Review: Sex-Specific Aspects in the Bariatric Treatment of Severely Obese Women
Abstract
:1. Introduction
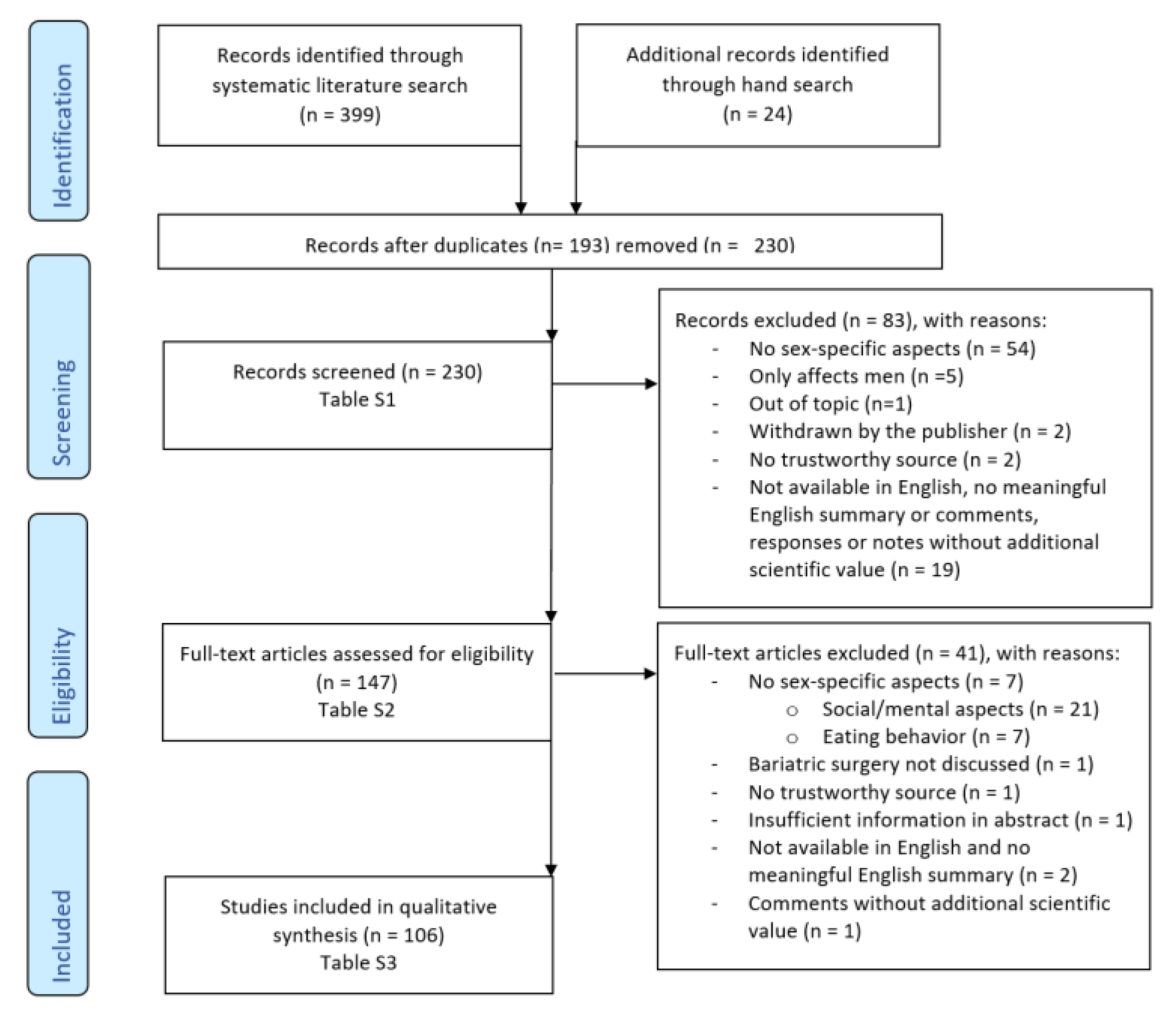
2. Methods
2.1. Electronic Literature Search in Scientific Databases
2.2. Selection and Inclusion Criteria
2.3. Summarizing into Main Categories
3. Results
- Female sexuality and sexual function
- Contraception
- Fertility
- Pregnancy and breastfeeding
- Sexual hormones and polycystic ovary syndrome (PCOS)
- Menopause and osteoporosis
- Pelvic floor disorders and urinary incontinence
- Female-specific cancer
- Metabolism, outcome, and quality of life
- Other
- Social and mental health aspects
- Eating behavior and disorders
3.1. Female Sexuality and Sexual Function
- There is strong evidence for improved sexual function as well as sexual quality of life and satisfaction with sexual life in obese women due to bariatric surgery.
- Although the prevalence of female sexual dysfunction decreases after bariatric surgery, it is still higher than in non-obese women.
- While there is an improvement of sexual function due to reduced weight, other aspects such as excessive hanging skin and social or mental aspects may be important for the sexual life of women after bariatric surgery.
- In the aftercare of bariatric surgery, changes in sexual behavior must be considered. There should be sufficient education of patients in terms of behaviors that pose risks for unintentional pregnancies and sexually transmitted infections.
3.2. Contraception
- There is a lack of evidence regarding the effectiveness of oral contraceptives among women undergoing bariatric surgery.
- In order to avoid any risk that comes along with (unplanned) pregnancy, especially in the postoperative period, women who have bariatric procedures are discouraged from using oral contraceptives.
- In clinical practice, contraceptive and pregnancy counseling by bariatric physicians is often not performed at all or falls short.
- Contraceptive counseling can improve the use of safe contraceptive methods and therefore avoid risky pregnancies.
- Better awareness of or knowledge about contraceptive needs after bariatric surgery by physicians can improve the quality of contraceptive counseling.
3.3. Fertility
- Obesity is linked to infertility by complex mechanisms and associations.
- There is evidence for significant improvement of various factors associated with infertility due to bariatric surgery.
- In the treatment of infertility in women, bariatric surgery is becoming an increasingly important issue.
- In order to improve the evidence, further prospective epidemiological research is required.
3.4. Pregnancy and Breastfeeding
- Obesity-related pregnancy risk can decrease due to weight loss as a result of bariatric surgery.
- After bariatric surgery, there is an increased risk of micronutrient deficiencies in pregnant women. Sufficient supplementation and monitoring of pregnant women, especially after bariatric procedures that include malabsorptive aspects, is recommended.
