soc. Prev. Community Dent. 2018, 8, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Kullander, S.; Sonesson, B. Studies on saliva in menstruating, pregnant and post-menopausal women. Acta Endocrinol. 1965, 48, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A.; Patton, L.L.; Tylenda, C.A. An assessment of salivary function in healthy pre-menopausal females. J. Gerontol. 1991, 46, M11–M15. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, Y.H.; Samaranayake, L.P.; Wu, P.C.; So, M. The antifungal e_ect of lactoferrinandlysozymeon Candida krusei and Candida albicans. APMIS Oral Biol. 1997, 105, 875–883. [Google Scholar]
- Wu, T.; Samaranayake, L.P.; Leung, W.K.; Sullivan, P.A. Inhibition of growth and secreted aspartyl proteinase production in Candida albicans by lysozyme. J. Med. Microbiol. 1999, 48, 721–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, C.K.; Dodds, M.W.; Zuo, P.; Johnson, D.A. A population-based study of salivary lysozyme concentrations and candidal counts. Arch. Oral Biol. 2018, 42, 25–31. [Google Scholar] [CrossRef]
- Rudney, J.D. Does variability in salivary protein concentrations influence oral microbial ecology and oral health? Crit. Rev. Oral Biol. Med. 1995, 6, 343–367. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Komiya-Ito, A.; Arataki, T.; Furuya, Y.; Yajima, Y.; Yamada, S.; Okuda, K.; Kato, T. Relationship between antimicrobial protein levels in whole saliva andperiodontitis. J.Periodontol. 2008, 79, 316–322. [Google Scholar] [CrossRef]
- Chorzewski, M.; Orywal, K.; Sierpinska, T.; Golebiewska, M. Salivaryprotective factors in patients suffering from decompensated type 2diabetes. Adv. Med. Sci. 2017, 62, 211–215. [Google Scholar] [CrossRef]
- Lal, K.; Pollock, J.J.; Santarpia, R.P.; Heller, H.M.; Kaufman, H.W.; Fuhrer, J.; Steigbigel, R.T. Pilot study comparing the salivary cationic protein concentrations in healthy adults and AIDS patients: Correlation with antifungal activity. J. Acquir. Immune Defic. Syndr. 1992, 5, 904–914. [Google Scholar]
- Sinor, Z.; Azirrawani, A. Association between salivary parameters and periodontal disease. In. Med. J. 2013, 20, 1–5. [Google Scholar]
