1. Introduction
Ototoxicity is a reaction to the pharmaceutical drugs used to treat communicable diseases that affects the cochlea or auditory nerve, which is characterized by vestibular or cochlea dysfunction [
1]. The prevalence of medication-induced hearing loss has tripled over the years and this is attributed to increased usage of ototoxic-medications. Ototoxic medications play an important role in modern medicine; however, they have the capacity to cause significant morbidity [
2]. This is particularly problematic because one of the key challenges encountered in ototoxicity assessment and management is a delay in diagnosing ototoxicity; with diagnosis occurring too late when the hearing loss has become severe and affects the speech frequencies [
1].
There are over 600 categories of medications that may cause ototoxicity, and this includes aminoglycosides antibiotics, platinum-based chemotherapeutic agents, loop diuretics, macrolide antibiotics and antimalarials [
1]. Ototoxicity affects people of all ages, but it is problematic to document the global scale of its occurrence due to the varied research methodologies followed, especially in low and middle income (LAMI) countries; and the diverse criteria used to define ototoxicity. Published evidence has looked at defining ototoxicity and developing early detection and monitoring guidelines to allow for considerations of treatment alterations in order to lessen or even prevent irreversible drug-induced hearing loss. Although many of these guidelines exist, their practicality, especially in LAMI countries, remains questionable, due to several factors.
In South Africa, a LAMI country with limited resources for basic healthcare and basic services, there has been no standardized practice for ototoxicity detection and monitoring. Until as recently as 2018, audiologists were adapting protocols from high income countries, such as the United States of America, to implement within the South African context, and this has often been with no or limited positive outcomes [
3]. This practice led to inconsistent service delivery confusion in terms of defining ototoxicity as well as inconsistent treatment options available to patients. In 2018, the Health Professions Council of South Africa’s Speech Language and Hearing Professions Board (HPCSA) [
4] issued national guidelines for ototoxicity assessment and management. Current authors, however, believe that the efficient implementation of these guidelines requires definition of the current practices around ototoxicity assessment and management for this population, and establishing how the current practice compares to the recommended guidelines. Research into this area will identify the gaps, define areas for improvement, and highlight areas requiring increased awareness and training to enhance both quantity and quality of lives of patients on ototoxic medications.
Landier [
5] approximated the occurrence of ototoxicity in patients on ototoxic-medications to be between 4% to 90% people in the United States. These medications are used to treat cancer, human immunodeficiency virus (HIV), tuberculosis (TB) and other communicable diseases. A 2016 global report estimated that 10.4 million people were diagnosed with TB in 2016. Of these people, 90% were adults and 74% were residing in African countries [
6]. Furthermore, the World Health Organization (WHO) [
7] estimates that there are 33 million persons living with HIV in Eastern and Southern Africa, and reports a steady increase in persons living with cancer.
Despite efforts to control the amount of new cases, there was an increase in new TB cases that are thought to have occurred in 2012 [
8,
9]. Additionally, 65% of the patients had hypertension, 22% had multi-morbidity, and a high number of young people were on antiretroviral therapy [
10]. HIV, TB, and cancer remain three of South Africa’s greatest burdens of disease. The dramatic increase in the number of people with communicable diseases has led to an increase in the usage of ototoxic-medications thus a heightened need for efficient ototoxicity monitoring and management protocols within this context.
A 2010 South African study by Khoza-Shangase [
11] explored the need for ototoxicity monitoring in patients with HIV/AIDS. The study highlighted that the lack of informational counselling, resources, staff, and economic challenges affect the adequate services dedicated to ototoxicity monitoring. Therefore, very few of the patients on ototoxic-medications receive audiological monitoring. The study raised the need for ototoxicity assessment and management within this LAMI country population [
11].
Although ototoxicity is argued to be non-life-threatening, it affects communication and health-related quality of life indicators with significant educational, occupational, and social consequences [
3,
11]. In children, ototoxicity can impact language, speech, social and cognitive development which could negatively impact on school performance and psychosocial functioning [
4]. The aim of ototoxicity rehabilitation should be early identification, continual monitoring, minimizing or preventing hearing impairment and planning of appropriate early rehabilitative measures [
11].