- Due to the extreme weight loss, there is increased fetal risk in the postoperative period. Clinical guidelines recommend contraception/avoiding pregnancy for at least 12–24 months after surgery, depending on the source.
- There might be a lack of evidence for the validity of oral glucose tolerance testing to diagnose gestational diabetes in women with a history of bariatric surgery.
- Breastfeeding can be a protective factor in the development of obesity and is important for the newborn.
- There is no indication of problems with breast milk after bariatric surgery. Therefore, the WHO’s recommendations for breastfeeding also apply to women with a history of bariatric surgery.
3.5. Sexual Hormones and Polycystic Ovary Syndrome (PCOS)
- PCOS and other complex dysregulations of the female sex hormone balance are associated with obesity.
- Weight loss due to bariatric surgery improves PCOS significantly and can regulate hormonal disorders such as obesity-related gonadal disorders.
- In the treatment of obesity-related gonadal disorders/PCOS in severely obese women, bariatric surgery should be taken into consideration as a therapeutic option.
- Regarding the nonspecific inflammatory marker CRP and the metabolic protecting hormone adiponectin, first scientific results indicate positive effects due to bariatric surgery.
3.6. Menopause and Osteoporosis
- Although there is increased intake of macronutrients, severely obese women often suffer from micronutrient deficiencies, especially vitamin D. Even before bariatric surgery, severely obese women represent a vulnerable group in terms of the development of osteoporosis.
- Due to continued diminished intake and affected absorption, bariatric surgery can increase that risk.
- Prophylactic substitution of calcium is recommended; vitamin D and parathyroid hormones should be observed closely in the postoperative period.
- There is a lack of evidence regarding the influence of bariatric surgery on menopausal symptoms. Initial research indicates a positive effect.
3.7. Pelvic Floor Disorders and Urinary Incontinence
- Pelvic floor disorders, and particularly urinary incontinence, are associated with obesity.
- Pelvic floor disorders and urinary incontinence can be improved by weight loss after bariatric surgery.
- There is a lack of research regarding the effects of bariatric surgery on prolapse symptoms and anal incontinence.
- In order to improve the evidence, further prospective epidemiological research with longer follow-up periods and more cases is required.
3.8. Female-Specific Cancer
- Obesity is associated with increased risk of female-specific cancer, such as breast and endometrial cancer.
- Weight loss as a result of bariatric surgery decreases the incidence of endometrial and breast cancer in severely obese women.
- First research indicates that bariatric surgery can lead to regression and healing of endometrial hyperplasia, a precancerous condition that can develop into endometrial cancer.
3.9. Metabolism, Outcome, and Quality of Life
- Although the prevalence of obesity is equal in men and women or even higher in men, men represent a minority among patients who undergo bariatric surgery.
- If treated, male patients have a higher BMI and comorbid risk profile on average. As a result, the benefit of the bariatric procedure is as high as it is in women. Nevertheless, satisfaction with the operation is greater in men.
- Further research about sex-specific aspects in the outcome of bariatric surgery, especially regarding metabolic aspects, is needed.
3.10. Other
- Similar to the decision for bariatric surgery, sex-specific aspects might affect the decision to undergo plastic surgery such as abdominoplasty after weight loss due to bariatric surgery.
- Abdominoplasty to reduce excessive hanging skin after bariatric surgery can improve quality of life similarly in men and women.
4. Discussion
4.1. Summary
4.2. Limitations
4.3. Further Approaches
4.4. Valorization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMH | anti-Mullerian hormone |
| BMI | body mass index (kg/m2) |
| CRP | C-reactive protein |
| DHEAS | DHEAS |
| FSFI | Female Sexual Function Index |
| HbA1C | hemoglobin A1C |
| ICIQ-IU | Incontinence Questionnaire-Urinary Incontinence |
| non-HDL-C | non-high-density lipoprotein cholesterol |
| PFDI | Pelvic Floor Disability Index |
| SD | standard deviation |
| WHO | World Health Organization |
References
- World Health Organization (WHO) WHO | Controlling the global obesity epidemic. Available online: https://www.who.int/nutrition/topics/obesity/en/ (accessed on 27 September 2019).
- World Health Organization (WHO) Obesity and overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 27 September 2019).
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism. 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, J.J.; El-Hamdouchi, A.; Diouf, A.; Monyeki, A.; Somda, S.A. Determining the worldwide prevalence of obesity. Lancet 2018, 391, 1773–1774. [Google Scholar] [CrossRef]
- Thielman, J.; Harrington, D.; Rosella, L.C.; Manson, H. Prevalence of age-specific and sex-specific overweight and obesity in Ontario and Quebec, Canada: a cross-sectional study using direct measures of height and weight. BMJ Open 2018, 8. [Google Scholar] [CrossRef]
- The GBD 2015 Obesity Collaborators Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27.
- World Health Organization (WHO) Obesity. Available online: https://www.who.int/topics/obesity/en/ (accessed on 28 September 2019).
- American Society for Metabolic and Bariatric Surgery Guidelines for bariatric surgery as part of the ASMBS Professional Resource Center. Available online: https://asmbs.org/resource-categories/guidelines-recommendations (accessed on 6 November 2019).
- Angrisani, L.; Santonicola, A.; Iovino, P.; Formisano, G.; Buchwald, H.; Scopinaro, N. Bariatric Surgery Worldwide 2013. Obes. Surg. 2015, 25, 1822–1832. [Google Scholar] [CrossRef]
- Chirurgische Arbeitsgemeinschaft für Adipositaschirurgie S3 Leitlinie: Chirurgie der Adipositas und metabolischer Erkrankungen; AWMF online. Available online: https://www.awmf.org/uploads/tx_szleitlinien/088-001l_S3_Chirurgie-Adipositas-metabolische-Erkrankugen_2018-02.pdf (accessed on 6 November 2019).
- Higgins, J.; Green, S. Chapter 4: Guide to the contents of a Cochrane protocol and review. In Cochrane Handbook for Systematic Reviews of Intervention; The Cochrane Collaboration: London, UK; Available online: https://training.cochrane.org/handbook/current (accessed on 2 November 2019).
