Exposure to Traffic-Related Particulate Matter 2.5 Triggers Th2-Dominant Ocular Immune Response in a Murine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Collection and Recovery of Traffic-Related PM2.5
2.3. RNA Isolation and Real-Time Polymerase Chain Reaction
2.4. CD11b-Positive Cellular Infiltration by Immunohistochemical Staining
2.5. Periodic Acid Schiff (PAS) Staining for Conjunctival Goblet Cells
2.6. Single-Cell Isolation from Draining Lymph Nodes and Conjunctivae For Flow Cytometric Analysis
2.7. In Vitro Stimulation of T Cells
2.8. Quantitation of IgE in Serum
2.9. TdT-Mediated dUTP Nick End Labeling (TUNEL) Assay
2.10. Statistical Analysis
3. Results
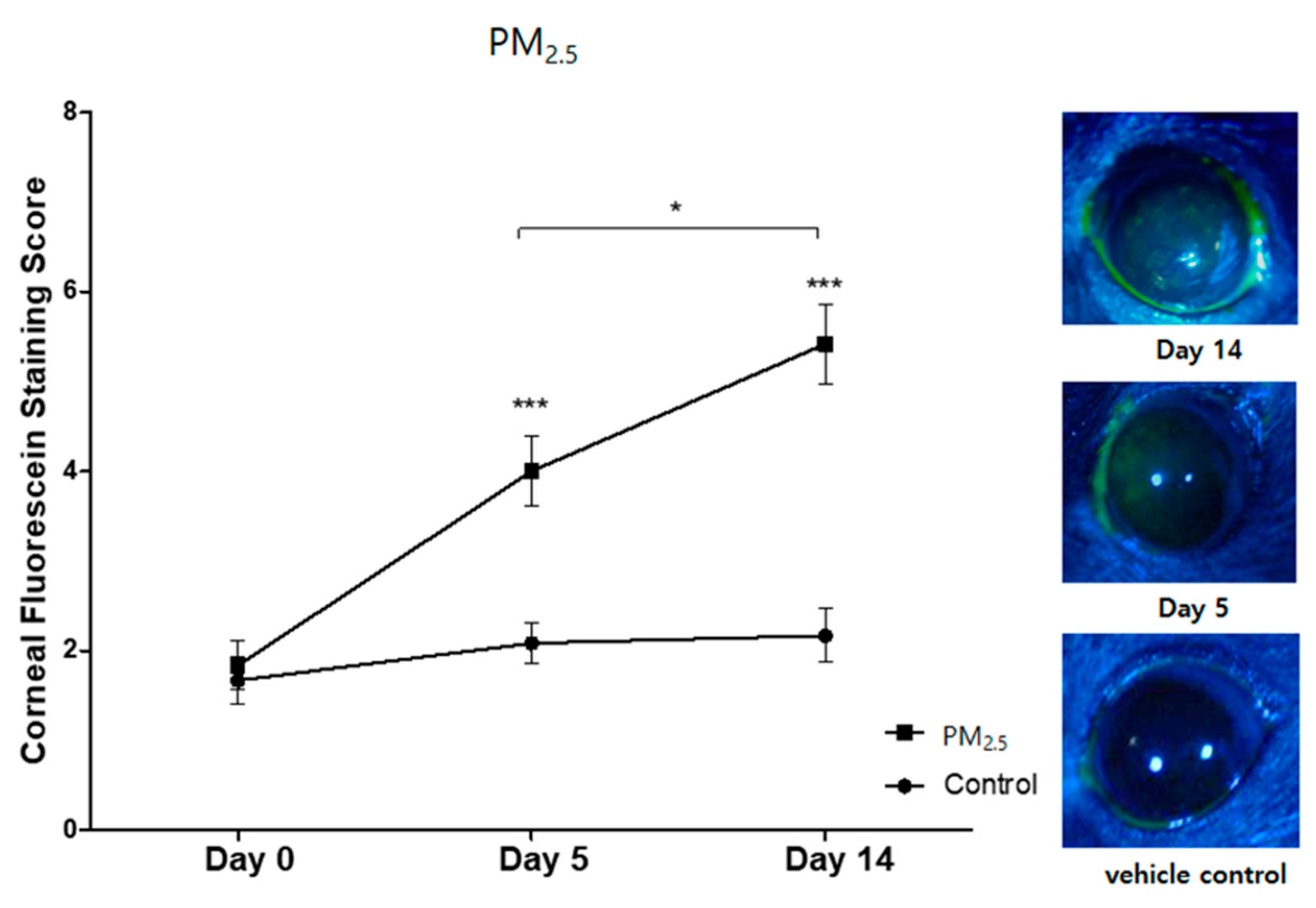
3.1. Clinical Signs of Ocular Surface Injury
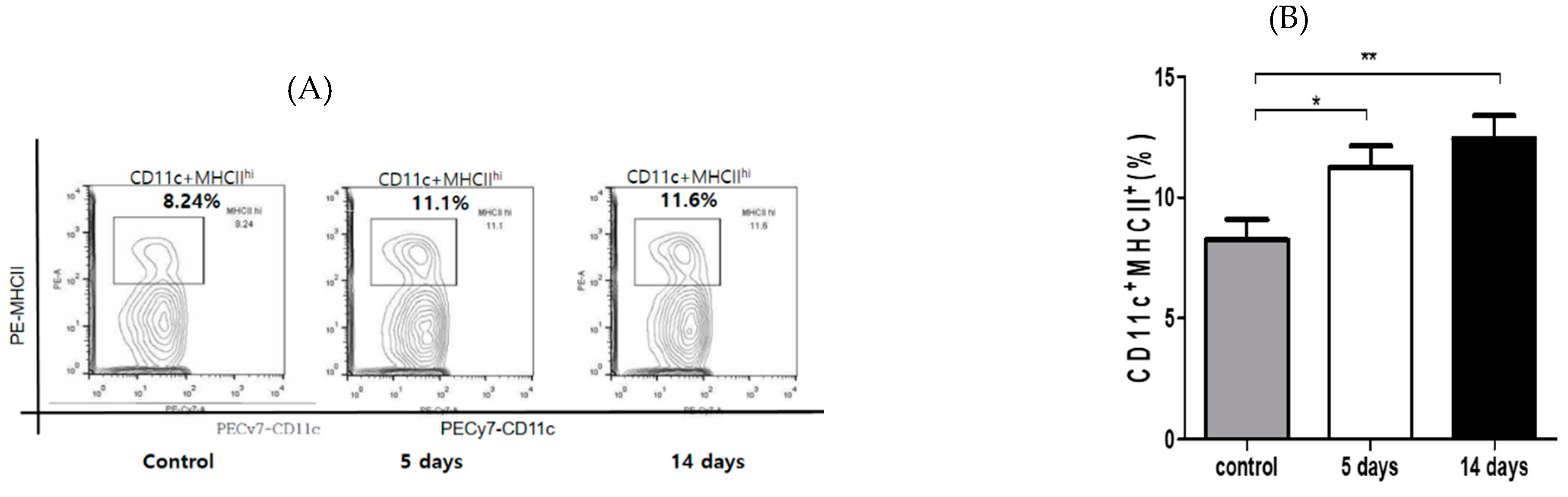
3.2. Inflammatory Cells’ Infiltration to Cornea and Conjunctiva
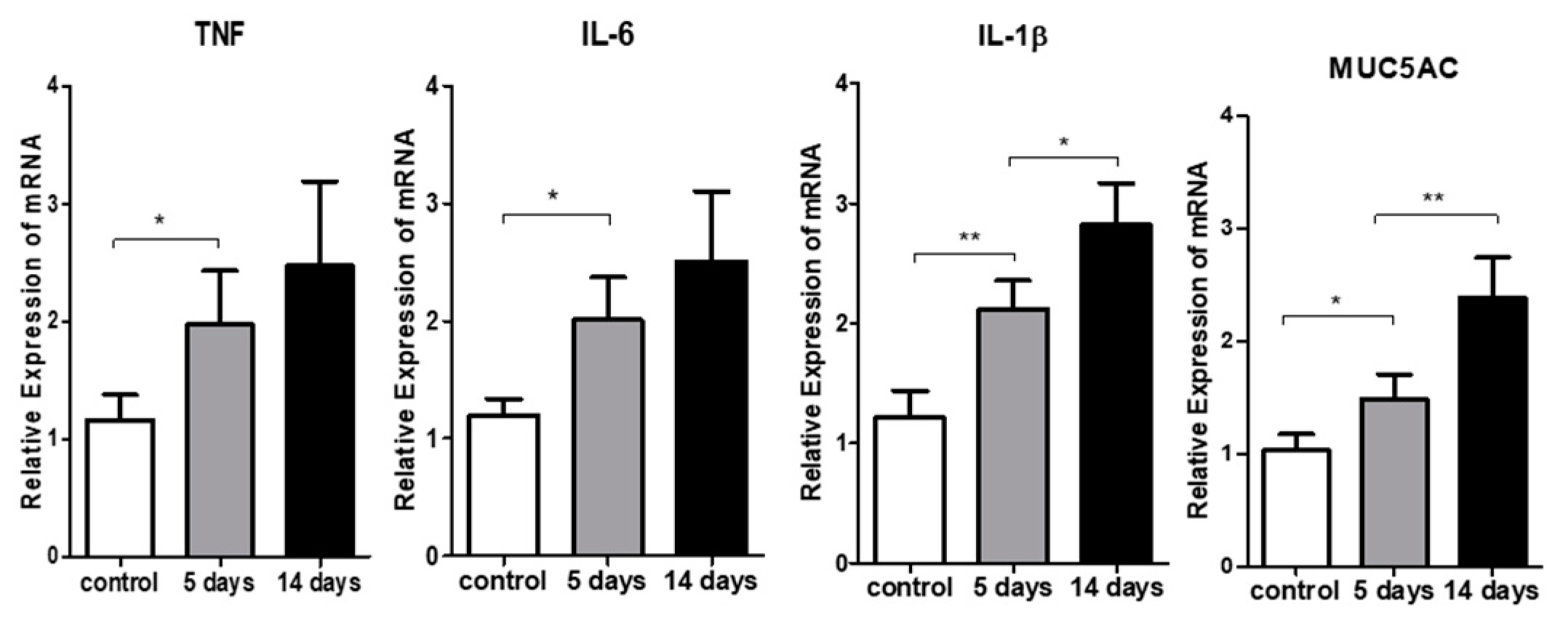
3.3. Inflammatory Cytokine Expression in the Ocular Surface
3.4. Effect of PM2.5 on Apoptosis of the Corneal Epithelial Cells
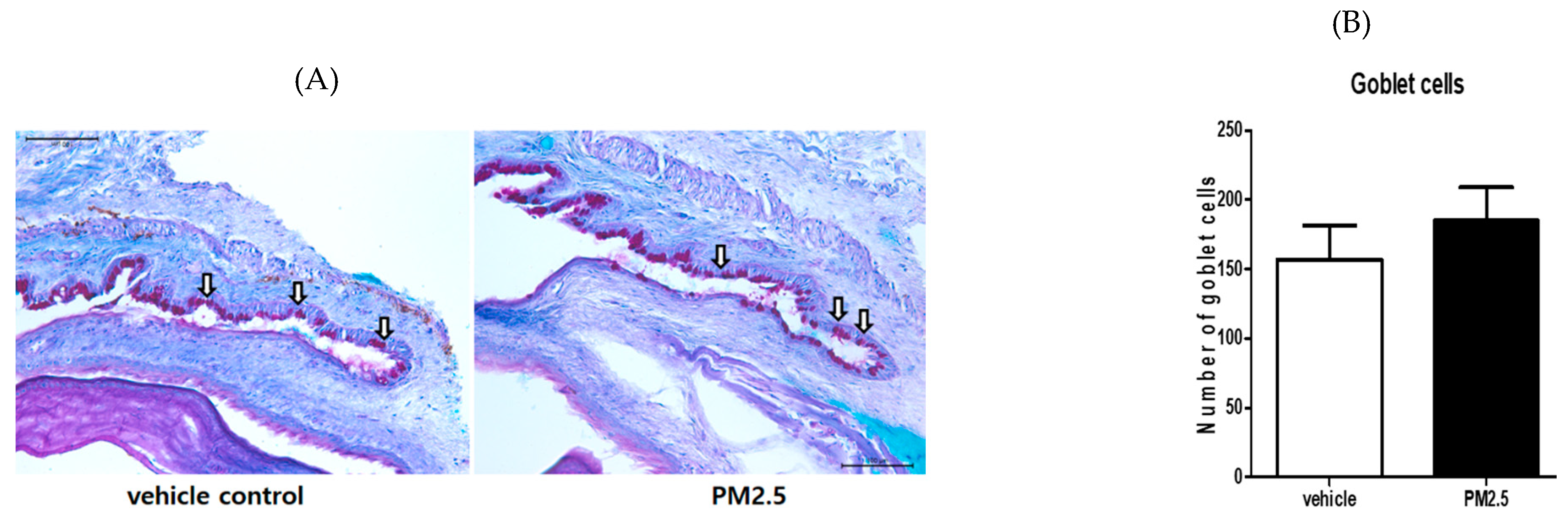
3.5. PM2.5 Did Not Decrease the Number of Goblet Cells in the Conjunctiva
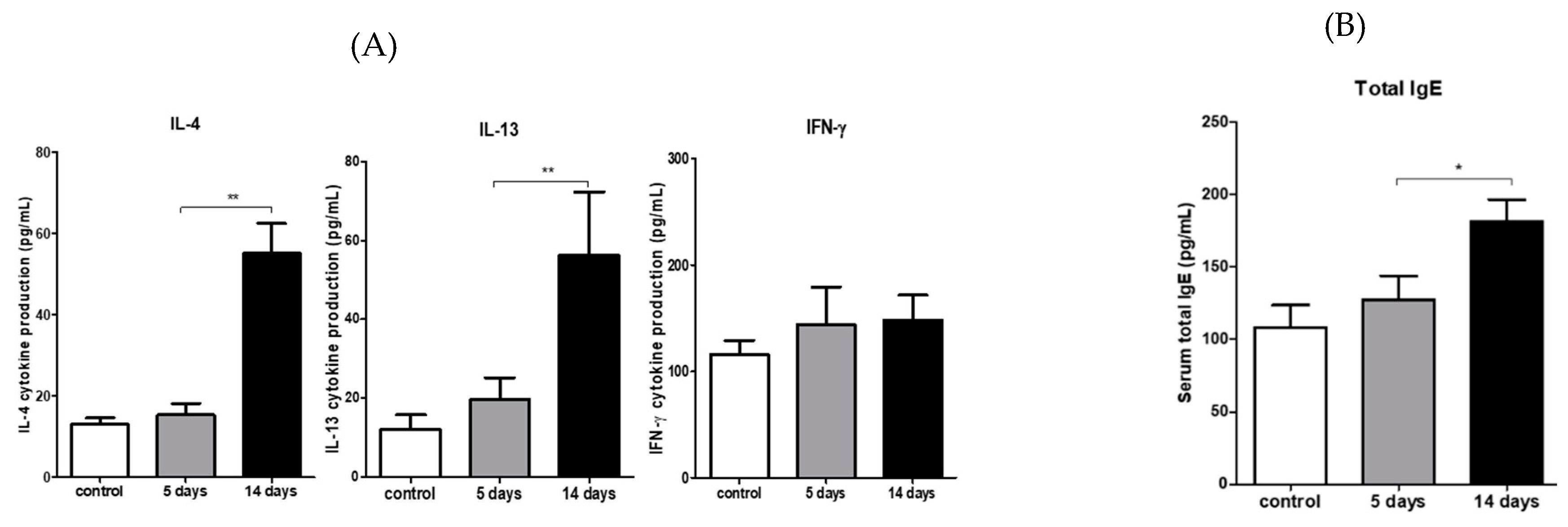
3.6. PM2.5 Induced Type 2 CD4 T-Cell Immune Responses in the Draining LNs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A.; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018, 115, 9592–9597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achilleos, S.; Kioumourtzoglou, M.-A.; Wu, C.-D.; Schwartz, J.D.; Koutrakis, P.; Papatheodorou, S.I. Acute effects of fine particulate matter constituents on mortality: A systematic review and meta-regression analysis. Environ. Int. 2017, 109, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Boogaard, H.; Walker, K.; Cohen, A.J. Air pollution: The emergence of a major global health risk factor. Int. Health 2019, 11, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Li, J.; Yang, Q.; Wu, A.; Qu, D.