The biggest challenge encountered in ototoxicity assessment and management, is a delay in diagnosing ototoxicity due to its varied and highly inconsistent presentation [
1,
3,
4]. Other factors like age, medical conditions and cognitive levels, and culturally influenced health-seeking behaviors may delay the early detection [
1,
2,
8]. According to the HPCSA guidelines [
4], ototoxicity assessment should begin with informational counselling which entails making patients aware of significant symptoms which need to be reported to attending audiologists and physicians. Ototoxicity often progresses undetected until significant deterioration in frequencies for speech is noted, therefore ototoxicity is better diagnosed clinically by comparing audiometric results done before and after the administration of drugs.
International ototoxicity monitoring guidelines have been developed for high-income countries These guidelines set the stage for what should be done within these countries, and the current authors argue that such protocols are suitable for contexts that are well-resourced with equipment, personnel, collaboration amongst team members, as well as efficient informational control and efficient healthcare services. In LAMI countries, the implementation of these protocols has challenges, unless contextual realities are confronted and taken cognizance of. This implementation will need to be guided by support from contextualized research evidence.
The HPCSA [
4] guidelines, which are comparable with other relevant guidelines outside South Africa, e.g., ASHA and AAA guidelines, recommend that the assessment and management of ototoxicity begins with pre-treatment counselling concerning the risk of ototoxic effects from the medication. These guidelines also recommend that the patient should receive baseline measures where their current hearing thresholds are obtained. These baseline findings are to be used later for comparison with the during and post-treatment hearing thresholds. The baseline measures should include case history, otoscopy, pure-tone audiometry (frequencies between 250Hz−8000Hz in addition to 9000Hz and 125000Hz and beyond) and diagnostic distortion product otoacoustic emissions (DPOAEs) [
4]. The guidelines further recommend that after the patient’s pre-treatment thresholds have been obtained, the patient should receive bi-weekly audiological evaluations. These evaluations should comprise of bilateral otoscopic examinations, bilateral pure tones (including supra-threshold testing) and bilateral diagnostic DPOAEs. Should the patient’s hearing thresholds be worse, relative to the baseline thresholds, a comprehensive audiological assessment should be carried out within the next 24 hours or before the next administration of medications [