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef] [Green Version]
- Pichlerova, D.; Bob, P.; Zmolikova, J.; Herlesova, J.; Ptacek, R.; Laker, M.K.; Raboch, J.; Fait, T.; Weiss, P. Sexual Dysfunctions in Obese Women Before and After Bariatric Surgery. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 3108–3114. [Google Scholar] [CrossRef]
- Janik, M.R.; Bielecka, I.; Paśnik, K.; Kwiatkowski, A.; Podgórska, L. Female Sexual Function Before and After Bariatric Surgery: a Cross-Sectional Study and Review of Literature. Obes. Surg. 2015, 25, 1511–1517. [Google Scholar] [CrossRef] [Green Version]
- Nimbi, F.M.; Paone, E.; Pierro, L.; Tripodi, F.; Fabrizi, A. Sexual Health in Obese Women Asking for Bariatric Surgery. J. Sex. Med. 2017, 14, e289. [Google Scholar] [CrossRef]
- Rosen, R.; Brown, C.; Heiman, J.; Leiblum, S.; Meston, C.; Shabsigh, R.; Ferguson, D.; D’Agostino, R. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marital Ther. 2000, 26, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Steffen, K.J.; King, W.C.; White, G.E.; Subak, L.L.; Mitchell, J.E.; Courcoulas, A.P.; Flum, D.R.; Strain, G.; Sarwer, D.B.; Kolotkin, R.L.; et al. Sexual Functioning of Men and Women with Severe Obesity Prior to Bariatric Surgery. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2017, 13, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Steffen, K.J.; King, W.C.; White, G.E.; Subak, L.L.; Mitchell, J.E.; Courcoulas, A.P.; Flum, D.R.; Strain, G.; Sarwer, D.B.; Kolotkin, R.L.; et al. Changes in Sexual Functioning in Women and Men in the 5 Years After Bariatric Surgery. JAMA Surg. 2019, 154, 487–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, C.F.A.; dos Santos, P.O.; de Oliveira, R.A.; Leite-Filho, H.; de Almeida Oliveira, A.F.; Bagano, G.O.; Lima Junior, E.B.; Miranda, E.P.; de Bessa Junior, J.; Barroso Junior, U. Changes in Sexual Function and Positions in Women With Severe Obesity After Bariatric Surgery. Sex. Med. 2018, 7, 80–85. [Google Scholar] [CrossRef] [Green Version]
- Sarwer, D.B.; Wadden, T.A.; Spitzer, J.C.; Mitchell, J.E.; Lancaster, K.; Courcoulas, A.; Gourash, W.; Rosen, R.C.; Christian, N.J. 4-Year Changes in Sex Hormones, Sexual Functioning, and Psychosocial Status in Women Who Underwent Bariatric Surgery. Obes. Surg. 2018, 28, 892–899. [Google Scholar] [CrossRef]
- Cherick, F.; Te, V.; Anty, R.; Turchi, L.; Benoit, M.; Schiavo, L.; Iannelli, A. Bariatric Surgery Significantly Improves the Quality of Sexual Life and Self-esteem in Morbidly Obese Women. Obes. Surg. 2019, 29, 1576–1582. [Google Scholar] [CrossRef]
- Lechmiannandan, S.; Panirselvam, M.; Muninathan, P.; Hussin, N.; Rajan, R.; Sidi, H.; Kosai, N.R.; Vinayak, C.R. Resolution of Female Sexual Dysfunction (FSD) Among the Obese Multiethnic Malaysian Women Now a Reality with Bariatric Surgery: a Prospective Pilot Study in Malaysia. Obes. Surg. 2019, 29, 1571–1575. [Google Scholar] [CrossRef]
- Ramalho, S.; Bastos, A.P.; Silva, C.; Vaz, A.R.; Brandão, I.; Machado, P.P.P.; Conceição, E. Excessive Skin and Sexual Function: Relationship with Psychological Variables and Weight Regain in Women After Bariatric Surgery. Obes. Surg. 2015, 25, 1149–1154. [Google Scholar] [CrossRef]
- Zeller, M.H.; Brown, J.L.; Reiter-Purtill, J.; Sarwer, D.B.; Black, L.; Jenkins, T.M.; McCracken, K.A.; Courcoulas, A.P.; Inge, T.H.; Noll, J.G.; et al. Sexual behaviors, risks, and sexual health outcomes for adolescent females following bariatric surgery. Surg. Obes. Relat. Dis. 2019, 15, 969–978. [Google Scholar] [CrossRef]
- Guttmacher Institute Contraceptive Use in the United States. Available online: https://www.guttmacher.org/fact-sheet/contraceptive-use-united-states (accessed on 16 October 2019).
- Ined - Institut national d’études démographiques What are the most widely used contraceptive methods across the world? Available online: https://www.ined.fr/en/everything_about_population/demographic-facts-sheets/faq/most-widely-used-contraceptive-methods-world/ (accessed on 16 October 2019).
- World Health Organization (WHO) Family planning/Contraception. Available online: https://www.who.int/news-room/fact-sheets/detail/family-planning-contraception (accessed on 16 October 2019).