-Y.; Wang, Y.; Ye, L.; Bao, J.; Shao, Y. Air pollutant particulate matter 2.5 induces dry eye syndrome in mice. Sci. Rep. 2018, 8, 17828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srimuruganandam, B.; Nagendra, S.M.S. Source characterization of PM10 and PM2.5 mass using a chemical mass balance model at urban roadside. Sci. Total. Environ. 2012, 433, 8–19. [Google Scholar] [CrossRef]
- Huang, K.L.; Liu, S.Y.; Chou, C.C.; Lee, Y.H.; Cheng, T.J. The effect of size-segregated ambient particulate matter on Th1/Th2-like immune responses in mice. PLoS ONE 2017, 12, e0173158. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, J.; Neas, L.M. Fine particles are more strongly associated than coarse particles with acute respiratory health effects in schoolchildren. Epidemiology 2000, 11, 6–10. [Google Scholar] [CrossRef]
- Wang, J.; Fonn, D.; Simpson, T.L.; Jones, L.W. Precorneal and pre- and postlens tear film thickness measured indirectly with optical coherence tomography. Investig. Opthalmol. Vis. Sci. 2003, 44, 2524–2528. [Google Scholar] [CrossRef] [Green Version]
- Novaes, P.; Saldiva, P.H.; Matsuda, M.; Macchione, M.; Rangel, M.P.; Kara-José, N.; Berra, A. The effects of chronic exposure to traffic derived air pollution on the ocular surface. Environ. Res. 2010, 110, 372–374. [Google Scholar] [CrossRef]
- Malerbi, F.K.; Martins, L.C.; Saldiva, P.H.; Braga, A. Ambient levels of air pollution induce clinical worsening of blepharitis. Environ. Res. 2012, 112, 199–203. [Google Scholar] [CrossRef]
- Galor, A.; Kumar, N.; Feuer, W.; Lee, D.J. Environmental factors affect the risk of dry eye syndrome in a United States veteran population. Ophthalmolgy 2014, 121, 972–973.e1. [Google Scholar] [CrossRef] [PubMed]