4]. The determination criteria (grading) for changes in the patient’s hearing, due to treatment, should be decided upon beforehand. Once the grading has been decided upon, the severity of the shift has to be graded according to the adverse event scale that is specific to hearing [
4]. Moreover, the HPCSA guidelines state that follow-up evaluations should be repeated once a month if a shift was detected, until the hearing thresholds stabilize, and no further change is detected.
According to the HPCSA guidelines, the medical management and aural rehabilitation of the patient includes a multidisciplinary team [
4] that has a key objective of adopting a comprehensive management plan for the patient, which includes developing non-ototoxic agents as one of its goals [
4]. For the HPCSA [
4] guidelines to be implemented efficiently, the current authors believe it is important to establish the current practice and identify gaps in this practice in order to be able to implement contextually responsive interventions for programme practicability and feasibility; hence this study.
3. Results and Discussion
3.1. Description of the Sample
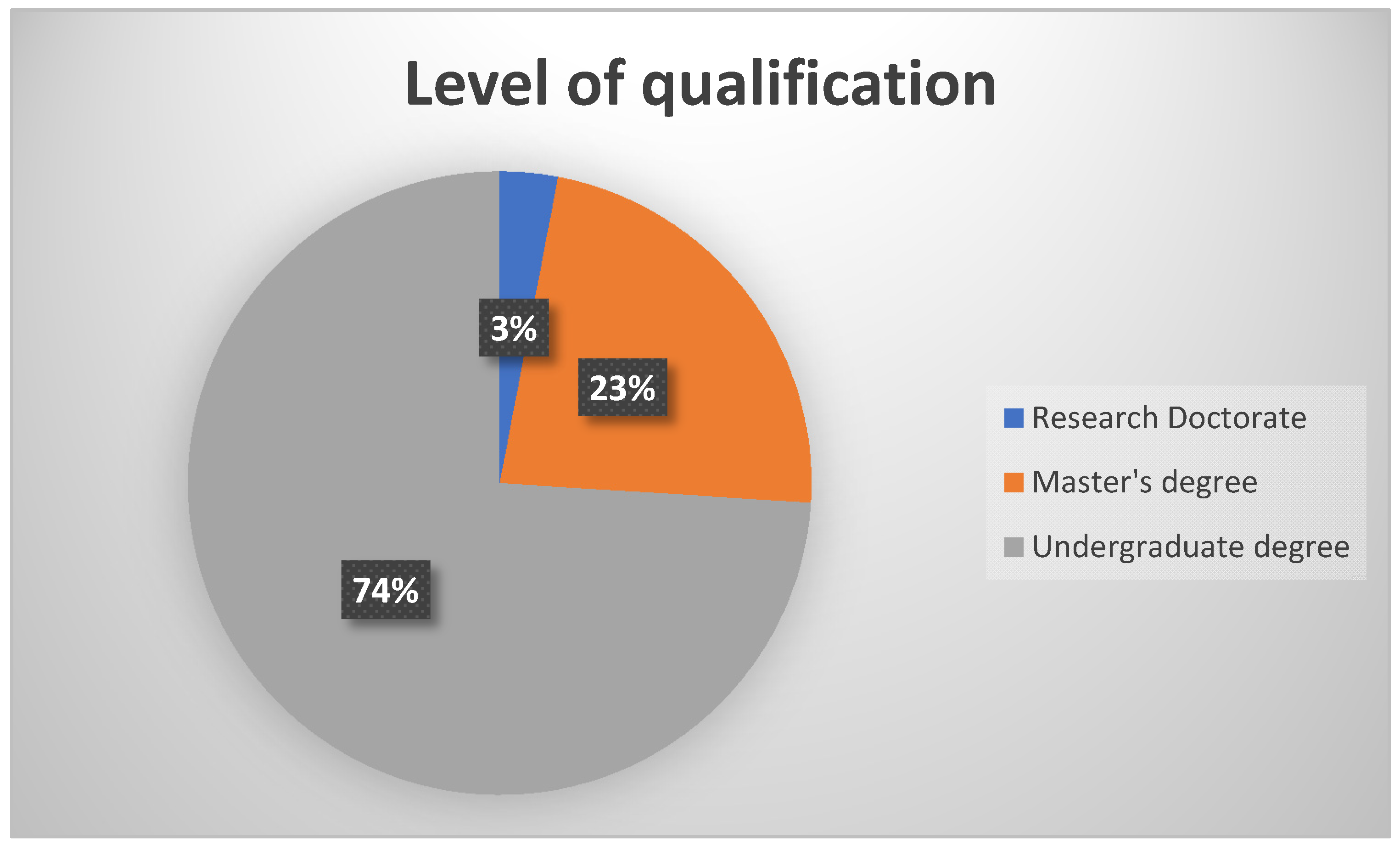
A total of 31 participants responded to the survey. As depicted in
Figure 1, 74% (n = 23) of the participants had an undergraduate degree in audiology or speech therapy and audiology, with the rest (n = 8) having obtained a postgraduate degree in audiology (either a Master’s degree or a Research Doctorate).
This demographic profile is representative of the South African audiology community which has more practitioners with an undergraduate qualification than post-graduate qualification. In South Africa, a postgraduate qualification is not a requirement for registration for clinical practice. Of the total sample, 71% (n = 22) of the participants reported to work in the public health sector, with a third 29% (n = 9) working in the private health sector. The fact that a majority of the sample was in the public sector is a positive finding for the current study as this is the sector that provides clinical services to at least 80% of the South African population, Therefore, evidence from this group has greater applicability in terms of guidelines implementation to the majority of the country’s citizens. It is acknowledged that this sector placement profile has no relationship to the actual placement of audiologists in the South African context. It is merely a feature of who completed the survey. Anecdotally from National Forums, as these data are not collected by the HPCSA, a large majority of audiologists in South Africa are in the private sector, with documented capacity versus demand challenges in the public sector.