- Schlatter, J. Oral Contraceptives after Bariatric Surgery. Obes. Facts 2017, 10, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginstman, C.; Frisk, J.; Carlsson, B.; Ärlemalm, A.; Hägg, S.; Brynhildsen, J. Plasma concentrations of etonogestrel in women using oral desogestrel before and after Roux-en-Y gastric bypass surgery: a pharmacokinetic study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Damhof, M.A.; Pierik, E.; Krens, L.L.; Vermeer, M.; van Det, M.J.; van Roon, E.N. Assessment of Contraceptive Counseling and Contraceptive Use in Women After Bariatric Surgery. Obes. Surg. 2019, 29, 4029–4035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merki-Feld, G.S.; Skouby, S.; Serfaty, D.; Lech, M.; Bitzer, J.; Crosignani, P.G.; Cagnacci, A.; Sitruk-Ware, R. European Society of Contraception Statement on Contraception in Obese Women. Eur. J. Contracept. Reprod. Health Care 2015, 20, 19–28. [Google Scholar] [CrossRef]
- Graham, Y.N.H.; Mansour, D.J.; Small, P.K.; Fraser, I.S. Contraceptive practices and menstrual patterns in women aged 18–50 years awaiting bariatric surgery. BMJ Sex. Reprod. Health 2018, 44, 228–230. [Google Scholar] [CrossRef] [Green Version]
- Shah, J.P.; Jatlaoui, T.C.; Zapata, L.B.; Curtis, K.M.; Pagano, H.P.; Whiteman, M.K. Health Care Provider Knowledge Regarding Oral Contraception Effectiveness for Women with a History of Bariatric Malabsorptive Procedures. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2019, 15, 1355–1361. [Google Scholar] [CrossRef]
- Michalsky, M.; Eneli, I.; McCracken, K. 24. Contraceptive Use Among Adolescent Female Metabolic and Bariatric Surgery Patients. J. Pediatr. Adolesc. Gynecol. 2019, 32, 204. [Google Scholar] [CrossRef]
- Casas, R.; Bourjeily, G.; Vithiananthan, S.; Tong, I. Contraceptive use in women undergoing bariatric surgery. Obes. Res. Clin. Pract. 2014, 8, e608–e613. [Google Scholar] [CrossRef]
- Mengesha, B.M.; Carter, J.T.; Dehlendorf, C.E.; Rodriguez, A.J.; Steinauer, J.E. Perioperative pregnancy interval, contraceptive counseling experiences, and contraceptive use in women undergoing bariatric surgery. Am. J. Obstet. Gynecol. 2018, 219, 81.e1–81.e9. [Google Scholar] [CrossRef] [Green Version]
- Mengesha, B.; Griffin, L.; Nagle, A.; Kiley, J. Assessment of contraceptive needs in women undergoing bariatric surgery. Contraception 2016, 94, 74–77. [Google Scholar] [CrossRef]
- Menke, M.N.; King, W.C.; White, G.E.; Gosman, G.G.; Courcoulas, A.P.; Dakin, G.F.; Flum, D.R.; Orcutt, M.J.; Pomp, A.; Pories, W.J.; et al. Conception rates and contraceptive use after bariatric surgery among women with infertility: Evidence from a prospective multicenter cohort study. Surg. Obes. Relat. Dis. 2019, 15, 777–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambineri, A.; Laudisio, D.; Marocco, C.; Radellini, S.; Colao, A.; Savastano, S. Female infertility: which role for obesity? Int. J. Obes. Suppl. 2019, 9, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Charalampakis, V.; Tahrani, A.A.; Helmy, A.; Gupta, J.K.; Singhal, R. Polycystic ovary syndrome and endometrial hyperplasia: an overview of the role of bariatric surgery in female fertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 207, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Al kabbi, M.S.; Al-Taee, H.A.; Al Hussaini, S.K. Impact of Bariatric surgery on antimularian hormone in reproductive age women. Middle East Fertil. Soc. J. 2018, 23, 273–277. [Google Scholar] [CrossRef]
- Chiofalo, F.; Ciuoli, C.; Formichi, C.; Selmi, F.; Forleo, R.; Neri, O.; Vuolo, G.; Paffetti, P.; Pacini, F. Bariatric Surgery Reduces Serum Anti-mullerian Hormone Levels in Obese Women With and Without Polycystic Ovarian Syndrome. Obes. Surg. 2017, 27, 1750–1754. [Google Scholar] [CrossRef]
- Durlinger, A.; Visser, J.; Themmen, A. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction 2002. [Google Scholar] [CrossRef]
- Grynnerup, A.G.-A.; Lindhard, A.; Sørensen, S. The role of anti-Müllerian hormone in female fertility and infertility–an overview. Acta Obstet. Gynecol. Scand. 2012, 91, 1252–1260. [Google Scholar] [CrossRef]
- Bhandari, S.; Ganguly, I.; Bhandari, M.; Agarwal, P.; Singh, A.; Gupta, N.; Mishra, A. Effect of sleeve gastrectomy bariatric surgery-induced weight loss on serum AMH levels in reproductive aged women. Gynecol. Endocrinol. 2016, 32, 799–802. [Google Scholar] [CrossRef]
- Vincentelli, C.; Maraninchi, M.; Valéro, R.; Béliard, S.; Maurice, F.; Emungania, O.; Berthet, B.; Lombard, E.; Dutour, A.; Gaborit, B.; et al. One-year impact of bariatric surgery on serum anti-Mullerian-hormone levels in severely obese women. J. Assist. Reprod. Genet. 2018, 35, 1317–1324. [Google Scholar] [CrossRef]
- Milone, M.; Sosa Fernandez, L.M.; Sosa Fernandez, L.V.; Manigrasso, M.; Elmore, U.; De Palma, G.D.; Musella, M.; Milone, F. Does Bariatric Surgery Improve Assisted Reproductive Technology Outcomes in Obese Infertile Women? Obes. Surg. 2017, 27, 2106–2112. [Google Scholar] [CrossRef]
- Edison, E.; Whyte, M.; van Vlymen, J.; Jones, S.; Gatenby, P.; de Lusignan, S.; Shawe, J. Bariatric Surgery in Obese Women of Reproductive Age Improves Conditions That Underlie Fertility and Pregnancy Outcomes: Retrospective Cohort Study of UK National Bariatric Surgery Registry (NBSR). Obes. Surg. 2016, 26, 2837–2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, H.O. Improvement in Fertility after Bariatric Surgery in Obese Females with Polycystic Ovarian Syndrome: Based on Four Years of Experience in Two Centers in Sulaimani Governorate, Kurdistan Region/Iraq. Bariatr. Surg. Pract. Patient Care 2017, 12, 162–169. [Google Scholar] [CrossRef]
- Soares Júnior, J.M.; Lobel, A.; Ejzenberg, D.; Serafıni, P.C.; Baracat, E.C. Bariatric surgery in infertile women with morbid obesity: defınitive solution? Rev. Assoc. Médica Bras. 2018, 64, 565–567. [Google Scholar] [CrossRef]