- Versura, P.; Profazio, V.; Cellini, M.; Torreggiani, A.; Caramazza, R. Eye discomfort and air pollution. Ophthalmolgy 1999, 213, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Vitar, R.M.L.; Tau, J.; Reides, C.G.; Berra, A.; Ferreira, S.; Llesuy, S. Evaluation of oxidative stress markers in human conjunctival epithelial cells exposed to Diesel Exhaust Particles (DEP). Investig. Opthalmol. Vis. Sci. 2015, 56, 7058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tau, J.; Novaes, P.; Matsuda, M.; Tasat, D.; Saldiva, P.H.; Berra, A. Diesel exhaust particles selectively induce both proinflammatory cytokines and mucin production in cornea and conjunctiva human cell lines. Investig. Opthalmol. Vis. Sci. 2013, 54, 4759–4766. [Google Scholar] [CrossRef]
- Eom, Y.; Song, J.S.; Lee, H.K.; Kang, B.; Kim, H.C.; Lee, H.K.; Kim, H.M. The effect of ambient titanium dioxide microparticle exposure to the ocular surface on the expression of inflammatory cytokines in the eye and cervical lymph nodes. Investig. Opthalmol. Vis. Sci. 2016, 57, 6580–6590. [Google Scholar] [CrossRef] [Green Version]
- Federico, K.; Claudio, A.B.; Carlos, C.D.; Annette, P.U.; Sophie, B.; Heather, A.R.; Markus, A. Contributions to cities’ ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmos. Environ. 2015, 120, 475–483. [Google Scholar]
- Torricelli, A.; Novaes, P.; Matsuda, M.; Braga, A.; Saldiva, P.H.; Alves, M.R.; Monteiro, M.L.R. Correlation between signs and symptoms of ocular surface dysfunction and tear osmolarity with ambient levels of air pollution in a large metropolitan area. Cornea 2013, 32, e11–e15. [Google Scholar] [CrossRef]
- Wolkoff, P. Ocular discomfort by environmental and personal risk factors altering the precorneal tear film. Toxicol. Lett. 2010, 199, 203–212. [Google Scholar] [CrossRef]
- Lemp, A. Report of the national eye institute/industry workshop on clinical trials in dry eyes. CLAO J. 1995, 21, 221–232. [Google Scholar]
- Yoon, S.; Han, S.; Jeon, K.-J.; Kwon, S. Effects of collected road dusts on cell viability, inflammatory response, and oxidative stress in cultured human corneal epithelial cells. Toxicol. Lett. 2017, 284, 152–160. [Google Scholar] [CrossRef]