3.2. Performance of Baseline Measures
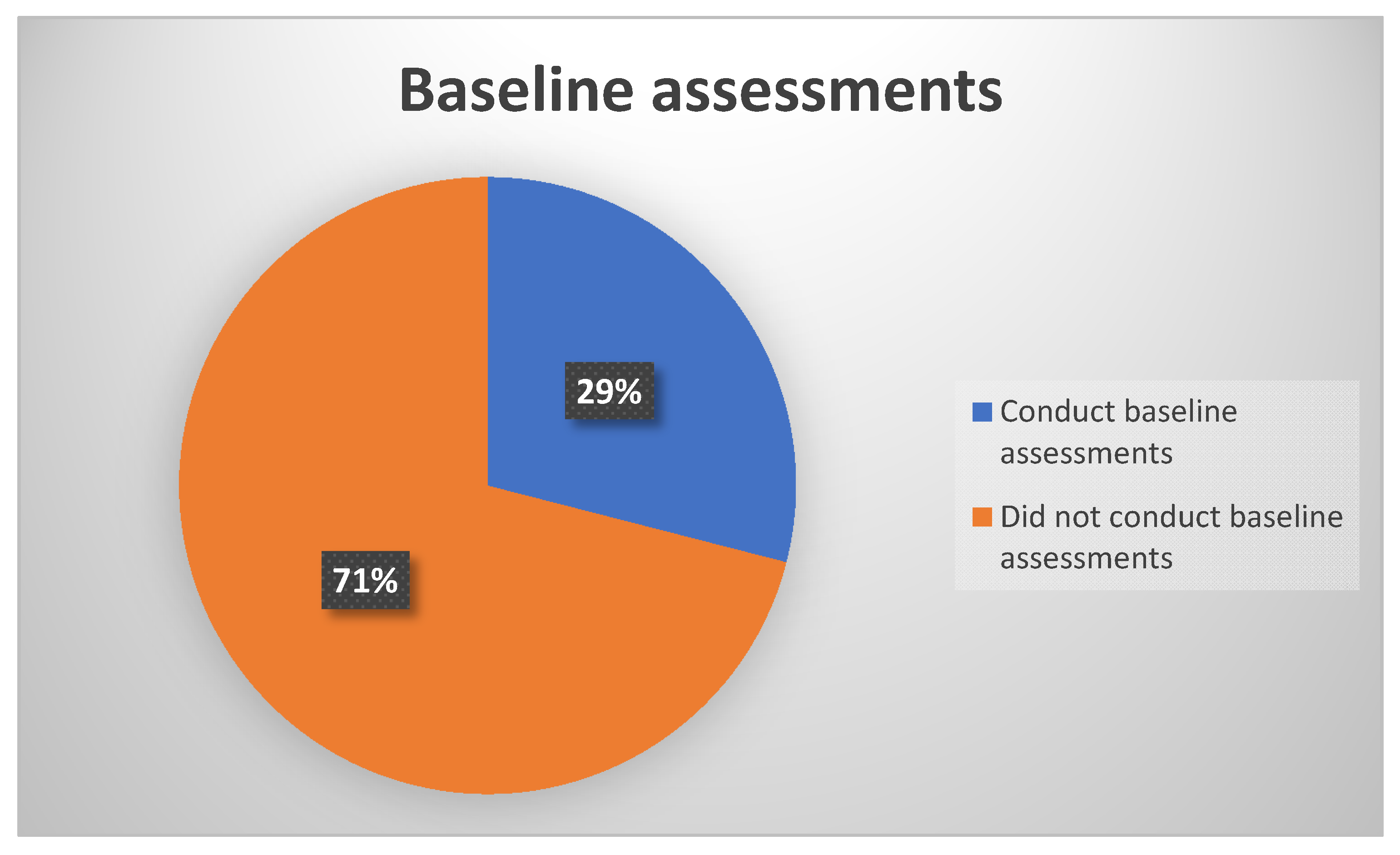
In looking at the practice of performing baseline assessments prior to treatment initiation or within 24 hours post treatment initiation, results, as depicted in
Figure 2, showed that more than half of the participants 71% (n = 22) did not conduct baseline assessments prior to treatment initiation for all their patients on treatment with ototoxic drugs. Consequently, only a third 29% (n = 9) of the participants reported conducting baseline assessments for 80%−100% of their patients’ assessments. This 29% comprised of audiologists who work in public sector facilities dedicated to the treatment of TB, where their main job is to conduct ototoxicity assessments and management.
The findings of the majority of participants not conducting baseline measures is contrary to international standards, such as those by the AAA [
18], as well as HPCSA [
4] guidelines. These guidelines suggest that all patients should receive baseline assessments prior to treatment with ototoxic medication. The implications of this omission are significant, as without a baseline assessment there are no hearing thresholds available to compare performance to, to determine the changes in hearing sensitivity, and to implement preventive measures should an ototoxic hearing loss occur. Furthermore, lack of baseline measures prevents early detection and identification, as well as early intervention of ototoxicity. Ultimately, this gap in practice limits opportunities for the adjustment of medication dose and/or frequency, the implementation of early rehabilitative strategies for enhanced patient’s quality of life, as well as patient counselling about possible otologic side effects. Patient counselling enhances treatment adherence [
8,
11,
19], which is key to treatment success.