- Dolin, C.; Ude Welcome, A.O.; Caughey, A.B. Management of Pregnancy in Women Who Have Undergone Bariatric Surgery. Obstet. Gynecol. Surv. 2016, 71, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Harreiter, J.; Schindler, K.; Bancher-Todesca, D.; Göbl, C.; Langer, F.; Prager, G.; Gessl, A.; Leutner, M.; Ludvik, B.; Luger, A.; et al. Management of Pregnant Women after Bariatric Surgery. J. Obes. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.-P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet Lond. Engl. 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Guelinckx, I.; Devlieger, R.; Beckers, K.; Vansant, G. Maternal obesity: pregnancy complications, gestational weight gain and nutrition. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2008, 9, 140–150. [Google Scholar] [CrossRef]
- McCall, S.J.; Li, Z.; Kurinczuk, J.J.; Sullivan, E.; Knight, M. Maternal and perinatal outcomes in pregnant women with BMI >50: An international collaborative study. PLoS ONE 2019, 14, e0211278. [Google Scholar] [CrossRef] [Green Version]
- Stothard, K.J.; Tennant, P.W.G.; Bell, R.; Rankin, J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA 2009, 301, 636–650. [Google Scholar] [CrossRef]
- Abenhaim, H.A.; Alrowaily, N.; Czuzoj-Shulman, N.; Spence, A.R.; Klam, S.L. Pregnancy outcomes in women with bariatric surgery as compared with morbidly obese women. J. Matern. Fetal Neonatal Med. 2016, 29, 3596–3601. [Google Scholar] [CrossRef]
- Galazis, N.; Docheva, N.; Simillis, C.; Nicolaides, K.H. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 181, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Jans, G.; Matthys, C.; Bel, S.; Ameye, L.; Lannoo, M.; Van der Schueren, B.; Dillemans, B.; Lemmens, L.; Saey, J.-P.; van Nieuwenhove, Y.; et al. AURORA: bariatric surgery registration in women of reproductive age - a multicenter prospective cohort study. BMC Pregnancy Childbirth 2016, 16, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maric, T.; Kanu, C.; Muller, D.; Tzoulaki, I.; Johnson, M.; Savvidou, M. OP13.09: Birthweight and intrauterine growth patterns of fetuses of pregnant women post-bariatric surgery. Ultrasound Obstet. Gynecol. 2017, 50, 90. [Google Scholar] [CrossRef] [Green Version]
- Stuart, A.; Källen, K. Risk of Abdominal Surgery in Pregnancy Among Women Who Have Undergone Bariatric Surgery. Obstet. Gynecol. 2017, 129, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Balestrin, B.; Urbanetz, A.A.; Barbieri, M.M.; Paes, A.; Fujie, J. Pregnancy After Bariatric Surgery: A Comparative Study of Post-Bariatric Pregnant Women Versus Non-Bariatric Obese Pregnant Women. Obes. Surg. 2019, 29, 3142–3148. [Google Scholar] [CrossRef]
- Ciangura, C.; Coupaye, M.; Deruelle, P.; Gascoin, G.; Calabrese, D.; Cosson, E.; Ducarme, G.; Gaborit, B.; Lelièvre, B.; Mandelbrot, L.; et al. Clinical Practice Guidelines for Childbearing Female Candidates for Bariatric Surgery, Pregnancy, and Post-partum Management After Bariatric Surgery. Obes. Surg. 2019, 1–13. [Google Scholar] [CrossRef]
- Leclercq, W.K.G.; Van Sambeek, A.; Uittenbogaart, M.; Niemarkt, H.J.; Bongers, M.Y.; Van Laar, J.O.E.H. Abdominal pain in a pregnant woman who had gastric reduction surgery: Risks associated with a history of bariatric surgery. Ned. Tijdschr. Geneeskd. 2018, 162. [Google Scholar]
- Kumari, A.; Nigam, A. Bariatric Surgery in Women: A Boon Needs Special Care During Pregnancy. J. Clin. Diagn. Res. JCDR 2015, 9, QE01–QE05. [Google Scholar] [CrossRef]
- Parent, B.; Martopullo, I.; Weiss, N.S.; Khandelwal, S.; Fay, E.E.; Rowhani-Rahbar, A. Bariatric Surgery in Women of Childbearing Age, Timing Between an Operation and Birth, and Associated Perinatal Complications. JAMA Surg. 2017, 152, 128. [Google Scholar] [CrossRef]
- Newlan, L.; Geraghty, S. Efficacy of oral glucose tolerance testing of pregnant women post bariatric surgery. Br. J. Midwifery 2018, 26, 704–707. [Google Scholar] [CrossRef]
- Whyte, M.; Johnson, R.; Cooke, D.; Hart, K.; McCormack, M.; Shawe, J. Diagnosing gestational diabetes mellitus in women following bariatric surgery: A national survey of lead diabetes midwives. Br. J. Midwifery 2016, 24, 434–438. [Google Scholar] [CrossRef]
- Parker, M.H.; Berghella, V.; Nijjar, J.B. Bariatric surgery and associated adverse pregnancy outcomes among obese women. J. Matern. Fetal Neonatal Med. 2016, 29, 1747–1750. [Google Scholar] [CrossRef] [PubMed]
- Smid, M.C.; Dotters-Katz, S.K.; Mcelwain, C.-A.; Volckmann, E.T.; Schulkin, J.; Stuebe, A.M. Pregnancy After Bariatric Surgery: National Survey of Obstetrician’s Comfort, Knowledge, and Practice Patterns. Obes. Surg. 2017, 27, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Neha, S.; Power, A. Managing women in pregnancy after bariatric surgery: the midwife as the co-ordinator of care. Br. J. Midwifery 2019, 27, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Gadgil, M.D.; Chang, H.-Y.; Richards, T.M.; Gudzune, K.A.; Huizinga, M.M.; Clark, J.; Bennett, W. Laboratory testing for and diagnosis of nutritional deficiencies in pregnancy before and after bariatric surgery. J. Womens Health 2014, 23, 129–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garretto, D.; Kim, Y.-K.; Quadro, L.; Rhodas, R.R.; Pimentel, V.; Crnosija, N.A.; Nie, L.; Bernstein, P.; Tropper, P.; Neal-Perry, G.S. Vitamin A and β-carotene in pregnant and breastfeeding post-bariatric women in an urban population. J. Perinat. Med. 2019, 47, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Hazart, J.; Le Guennec, D.; Accoceberry, M.; Lemery, D.; Mulliez, A.; Farigon, N.; Lahaye, C.; Miolanne-Debouit, M.; Boirie, Y. Maternal Nutritional Deficiencies and Small-for-Gestational-Age Neonates at Birth of Women Who Have Undergone Bariatric Surgery. J. Pregnancy 2017, 2017. [Google Scholar] [CrossRef]