- Han, J.Y.; Kang, B.; Eom, Y.; Kim, H.M.; Song, J.S. Comparing the effects of particulate matter on the ocular surfaces of normal eyes and a dry eye rat model. Cornea 2017, 36, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Chauhan, S.K.; Zhang, Q.; Dana, R. Amelioration of murine dry eye disease by topical antagonist to chemokine receptor 2. Arch. Ophthalmol. 2009, 127, 882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Hattori, T.; Park, E.Y.; Stevenson, W.; Chauhan, S.K.; Dana, R. Expression of toll-like receptor 4 contributes to corneal inflammation in experimental dry eye disease. Investig. Opthalmol. Vis. Sci. 2012, 53, 5632–5640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlereth, S.; Lee, H.S.; Khandelwal, P.; Saban, D.R. Blocking CCR7 at the ocular surface impairs the pathogenic contribution of dendritic cells in allergic conjunctivitis. Am. J. Pathol. 2012, 180, 2351–2360. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Chauhan, S.K.; Okanobo, A.; Nallasamy, N.; Dana, R. Therapeutic efficacy of topical epigallocatechin gallate in murine dry eye. Cornea 2011, 30, 1465–1472. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.K.; Gupta, S.C.; Agarwal, R.; Sushma, S.; Agrawal, S.S.; Saxena, R. A multicentric case-control study on the impact of air pollution on eyes in a metropolitan city of India. Indian J. Occup. Environ. Med. 2007, 11, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Yamaguchi, T. Inflammatory response in dry eye. Investig. Opthalmol. Vis. Sci. 2018, 59, DES192–DES199. [Google Scholar] [CrossRef] [Green Version]
- Pflugfelder, S.C.; Corrales, R.M.; De Paiva, C.S. T helper cytokines in dry eye disease. Exp. Eye Res. 2013, 117, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.K.; El Annan, J.; Ecoiffier, T.; Goyal, S.; Zhang, Q.; Saban, D.R.; Dana, R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression1. J. Immunol. 2009, 182, 1247–1252. [Google Scholar] [CrossRef] [Green Version]