3.3. Frequency of Assessments
As far as the frequency of ototoxicity monitoring was concerned, no participants reported conducting the recommended bi-weekly monitoring to all patients requiring it. A majority 71% (n = 22) of the sample indicated conducting bi-weekly audiological monitoring on less than 10% of their patients requiring ototoxicity monitoring, while the rest of the sample 29% (n = 9) reported that more than 50%, of their patients receive bi-weekly audiological monitoring. These are the same participants who reported conducting baseline assessments.
These findings are concerning as they continue to reveal a significant weakness in the translation of policies/guidelines into practice. Such practice limits early identification of ototoxic changes and impedes medical intervention to conserve hearing function in this population. Govender and Paken [
3] found similar findings in their study in the same context. These authors found that most audiologists do not conduct bi-weekly audiological monitoring due to lack of resources or patients that only arrive for audiological monitoring once the hearing impairment is severe. In another study, Harris, Peer and Fagan [
8], found that there is huge paucity in audiological monitoring in ototoxicity and this is due to lack of knowledge of guidelines, financial pressure and budgetary demands from life-threatening and/or communicable diseases. Without regular audiological monitoring, hearing threshold shifts will be more difficult to detect thus leading to delayed intervention. This will lead to increased burden of disease and impact on the patients’ quality of life, which are all preventable with adherence to evidence-based practice [
20].
3.4. Types of Assessment Measures Used
As far as the types of assessment measures used to assess patients with a high-risk for ototoxicity were concerned, the findings, as depicted in
Table 1, indicate that there is generally no standard consistent battery of assessments that is used. The results indicate that 70% (n = 21) of the participants use ultrahigh frequency pure tone audiometry, with DPOAEs included by approximately 30% (n = 10) of the participants. Otoscopy and immittance audiometry are the only measures that were found to be the most widely and consistently used with close to 100% (n = 30) of the participants reporting using them.
Findings with regards to equipment used also revealed that the type of equipment used for each patient could change over the period of monitoring, so inconsistent comparative data were available rendering the ototoxicity monitoring programmes insufficient and inefficient. Current findings raise important implications for resource procurement for clinics where ototoxicity is part of the caseload seen. Furthermore, they raise implications about ensuring consistent use of reliable and valid measures for repeated measures to ensure ability to closely compare and contrast the results as part of the monitoring protocol. The high, prevalent use of otoscopy and immittance audiometry on their own in this population is of concern since outer and middle ear function are not the main concern in general ototoxicity monitoring, unless these measures are coupled with additional measures. Immittance is an important measure when used within a test battery approach that includes DPOAEs, where conductive hearing loss may be present, and its presence affects the sensitivity and validity of otoacoustic emissions.
3.5. Criteria Used
According to the HPCSA [
4] guidelines, audiologists should have criteria to determine changes in patients’ hearing due to ototoxic medication, and this should be decided on before the patient receives the medication. The HPCSA criteria state the following: ≥ 20 dB pure tone threshold shift at a single frequency, ≥ 10 dB shift at two consecutive frequencies or threshold response shifting to “no response” at three consecutive frequencies. Once the presence of an ototoxic shift has been identified; the adverse effect on hearing ability must be graded in accordance with an adverse event scale, specific to hearing. In the HPCSA guidelines, this includes the use of four grades—where grade 1 represents a threshold shift or loss of 15–25dB relative to baseline, averaged at two or more contiguous frequencies in at least one ear, and grade 4 depicts profound bilateral hearing loss >90dBHL. In this study, 77% (n = 24) of the sample reported using the grading system suggested by the HPCSA, but it is important to note that 23% (n = 7) of the sample did not use any system to grade the changes in hearing sensitivity. In terms of the HPCSA grading criteria used to grade the severity, 11% (n = 6) of the participants reported not using this grading criteria and approximately 89% (n = 25) use the suggested criteria to grade the severity of the hearing impairment once a shift had been identified. The use of a grading system and severity criteria, singly or in combination, where other aspects of the ototoxicity monitoring protocol are not in place has minimal value or benefit in this context. Specifically, where a) baseline measures have not been obtained, or b) baseline measures have been obtained, but inappropriate assessment measures were used, and c) frequency of monitoring was not regular. These limitations in an ototoxicity monitoring protocol impact analysis of data, therefore preventing the appropriate use of a grading system for decision making. The lack of evidence-based practice has cascading effects for the ototoxicity monitoring and management programme.