- Maslin, K.; James, A.; Brown, A.; Bogaerts, A.; Shawe, J. What Is Known About the Nutritional Intake of Women during Pregnancy Following Bariatric Surgery? A Scoping Review. Nutrients 2019, 11, 2116. [Google Scholar] [CrossRef] [Green Version]
- Shawe, J.; Ceulemans, D.; Akhter, Z.; Neff, K.; Hart, K.; Heslehurst, N.; Štotl, I.; Agrawal, S.; Steegers-Theunissen, R.; Taheri, S.; et al. Pregnancy after bariatric surgery: Consensus recommendations for periconception, antenatal and postnatal care. Obes. Rev. 2019, 20, 1507–1522. [Google Scholar] [CrossRef] [Green Version]
- Bartholomay, L.M.; Berlin, K.; McInerney, M.; Garcia, L. Vitamin K Status in Women of Childbearing Years Before or After Bariatric Surgery. Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef]
- Jans, G.; Matthys, C.; Bogaerts, A.; Ameye, L.; Delaere, F.; Roelens, K.; Loccufier, A.; Logghe, H.; De Becker, B.; Verhaeghe, J.; et al. Depression and Anxiety: Lack of Associations with an Inadequate Diet in a Sample of Pregnant Women with a History of Bariatric Surgery-a Multicenter Prospective Controlled Cohort Study. Obes. Surg. 2018, 28, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- Monshi, B.; Stockinger, T.; Vigl, K.; Richter, L.; Weihsengruber, F.; Rappersberger, K. Phrynoderma and acquired acrodermatitis enteropathica in breastfeeding women after bariatric surgery. JDDG J. Dtsch. Dermatol. Ges. 2015, 13, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.L.; Faria, O.P.; de Gouvêa, H.R.; Amato, A.A. Supplementation Adherence and Outcomes Among Pregnant Women After Bariatric Surgery. Obes. Surg. 2019, 29, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Neovius, M.; Stephansson, O. Careful monitoring of fetal growth and maternal nutritional status should be practiced in pregnant women with a history of bariatric surgery. BMJ Evid.-Based Med. 2016, 21, 233. [Google Scholar] [CrossRef]
- Jans, G.; Devlieger, R.; Preter, V.D.; Ameye, L.; Roelens, K.; Lannoo, M.; der Schueren, B.V.; Verhaeghe, J.; Matthys, C.; De Preter, V.; et al. Bariatric Surgery Does Not Appear to Affect Women’s Breast-Milk Composition. J. Nutr. 2018, 148, 1096–1102. [Google Scholar] [CrossRef]
- Gimenes, J.C.; Nicoletti, C.F.; de Souza Pinhel, M.A.; Cortes-Oliveira, C.; Salgado Júnior, W.; Nonino, C.B. Nutritional Status of Children from Women with Previously Bariatric Surgery. Obes. Surg. 2018, 28, 990–995. [Google Scholar] [CrossRef]
- Opray, N.; Grivell, R.M.; Deussen, A.R.; Dodd, J.M. Directed preconception health programs and interventions for improving pregnancy outcomes for women who are overweight or obese. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Robson, S.; Daniels, B.; Rawlings, L. Bariatric surgery for women of reproductive age. BJOG Int. J. Obstet. Gynaecol. 2016, 123, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Sam, S. Obesity and Polycystic Ovary Syndrome. Obes. Manag. 2007, 3, 69–73. [Google Scholar] [CrossRef]
- Christ, J.; Falcone, T. Changes in ovarian morphology associated with bariatric surgery among women with polycystic ovary syndrome (PCOS). Fertil. Steril. 2016, 106, e32–e33. [Google Scholar] [CrossRef]
- Christ, J.P.; Falcone, T. Bariatric Surgery Improves Hyperandrogenism, Menstrual Irregularities, and Metabolic Dysfunction Among Women with Polycystic Ovary Syndrome (PCOS). Obes. Surg. 2018, 28, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Morreale, H.F.; Santacruz, E.; Luque-Ramírez, M.; Botella Carretero, J.I. Prevalence of “obesity-associated gonadal dysfunction” in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Hum. Reprod. Update 2017, 23, 390–408. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.E.; Jain, S.K. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int. J. Mol. Sci. 2017, 18. [Google Scholar]
- Abiad, F.; Khalife, D.; Safadi, B.; Alami, R.; Awwad, J.; Khalifeh, F.; Ghazeeri, G. The effect of bariatric surgery on inflammatory markers in women with polycystic ovarian syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2018, 12, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, D.F.; Boekholdt, S.M.; Arsenault, B.J.; Ahmadi-Abhari, S.; Wareham, N.J.; Stroes, E.S.G.; Khaw, K. C-Reactive Protein Identifies Low-Risk Metabolically Healthy Obese Persons: The European Prospective Investigation of Cancer–Norfolk Prospective Population Study. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, M.-X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Wright, N.C.; Saag, K.G.; Dawson-Hughes, B.; Khosla, S.; Siris, E.S. The impact of the new National Bone Health Alliance (NBHA) diagnostic criteria on the prevalence of osteoporosis in the USA. Osteoporos. Int. 2017, 28, 1225–1232. [Google Scholar] [CrossRef]
- Luhrs, A.R.; Davalos, G.; Lerebours, R.; Yoo, J.; Park, C.; Tabone, L.; Omotosho, P.; Torquati, A.; Portenier, D.; Guerron, A.D. Determining changes in bone metabolism after bariatric surgery in postmenopausal women. Surg. Endosc. 2019. [Google Scholar] [CrossRef]
- Casimiro, I.; Sam, S.; Brady, M.J. Endocrine implications of bariatric surgery: a review on the intersection between incretins, bone, and sex hormones. Physiol. Rep. 2019, 7. [Google Scholar] [CrossRef]
- Menegati, G.C.; de Oliveira, L.C.; Santos, A.L.A.; Cohen, L.; Mattos, F.; Mendonça, L.M.C.; Carneiro, J.R.I.; Farias, M.L.F.; Rosado, E.L. Nutritional Status, Body Composition, and Bone Health in Women After Bariatric Surgery at a University Hospital in Rio de Janeiro. Obes. Surg. 2016, 26, 1517–1524. [Google Scholar] [CrossRef]