- El Annan, J.; Chauhan, S.K.; Ecoiffier, T.; Zhang, Q.; Saban, D.R.; Dana, R. Characterization of effector T cells in dry eye disease. Investig. Opthalmol. Vis. Sci. 2009, 50, 3802–3807. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Chen, Y.; Chauhan, S.; Dana, R. Ocular surface immunity: Homeostatic mechanisms and their disruption in dry eye disease. Prog. Retin. Eye Res. 2012, 31, 271–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Paiva, C.S.; Villarreal, A.L.; Corrales, R.M.; Rahman, H.T.; Chang, V.Y.; Farley, W.J.; Stern, M.E.; Niederkorn, J.Y.; Li, D.-Q.; Pflugfelder, S.C. Dry Eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-γ. Investig. Opthalmol. Vis. Sci. 2007, 48, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- De Paiva, C.S.; Chotikavanich, S.; Pangelinan, S.B.; Pitcher, J.D.; Fang, B.; Zheng, X.; Ma, P.; Farley, W.J.; Siemasko, K.F.; Niederkorn, J.Y.; et al. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009, 2, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Irkeç, M.; Bozkurt, B. Molecular immunology of allergic conjunctivitis. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 534–539. [Google Scholar] [CrossRef]
- Perez, L.; Declercq, C.; Iñiguez, C.; Aguilera, I.; Badaloni, C.; Ballester, F.; Bouland, C.; Chanel, O.; Cirarda, F.B.; Forastiere, F.; et al. Chronic burden of near-roadway traffic pollution in 10 European cities (APHEKOM network). Eur. Respir. J. 2013, 42, 594–605. [Google Scholar] [CrossRef] [Green Version]
- Wong, G.W.; Chow, C.M. Childhood asthma epidemiology: Insights from comparative studies of rural and urban populations. Pediatr. Pulmonol. 2007, 43, 107–116. [Google Scholar] [CrossRef]
- Robinson, C.L.; Baumann, L.M.; Romero, K.; Combe, J.M.; Gómez, A.; Gilman, R.H.; Cabrera, L.; Gonzalvez, G.; Hansel, N.N.; Wise, R.A.; et al. Effect of urbanisation on asthma, allergy and airways inflammation in a developing country setting. Thorax 2011, 66, 1051–1057. [Google Scholar] [CrossRef] [Green Version]