3.6. Ototoxicity Management
With regards to the current management practices of patients with ototoxicity, results indicated that the majority of participants (65%) reported providing ototoxicity management within their own institutions. This management involves a combination of various options for 35% of the participants, specifically: a) inform a doctor to explore strategies for medical intervention, b) patient is given hearing aids or other amplification devices, c) patient is enrolled into an aural rehabilitation intervention, d) asking the nurse to monitor ototoxicity signs such as tinnitus as well as provide referrals, as well as e) informing the pharmacist to make recommendations for oto-protective treatments and less ototoxic medications. The rest of the sample reported the provision of hearing amplification (n = 8), aural rehabilitation (n = 6), monitoring patients’ hearing for the subsequent 3−6 months post-ototoxic medications treatment (n = 2) and the referral of the patients for diagnostic audiological assessment (n = 4). These management strategies are in line with international guidelines as well as HPCSA guidelines. Of the participants that reported making referrals for attending physicians/practitioners, almost all reported not receiving any positive collaboration from the practitioners concerned. This is consistent with Wium and Gerber [
21], who found that other health practitioners (i.e., doctors, nurses, pharmacists) are unaware of ototoxicity and the effects thereof, and this explains their reluctance to collaborate with audiologists. This raises implications for audiologists, who should increase awareness about ototoxicity amongst these professionals, and should strengthen their patients’ advocacy role for collaborative and efficacious management of patients on ototoxic medications.
3.7. Collaborative Work
In terms of whether the multidisciplinary approach is effective for ototoxicity assessment and management within the South African context, the results shown in
Table 2 reveal that there are a number of barriers with the effective implementation of this approach. Approximately 100% of the participants reported that the approach is vital in ototoxicity assessment and management practices, however, its efficiency is negatively impacted by a number of factors. In terms of effectiveness of the approach, 31% (n = 7) of the participants reported that the approach is effective only if team members are dedicated and work well together. Majority of the participants 69% (n = 24) felt that the approach was not effective because of various factors including a) lack of trust amongst professionals, b) the late referrals to audiologists, where patients are only referred once they have a severe hearing loss, as well as c) the large caseloads and the hectic schedules of clinicians. Moreover, participants reported that team members’ lack of knowledge regarding ototoxicity, communication amongst the team members as well as training in ototoxicity are some of the challenges that hinder appropriate ototoxicity management within a multidisciplinary team.
Lack of collaborative working relationships between the multidisciplines involved in management of patients on ototoxic medications is not a new finding. Khoza-Shangase and Jina [
22] found that although South African general practitioners appeared to be aware of ototoxicity and its symptoms, as well as the audiological services available to them, they did not make use of these audiological services as part of their patient care. In that study, it was concluded that general practitioners focus and place high priority on the patient’s diagnosis over an invisible side effect such as ototoxicity [
22]. In another study, Wium and Gerber [
21] found that practitioners do not make the referrals to audiologists due to time constraints, as well as due to insufficient knowledge from the practitioner and the patient about ototoxicity signs and symptoms. Internationally, current findings echo those from New Zealand where there is no nationally accepted ototoxicity monitoring programme practice pattern such that the state of monitoring is reported to be “poorly understood” [
23]. In a study by Steffens et al. [
24] in New Zealand, findings revealed a need for better collaboration between disciplines involved; with oncologists and audiologists being on opposing sides. For example, oncologists reported that information provided by audiologists guided oncology treatment decisions when possible, whilst audiologists suspected that their information was either not used or did not influence treatment decisions. In the current study, results seem to indicate that the multidisciplinary approach is theoretically seen as relevant and important, but its implementation almost impractical within the South African context. This situation might be changed by the impending National Health Insurance (NHI) implementation which has a health teams approach within a re-engineered primary health care model. This approach has preventive care, where ototoxicity prevention is located, as a priority [
25].
