The Risk of Gastrointestinal Bleeding between Non-Vitamin K Antagonist Oral Anticoagulants and Vitamin K Antagonists in the Asian Atrial Fibrillation Patients: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Inclusion Criteria
2.2. Data Extraction and Quality Assessment
2.3. Data Synthesis and Analysis
2.4. GRADE System, Meta-Regressions, and Sensitivity Analyses
3. Results
3.1. Study Search and Research Evaluation
3.2. Risk of Bias in Enrolled Articles
3.3. Gastrointestinal Bleeding Comparison and Possible Moderators
3.4. GRADE for Overall
3.5. GRADE for RCT Subgroup
3.6. GRADE for Retrospective Studies Subgroup
3.7. GRADE for the Subgroups of Different NOACs
3.8. Meta-Regressions and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Svennberg, E.; Engdahl, J.; Al-Khalili, F.; Friberg, L.; Frykman, V.; Rosenqvist, M. Mass screening for untreated atrial fibrillation: The STROKESTOP study. Circulation 2015, 131, 2176–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladstone, D.J.; Bui, E.; Fang, J.; Laupacis, A.; Lindsay, M.P.; Tu, J.V.; Silver, F.L.; Kapral, M.K. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke 2009, 40, 235–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henninger, N.; Goddeau, R.P.; Karmarkar, A.; Helenius, J.; McManus, D.D. Atrial fibrillation is associated with a worse 90-day outcome than other cardioembolic stroke subtypes. Stroke 2016, 47, 1486–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Tong, X.; George, M.G. Atrial fibrillation associated costs for stroke hospitalizations of medicare beneficiaries in the stroke belt of the United States. J. Atr. Fibrillation 2013, 5, 7–11. [Google Scholar] [PubMed]
- Chiang, C.E.; Wang, K.L.; Lip, G.Y. Stroke prevention in atrial fibrillation: An Asian perspective. Thromb. Haemost. 2014, 111, 789–797. [Google Scholar] [PubMed] [Green Version]
- Ageno, W.; Gallus, A.S.; Wittkowsky, A.; Crowther, M.; Hylek, E.M.; Palareti, G. Oral anticoagulant therapy: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest 2012, 141, e44S–e88S. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Cairns, J.A.; Mitchell, L.B.; Macle, L.; Stiell, I.G.; Gladstone, D.; McMurtry, M.S.; Connolly, S.; Cox, J.L.; Dorian, P.; et al. Focused update of the Canadian cardiovascular society guidelines for the management of atrial fibrillation. Can. J. Cardiol. 2014, 30, 1114–1130. [Google Scholar] [CrossRef]
- Heidbuchel, H.; Verhamme, P.; Alings, M.; Antz, M.; Hacke, W.; Oldgren, J.; Sinnaeve, P.; Camm, A.J.; Kirchhof, P. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: Executive summary. Eur. Heart J. 2013, 34, 2094–2106. [Google Scholar] [CrossRef] [Green Version]
- Boland, M.; Murphy, M.; Murphy, M.; McDermott, E. Acute-onset severe gastrointestinal tract hemorrhage in a postoperative patient taking rivaroxaban after total hip arthroplasty: A case report. J. Med. Case Rep. 2012, 6, 129. [Google Scholar] [CrossRef] [Green Version]
- Feinberg, J.; Grabowitz, L.; Rotman-Pikielny, P.; Berla, M.; Levy, Y. Dabigatran etexilate linked to fatal gastrointestinal hemorrhage. Isr. Med. Assoc. J. 2014, 16, 388–389. [Google Scholar]
- Holster, I.L.; Valkhoff, V.E.; Kuipers, E.J.; Tjwa, E.T.T.L. New oral anticoagulants increase risk for gastrointestinal bleeding: A systematic review and meta-analysis. Gastroenterology 2013, 145, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, F.; Jia, B.; Huang, P.; Lynn, H.S.; Zhang, W. Safety of the direct-acting anticoagulants in patients with atrial fibrillation: A meta-analysis. Thromb. Res. 2015, 135, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Lip, G.Y.H.; Lin, S.J.; Chiang, C.E. Non-vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: Meta-analysis. Stroke 2015, 46, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. Ed.) 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin. Res. Ed.) 2016, 355, i4919. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ (Clin. Res. Ed.) 2011, 343, d4002. [Google Scholar] [CrossRef] [Green Version]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE handbook: Introduction to GRADE handbook. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 10 January 2020).