- Mimura, T.; Ichinose, T.; Yamagami, S.; Fujishima, H.; Kamei, Y.; Goto, M.; Takada, S.; Matsubara, M. Airborne particulate matter (PM2.5) and the prevalence of allergic conjunctivitis in Japan. Sci. Total Environ. 2014, 487, 493–499. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [Green Version]
- Brandt, E.; Kovacic, M.B.; Lee, G.-B.; Gibson, A.M.; Acciani, T.H.; Le Cras, T.D.; Ryan, P.H.; Budelsky, A.L.; Hershey, G.K.K. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J. Allergy Clin. Immunol. 2013, 132, 1194–1204.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, S.; Liu, K.; Smith, C.; Bonito, A.J.; Steinman, R.M. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J. Exp. Med. 2004, 21, 1607–1618. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, A.R.; Bein, K.J.; Smiley-Jewell, S.; Pinkerton, K.E. Fine particulate matter (PM2.5) enhances allergic sensitization in BALB/cmice. J. Toxicol. Environ. Health Part A 2017, 80, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, M.; Barajas, B.; Sioutas, C.; Williams, M.A.; Nel, A.E. Nrf2 deficiency in dendritic cells enhances the adjuvant effect of ambient ultrafine particles on allergic sensitization. J. Innate Immun. 2013, 5, 543–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, M.A.; Rangasamy, T.; Bauer, S.M.; Killedar, S.; Karp, M.; Kensler, T.W.; Yamamoto, M.; Breysse, P.; Biswal, S.; Georas, S.N. Disruption of the transcription factor Nrf2 promotes pro-oxidative dendritic cells that stimulate Th2-like immunoresponsiveness upon activation by ambient particulate matter. J. Immunol. 2008, 181, 4545–4559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, C.-M.; Wang, C.-H.; Lee, M.-J.; He, J.-R.; Huang, H.-Y.; Chao, M.-W.; Chung, K.F.; Kuo, H.-P. Aryl hydrocarbon receptor activation by diesel exhaust particles mediates epithelium-derived cytokines expression in severe allergic asthma. Allergy 2018, 73, 2192–2204. [Google Scholar] [CrossRef] [PubMed]