3.8. Additional Analysis
In an attempt to establish if there were any trends in the data that could explain the outcomes in terms of current practice, factors such as level of qualification, place of employment, sector of employment, and length of employment were carefully qualitatively scrutinized. Of these factors potentially influencing adherence to guidelines, institution of employment, specifically employment in TB hospitals, seemed to have a positive influence on current practice, possibly due to the focused nature of the audiologists’ scope of practice in these hospitals as well as the availability of resources—as ototoxicity monitoring forms part of standard hospital care. This is a single big difference in these specialized hospitals, where resources are focused and dedicated. The level of education of the participants appeared to have no influence in current findings. This was not expected as ototoxicity assessment and management forms part of the HPCSA minimum standards for undergraduate qualification in Audiology in South Africa, with postgraduate training comprising of research only, research report and research coursework, with no clinical training at postgraduate level.
4. Conclusions
Up until recently there has been limited research that investigates the current ototoxicity assessment and management practices in South Africa. This is a serious gap in evidence on ototoxicity monitoring, which Campbell and Le Prell [
26] emphasize should cover detection and monitoring as well as grading of adverse events in any context. This is an important area for research because of the increased risk of communicable diseases that require ototoxic medication for treatment. The lack of clinical guidelines that have been developed for the South African context, until recently, has exacerbated this situation. Current findings indicating training in ototoxicity assessment and management for all audiologists has not translated well to the practices exhibited by the same audiologists within this South African context are concerning. Similar findings have been found in early hearing detection and intervention (EHDI) studies within the South African context, where implementation of knowledge has been found to be impeded by contextual challenges. In this study; specifically, practices around performance of baseline measures as well as frequency of assessments during monitoring were found to be less than optimal, and did not adhere to guidelines. Limited consistency in the types of assessment measures used in the assessment batteries was found, with inconsistent application of standardized criteria used to establish ototoxicity and the grading approaches adopted. Ototoxicity management is influenced by a number of barriers including poor collaborative work between the disciplines involved in this population.
This highlights factors such as lack of translating knowledge, policies and guidelines into practice, as well as a clinical environment that is not conducive to this translation. This is a consistent finding in most studies around policies, guidelines and regulations within the South African context, where what is on paper does not get translated into practice for various reasons; key to which are resource constraints. Current findings seem to suggest that sufficient graduate training and the existence of policies and guidelines do not guarantee implementation, since this could be impacted by a number of factors, including availability of equipment, workload, training of team members in ototoxicity, and so on. The lack of standardized valid and reliable test battery and sensitive assessment measures nationally, with poor regular monitoring and follow up of patients on ototoxic medications limits chances of early identification followed by intervention in this population. The limited collaborative work between audiologists and attending physicians (and the rest of the medical team involved in the management of these patients) to enable prevention of ototoxicity further negatively influences hearing conservation efforts for this already vulnerable population group. This prevents the execution of what Lord [
27] refers to as another key purpose of ototoxicity monitoring, over and above provision of feedback to the attending physician about the effects the treatment is having on the auditory system; that of setting expectations for the patient and his/her family about the communication issues that may result from the drug therapy.
Current findings provide some contextually relevant evidence that will contribute toward ototoxicity programmes’ strategic planning, implementation and monitoring. These findings are novel for the South African context as far as implementation of ototoxicity monitoring and management guidelines, on the heels of the 2018 release of the HPCSA guidelines [
4]. Furthermore, previous studies have had physicians as participants, have looked at practice in specific conditions such as cancer or TB, while this study focused on audiologists with general ototoxicity assessment and management perspective [
21,
22,
23,
24]. Nonetheless, these findings should take cognizance of the identified methodological weaknesses of the study. The small sample size of this study, although a finding on its own in terms of practicing audiologists’ interest in ototoxicity assessment and management, particularly in the private healthcare sector in South Africa, must be taken into consideration when interpreting current findings. Furthermore, the fact that reasons for why certain practices were not followed were not explored in the current study is an identified limitation and raises implications for future studies. These findings have significance for the clinical training (education) of all team members, continued professional development, policy formulation by healthcare policy makers, resource planning and allocation (including both equipment and relevant staff), as well as awareness campaigns around collaborative work within the South African context. All these implications have the aim of ensuring that current practice improves in order for the guidelines to be effectively and efficiently implemented. This is important as these guidelines have carefully taken the South African context into consideration without compromising the goal of ototoxicity monitoring programs. These implications also have a goal of ensuring that barriers identified in this study are addressed. Because the current study was an initial assessment of current practice in light of the HPCSA guidelines, future studies should collect data that can establish causal links between some of the barriers identified, e.g., lack of staff or equipment, and the quality of ototoxicity assessment and management practices.