- Ohshima, A.; Koyama, T.; Ogawa, A.; Zamami, Y.; Tanaka, H.Y.; Kitamura, Y.; Sendo, T.; Hinotsu, S.; Miller, M.W.; Kano, M.R. Oral anticoagulants usage in Japanese patients aged 18-74 years with non-valvular atrial fibrillation: A retrospective analysis based on insurance claims data. Fam. Pract. 2019, 36, 685–692. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.S.; Jang, E.; Yu, H.T.; Kim, T.H.; Uhm, J.S.; Kim, J.Y.; Sung, J.H.; Pak, H.N.; Lee, M.H.; et al. The optimal drug adherence to maximize the efficacy and safety of non-vitamin K antagonist oral anticoagulant in real-world atrial fibrillation patients. Europace 2019, 22, euz273. [Google Scholar] [CrossRef]
- Lin, H.M.D.; Lai, C.L.; Dong, Y.H.; Tu, Y.K.; Chan, K.A.; Suissa, S. Re-evaluating safety and effectiveness of dabigatran versus Warfarin in a nationwide data environment: A prevalent new-user design study. Drugs Real World Outcomes 2019, 6, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.R.; Choi, E.K.; Han, K.D.; Jung, J.H.; Oh, S.; Lip, G.Y.H. Optimal rivaroxaban dose in Asian patients with atrial fibrillation and normal or mildly impaired renal function. Stroke 2019, 50, 1140–1148. [Google Scholar] [CrossRef]
- Kim, H.M.; Choi, E.K.; Park, C.S.; Cha, M.J.; Lee, S.Y.; Kwon, J.M.; Oh, S. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in octogenarian patients with non-valvular atrial fibrillation. PLoS ONE 2019, 14, e0211766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, H.K.; Lee, K.H.; Park, H.W.; Yoon, N.S.; Kim, M.C.; Lee, N.; Kim, J.S.; Ahn, Y.; Jeong, M.H.; Park, J.C.; et al. Real world comparison of rivaroxaban and warfarin in Korean patients with atrial fibrillation: Propensity matching cohort analysis. Chonnam. Med. J. 2019, 55, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.S.; Yun, J.E.; Park, J.J.; Kim, Y.J.; Lee, J.; Kim, H.; Park, D.-W.; Nam, G.-B. Outcomes after use of standard—And low-dose non-vitamin K oral anticoagulants in Asian patients with atrial fibrillation. Stroke 2018, 50, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Choi, E.K.; Han, K.D.; Jung, J.H.; Oh, S.; Lip, G.Y.H. Edoxaban in Asian patients with atrial fibrillation: Effectiveness and safety. J. Am. Coll. Cardiol. 2018, 72, 838–853. [Google Scholar] [CrossRef]
- Lai, C.L.; Chen, H.M.; Liao, M.T.; Lin, T.T. Dabigatran, rivaroxaban, and warfarin in the oldest adults with atrial fibrillation in Taiwan. J. Am. Geriatr. Soc. 2018, 66, 1567–1574. [Google Scholar] [CrossRef]
- Lee, H.F.; Chan, Y.H.; Tu, H.T.; Kuo, C.T.; Yeh, Y.H.; Chang, S.H.; Wu, L.S.; See, L.C. The effectiveness and safety of low-dose rivaroxaban in Asians with non-valvular atrial fibrillation. Int. J. Cardiol. 2018, 261, 78–83. [Google Scholar] [CrossRef]
- Chan, Y.H.; See, L.C.; Tu, H.T.; Yeh, Y.H.; Chang, S.H.; Wu, L.S.; Lee, H.F.; Wang, C.L.; Kuo, C.F.; Kuo, C.T. Efficacy and safety of apixaban, dabigatran, rivaroxaban, and warfarin in Asians with nonvalvular atrial fibrillation. J. Am. Heart Assoc. 2018, 7, e008150. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.Y.; Lin, S.Y.; Cheng, S.H.; Wang, C.C. Effectiveness and safety of different Rivaroxaban dosage regimens in patients with non-valvular atrial fibrillation: A nationwide, population-based cohort study. Sci. Rep. 2018, 8, 3451. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Park, H.W.; Lee, N.; Hyun, D.Y.; Won, J.; Oh, S.S.; Park, H.J.; Kim, Y.; Cho, J.Y.; Kim, M.C.; et al. Optimal dose of dabigatran for the prevention of thromboembolism with minimal bleeding risk in Korean patients with atrial fibrillation. Europace 2017, 19, iv1–iv9. [Google Scholar] [CrossRef]