- Zilm, P.S.; Mira, A.; Bagley, C.J.; Rogers, A.H. Effect of alkaline growth pH on the expression of cell envelope proteins in Fusobacterium nucleatum. Microbiology 2010, 56, 1783–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewón, L.A.; Karjalainen, S.M.; Sainio, M.; Seppä, O. Calcium and other salivary factors in periodontitis-affected subjects prior to treatment. J. Clin. Periodontol. 1995, 22, 267–270. [Google Scholar] [CrossRef] [PubMed]
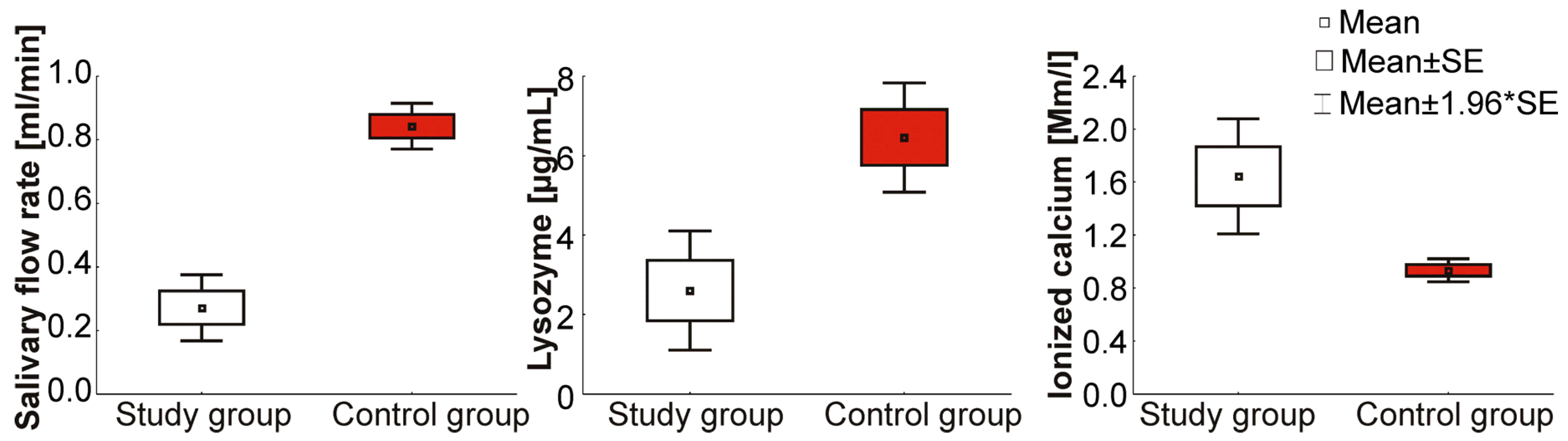
| Study Group (N = 9) | Control Group (N = 15) | p-Value | |
|---|---|---|---|
| pH | 0.3843 1 | ||
| Mean ± SD | 6.5 ± 1.1 | 7.5 ± 8.0 | |
| Range | 4.5–8.0 | 5.0–61.0 | |
| Me | 7.0 | 6.3 | |
| Salivary flow rate (mL/min) | 0.0001 1,* | ||
| Mean ± SD | 0.3 ± 0.2 | 0.8 ± 0.3 | |
| Range | 0.1–0.5 | 0.3–1.5 | |
| Me | 0.3 | 0.8 | |
| Lactoferrin (µg/mL) | 0.6717 1 | ||
| Mean ± SD | 6.1 ± 5.3 | 7.0 ± 8.8 | |
| Range | 1.2–17.3 | 1.1–61.7 | |
| Me | 4.0 | 5.6 | |
| Lysozyme (µg/mL) | 0.0019 1,* | ||
| Mean ± SD | 2.6 ± 2.3 | 6.5 ± 4.8 | |
| Range | 0.5–8.3 | 1.3–22.1 | |
| Me | 2.1 | 4.8 | |
| Immunoglobulin A (µg/mL) | 0.2944 1 | ||
| Mean ± SD | 416.4 ±389.0 | 515.8 ± 429.6 | |
| Range | 71.0–1 245.0 | 36.0–2 182.0 | |
| Me | 251.0 | 399.0 | |
| Ionized calcium (Mm/L) | 0.000 1,* | ||
| Mean ± SD | 1.6 ±0.7 | 0.9 ± 0.3 | |
| Range | 1.1–3.3 | 0.5–1.9 | |
| Me | 1.5 | 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cydejko, A.; Kusiak, A.; Grzybowska, M.E.; Kochańska, B.; Ochocińska, J.; Maj, A.; Świetlik, D. Selected Physicochemical Properties of Saliva in Menopausal Women—A Pilot Study. Int. J. Environ. Res. Public Health 2020, 17, 2604. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17072604
Cydejko A, Kusiak A, Grzybowska ME, Kochańska B, Ochocińska J, Maj A, Świetlik D. Selected Physicochemical Properties of Saliva in Menopausal Women—A Pilot Study. International Journal of Environmental Research and Public Health. 2020; 17(7):2604. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17072604
Chicago/Turabian StyleCydejko, Aleksandra, Aida Kusiak, Magdalena Emilia Grzybowska, Barbara Kochańska, Jolanta Ochocińska, Adrian Maj, and Dariusz Świetlik. 2020. "Selected Physicochemical Properties of Saliva in Menopausal Women—A Pilot Study" International Journal of Environmental Research and Public Health 17, no. 7: 2604. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17072604