- Goughnour, S.L.; Thurston, R.C.; Althouse, A.D.; Freese, K.E.; Edwards, R.P.; Hamad, G.G.; McCloskey, C.; Ramanathan, R.; Bovbjerg, D.H.; Linkov, F. Assessment of hot flushes and vaginal dryness among obese women undergoing bariatric surgery. Climacteric 2016, 19, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Rojas, P.; Basfi-fer, K.; Carrasco, F.; Inostroza, J.; Codoceo, J.; Valencia, A.; Papapietro, K.; Csendes, A.; Ruz, M. Micronutrient Deficiencies in Morbidly Obese Women Prior to Bariatric Surgery. Obes. Surg. 2016, 26, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.; Zheng, Y.; Huang, H.; Chen, L.; Cao, B. Effects of bariatric surgery on pelvic floor disorders in obese women: a meta-analysis. Arch. Gynecol. Obstet. 2017, 296, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaikh, G.K.; Ibrahim, L.; Al-Mandeel, H.; Alshaikh, R.; Syed, S.B. Association of obesity with pelvic floor disorders and its effect on the quality of life in women awaiting bariatric surgery. Kuwait Med. J. 2018, 50, 461–466. [Google Scholar]
- Kim, J.H.; Sun, H.Y.; Lee, H.Y.; Soh, M.J.; Park, S.; Kim, Y.J.; Song, Y.J. Improvement of voiding characteristics in morbidly obese women after bariatric surgery: A single-center study with a 1-year follow-up. Surg. Obes. Relat. Dis. 2017, 13, 836–841. [Google Scholar] [CrossRef]
- Knepfler, T.; Valero, E.; Triki, E.; Chilintseva, N.; Koensgen, S.; Rohr, S. Bariatric surgery improves female pelvic floor disorders. J. Visc. Surg. 2016, 153, 95–99. [Google Scholar] [CrossRef]
- Paka, C.; Hallock, J.; Trock, B.; Steele, K.; Wright, E.J. Urinary Incontinence and Pelvic Organ Prolapse Knowledge, Care-Seeking, and Embarrassment in Women Planning Bariatric Surgery: A Cross-sectional Study. Female Pelvic Med. Reconstr. Surg. 2019. Publish Ahead of Print. [Google Scholar] [CrossRef]
- Uruc, F.; Serkan, A.; Bekir, A.; Aytac, S.; Elif, U.; Özgür, H.Y.; Ahmet, Ü.; Caglar, Y. Effect of Bariatric Sleeve Gastrectomy Technique on Women’s Lower Urinary Tract Symptoms and Quality of Life: A Prospective Study. J. Urol. Surg. 2016, 3, 90–94. [Google Scholar] [CrossRef]
- Durigon, K.K.; La, V.R.; Marques, T.C.; de Souza Machado, C.; Langlois, F.C.; da Rosa Viana, P.; da Rosa Telles, L.H. Quality of Life and Urinary Incontinence Symptoms in Women Undergoing Bariatric Surgery: A Combined Case-Cohort Study. Female Pelvic Med. Reconstr. Surg. 2019. Publish Ahead of Print. [Google Scholar] [CrossRef]
- O’Boyle, C.J.; O’Sullivan, O.E.; Shabana, H.; Boyce, M.; O’Reilly, B.A. The Effect of Bariatric Surgery on Urinary Incontinence in Women. Obes. Surg. 2016, 26, 1471–1478. [Google Scholar] [CrossRef]
- Anglim, B.; O’Boyle, C.J.; O’Sullivan, O.E.; O’Reilly, B.A. The long-term effects of bariatric surgery on female urinary incontinence. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Afarideh, M.; Ghajar, A.; Nikdad, M.S.; Alibakhshi, A. Sex-Specific Aspects of Bariatric Surgery in Iran Are Far from Understood. J. Am. Coll. Surg. 2016, 223, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Leshem, A.; Shimonov, M.; Amir, H.; Gordon, D.; Groutz, A. Effects of Bariatric Surgery on Female Pelvic Floor Disorders. Urology 2017, 105, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Shimonov, M.; Groutz, A.; Schachter, P.; Gordon, D. Is bariatric surgery the answer to urinary incontinence in obese women? Neurourol. Urodyn. 2017, 36, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, I.; Tavakkoli, A.; Minassian, V. Pelvic Organ Prolapse and Urinary Incontinence in Women After Bariatric Surgery: 5-Year Follow-up. Female Pelvic Med. Reconstr. Surg. 2018, 24, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Kamangar, F.; Dores, G.M.; Anderson, W.F. Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World. J. Clin. Oncol. 2016. [Google Scholar] [CrossRef]
- Feigelson, H.; Caan, B.; Weinmann, S.; Leonard, A.; Powers, J.; Yenumula, P.; Arterburn, D.; Koebnick, C.; Altaye, M.; Schauer, D. Bariatric Surgery is Associated With Reduced Risk of Breast Cancer in Both Premenopausal and Postmenopausal Women. Ann. Surg. 2019. Publish Ahead of Print. [Google Scholar] [CrossRef]
- Modesitt, S.C.; Hallowell, P.T.; Slack-Davis, J.K.; Michalek, R.D.; Atkins, K.A.; Kelley, S.L.; Arapovic, S.; Shupnik, M.A.; Hoehn, K. Women at extreme risk for obesity-related carcinogenesis: Baseline endometrial pathology and impact of bariatric surgery on weight, metabolic profiles and quality of life. Gynecol. Oncol. 2015, 138, 238–245. [Google Scholar] [CrossRef]
- Benito, V.; López-Tomassetti, E.; Esparza, M.; Arencibia, O.; Andújar, M.; Prieto, M.; Lubrano, A. Bariatric Surgery: Does It Play a Role in Fertility-Preserving Treatment among Obese Young Women with Endometrial Cancer? J. Minim. Invasive Gynecol. 2015, 22, 906–909. [Google Scholar] [CrossRef]
- Needleman, B.J.; Noria, S.F. Metabolic and bariatric surgery offers benefits to women beyond weight loss. Gynecol. Oncol. 2015, 138, 221–222. [Google Scholar] [CrossRef]
- Upala, S.; Sanguankeo, A. Bariatric surgery and risk of postoperative endometrial cancer: a systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2015, 11, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Kitson, S.; Ryan, N.; MacKintosh, M.L.; Edmondson, R.; Duffy, J.M.; Crosbie, E.J. Interventions for weight reduction in obesity to improve survival in women with endometrial cancer. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Anveden, Å.; Taube, M.; Peltonen, M.; Jacobson, P.; Andersson-Assarsson, J.C.; Sjöholm, K.; Svensson, P.-A.; Carlsson, L.M.S. Long-Term Incidence of Female-Specific Cancer after Bariatric Surgery or Usual Care in the Swedish Obese Subjects Study. Gynecol. Oncol. 2017, 145, 224–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanguankeo, A.; Upala, S. Role of bariatric surgery in obese women with endometrial cancer risk. Am. J. Obstet. Gynecol. 2016, 214, 138–139. [Google Scholar] [CrossRef] [PubMed]