- Volpe, E.A.; Henriksson, J.T.; Wang, C.; Barbosa, F.L.; Zaheer, M.; Zhang, X.; Pflugfelder, S.C.; De Paiva, C.S. Interferon-gamma deficiency protects against aging-related goblet cell loss. Oncotarget 2016, 7, 64605–64614. [Google Scholar] [CrossRef] [Green Version]
- Novaes, P.; Saldiva, P.H.D.N.; Kara-José, N.; Macchione, M.; Matsuda, M.; Racca, L.; Berra, A. Ambient levels of air pollution induce goblet-cell hyperplasia in human conjunctival epithelium. Environ. Health Perspect. 2007, 115, 1753–1756. [Google Scholar] [CrossRef] [Green Version]
- Dartt, D.A. Control of mucin production by ocular surface epithelial cells. Exp. Eye Res. 2004, 78, 173–185. [Google Scholar] [CrossRef]
- Kondo, M.; Tamaoki, J.; Takeyama, K.; Nakata, J.; Nagai, A. Interleukin-13 induces goblet cell differentiation in primary cell culture from guinea pig tracheal epithelium. Am. J. Respir. Cell Mol. Boil. 2002, 27, 536–541. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.S.; Han, S.; Seo, J.-W.; Jeon, K.-J. Exposure to Traffic-Related Particulate Matter 2.5 Triggers Th2-Dominant Ocular Immune Response in a Murine Model. Int. J. Environ. Res. Public Health 2020, 17, 2965. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17082965
Lee HS, Han S, Seo J-W, Jeon K-J. Exposure to Traffic-Related Particulate Matter 2.5 Triggers Th2-Dominant Ocular Immune Response in a Murine Model. International Journal of Environmental Research and Public Health. 2020; 17(8):2965. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17082965
Chicago/Turabian StyleLee, Hyun Soo, Sehyun Han, Jeong-Won Seo, and Ki-Joon Jeon. 2020. "Exposure to Traffic-Related Particulate Matter 2.5 Triggers Th2-Dominant Ocular Immune Response in a Murine Model" International Journal of Environmental Research and Public Health 17, no. 8: 2965. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph17082965