- Neff, R.; Havrilesky, L.J.; Chino, J.; O’Malley, D.M.; Cohn, D.E. Bariatric surgery as a means to decrease mortality in women with type I endometrial cancer—An intriguing option in a population at risk for dying of complications of metabolic syndrome. Gynecol. Oncol. 2015, 138, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, A.M.; Maurer, K.; Cooper, K.R.; Rose, P.G.; Schauer, P.R.; Michener, C.M. Weight loss outcomes following referral of obese women with endometrial cancer and complex atypical hyperplasia to a bariatric specialist. Gynecol. Oncol. 2016, 141, 106. [Google Scholar] [CrossRef]
- Fernandez-Montoli, M.-E.; Sabadell, J.; Martínez-García, J.M.; Perez, N.A.C. Fertility-sparing treatment for atypical endometrial hyperplasia and endometrial cancer. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Hoel, E. Is Bariatric Surgery Protective against Breast and Endometrial Cancers for Women? (Cover story). Bariatr. Times 2015, 12, 10–12. [Google Scholar]
- Ganesan, K.; Choy, B.J.K. Comment on “Bariatric Surgery is Associated with Reduced Risk of Breast Cancer in Both Premenopausal and Postmenopausal Women”. Ann. Surg. 2019, 1. [Google Scholar] [CrossRef]
- Feigelson, H.; Caan, B.; Weinmann, S.; Leonard, A.C.; Powers, J.D.; Yenumula, P.R.; Arterburn, D.E.; Koebnick, C.; Altaye, M.; Schauer, D.P. Response to: Comment on “Bariatric Surgery is Associated with Reduced Risk of Breast Cancer in Both Premenopausal and Postmenopausal Women”. Ann. Surg. 2019, 1. [Google Scholar] [CrossRef]
- Grayson, B.E.; Gutierrez-Aguilar, R.; Sorrell, J.E.; Matter, E.K.; Adams, M.R.; Howles, P.; Karns, R.; Seeley, R.J.; Sandoval, D.A. Bariatric surgery emphasizes biological sex differences in rodent hepatic lipid handling. Biol. Sex Differ. 2017, 8, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy-Dalby, A.; Adam, S.; Ammori, B.J.; Syed, A.A. Weight loss and metabolic outcomes of bariatric surgery in men versus women—A matched comparative observational cohort study. Eur. J. Intern. Med. 2014, 25, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Kochkodan, J.; Telem, D.A.; Ghaferi, A.A. Physiologic and psychological gender differences in bariatric surgery. Surg. Endosc. 2018, 32, 1382–1388. [Google Scholar] [CrossRef] [PubMed]
- Stroh, C.; Weiner, R.; Wolff, S.; Knoll, C.; de Zwaan, M.; Manger, T.; Adipositas, K. Kommentar zu genderspezifischen Aspekten in der Adipositas- und metabolischen Chirurgie–Daten der Qualitätssicherungsstudie für operative Therapie der Adipositas. Zentralblatt Für Chir. - Z. Für Allg. Visz. Thorax- Gefäßchirurgie 2015, 140, 285–293. [Google Scholar] [CrossRef]
- Wee, C.C.; Davis, R.B.; Jones, D.B.; Apovian, C.A.; Chiodi, S.; Huskey, K.W.; Hamel, M.B. Sex, Race, and the Quality of Life Factors Most Important to Patients’ Well-Being Among Those Seeking Bariatric Surgery. Obes. Surg. 2016, 26, 1308–1316. [Google Scholar] [CrossRef] [Green Version]
- Young, M.T.; Phelan, M.J.; Nguyen, N.T. A Decade Analysis of Trends and Outcomes of Male vs Female Patients Who Underwent Bariatric Surgery. J. Am. Coll. Surg. 2016, 222, 226–231. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L.; Liu, M.; Liu, C. Effect of Bariatric Surgery on Urinary Incontinence in Obese Women: A Meta-analysis and Systematic Review. Female Pelvic Med. Reconstr. Surg. 2018. Publish Ahead of Print. [Google Scholar] [CrossRef]
- Ahmed, H.O.; Arif, S.H.; Abdulhakim, S.A.; Kakarash, A.; Ali Omer, M.A.; Nuri, A.M.; Omer, H.H.; Jalal, H.K.; Omer, S.H.; Muhammad, N.A. Gender difference in requesting abdominoplasty, after bariatric surgery: Based on five years of experience in two centers in Sulaimani Governorate, Kurdistan Region/Iraq. Int. J. Surg. 2018, 56, 155–160. [Google Scholar] [CrossRef]
- Biörserud, C.; Shams, K.; Elander, A.; Olsén, M.F. Self-image after bariatric surgery and its relationship to gender, excess skin and health-related quality of life. J. Plast. Surg. Hand Surg. 2018, 52, 288–293. [Google Scholar] [CrossRef]
- Horvath, C.M.; Jossen, J.; Kröll, D.; Nett, P.C.; Baty, F.; Brill, A.-K.; Ott, S.R. Prevalence and Prediction of Obstructive Sleep Apnea Prior to Bariatric Surgery—Gender-Specific Performance of Four Sleep Questionnaires. Obes. Surg. 2018, 28, 2720–2726. [Google Scholar] [CrossRef]
| Search Engine | Databases |
|---|---|
| EBSCO Publishing |
|
| Cochrane Library |
|
| PubMed |
|
| Scopus |
|
| Dates of Search | Database | Publication Years | Further Search Settings | Search Terms | Hits |
|---|---|---|---|---|---|
| 30/09/2019–05/10/2019 | MEDLINE | Last 5 years | In title | bariatric AND women | 113 |
| bariatric AND female | 21 | ||||
| bariatric AND sex | 11 | ||||
| bariatric AND gender | 9 | ||||
| 07/10/2019 | Academic Search Premier | Since 10/2014 | In title/subjects | bariatric AND women OR female OR woman OR females | 66 |
| bariatric AND sex OR gender | 10 | ||||
| 07/10/2019 | PsycInfo | Since 10/2014 | In title/subjects | bariatric AND women OR female OR woman OR females | 18 |
| bariatric AND sex OR gender | 2 | ||||
| 08/10/2019 | Cochrane | – | In title/subjects | bariatric | 13 |
| 08/10/2019 | Scopus | Since 2015 | In title | bariatric AND women OR female OR sex OR gender | 136 |
| Sum | 399 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jäger, P.; Wolicki, A.; Spohnholz, J.; Senkal, M. Review: Sex-Specific Aspects in the Bariatric Treatment of Severely Obese Women. Int. J. Environ. Res. Public Health 2020, 17, 2734. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17082734
Jäger P, Wolicki A, Spohnholz J, Senkal M. Review: Sex-Specific Aspects in the Bariatric Treatment of Severely Obese Women. International Journal of Environmental Research and Public Health. 2020; 17(8):2734. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17082734
Chicago/Turabian StyleJäger, Pia, Annina Wolicki, Johannes Spohnholz, and Metin Senkal. 2020. "Review: Sex-Specific Aspects in the Bariatric Treatment of Severely Obese Women" International Journal of Environmental Research and Public Health 17, no. 8: 2734. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17082734