- Cha, M.J.; Choi, E.K.; Han, K.D.; Lee, S.R.; Lim, W.H.; Oh, S.; Lip, G.Y.H. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants in Asian patients with atrial fibrillation. Stroke 2017, 48, 3040–3048. [Google Scholar] [CrossRef]
- Ho, J.C.S.; Chang, A.M.; Yan, B.P.; Yu, C.M.; Lam, Y.Y.; Lee, V.W.Y. Dabigatran compared with warfarin for stroke prevention with atrial fibrillation: Experience in Hong Kong. Clin. Cardiol. 2012, 35, E40–E45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronow, W.S.; Shamliyan, T.A. Comparative effectiveness and safety of rivaroxaban in adults with nonvalvular atrial fibrillation. Am. J. Ther. 2019, 26, e679–e703. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.; Aswapanyawongse, O.; Tacey, M.; Kitipornchai, T.; Ho, P.; Lim, H.Y. Real-world direct oral anticoagulants experience in atrial fibrillation: Falls risk and low dose anticoagulation are predictive of both bleeding and stroke risk. Intern. Med. J. 2019. [Google Scholar] [CrossRef] [PubMed]
- Pratt, N.L.; Ramsay, E.; Kalisch Ellett, L.M.; Duszynski, K.; Shakib, S.; Kerr, M.; Caughey, G.; Roughead, E.E. Comparative effectiveness and safety of low-strength and high-strength direct oral anticoagulants compared with warfarin: A sequential cohort study. BMJ Open 2019, 9, e026486. [Google Scholar] [CrossRef]
- Esteve-Pastor, M.A.; Rivera-Caravaca, J.M.; Roldán, V.; Orenes-Piñero, E.; Romiti, G.F.; Romanazzi, I.; Bai, Y.; Carmo, J.; Proietti, M.; Marín, F.; et al. Estimated effectiveness and safety of nonvitamin K antagonist oral anticoagulants compared with optimally acenocoumarol anticoagulated “Real-World” in patients with atrial fibrillation. Am. J. Cardiol. 2018, 122, 785–792. [Google Scholar] [CrossRef] [Green Version]
- Kato, E.T.; Giugliano, R.P.; Ruff, C.T.; Koretsune, Y.; Yamashita, T.; Kiss, R.G.; Nordio, F.; Murphy, S.A.; Kimura, T.; Jin, J.; et al. Efficacy and safety of edoxaban in elderly patients with atrial fibrillation in the ENGAGE AF-TIMI 48 trial. J. Am. Heart Assoc. 2016, 5, e003432. [Google Scholar] [CrossRef]
- Spencer, R.J.; Amerena, J.V. Rivaroxaban in the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation: Clinical implications of the ROCKET AF trial and its subanalyses. Am. J. Cardiovasc. Drugs 2015, 15, 395–401. [Google Scholar] [CrossRef]
- Sherwood, M.W.; Nessel, C.C.; Hellkamp, A.S.; Mahaffey, K.W.; Piccini, J.P.; Suh, E.-Y.; Becker, R.C.; Singer, D.E.; Halperin, J.L.; Hankey, G.J.; et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: ROCKET AF trial. J. Am. Coll. Cardiol. 2015, 66, 2271–2281. [Google Scholar] [CrossRef] [Green Version]
- Diener, H.C.; Halperin, J.L.; Fox, K.; Hankey, G.J. Stroke prevention with rivaroxaban in higher-risk populations with atrial fibrillation. Int. J. Clin. Pract. 2015, 69, 743–756. [Google Scholar] [CrossRef]
- Abe, J.; Umetsu, R.; Kato, Y.; Ueda, N.; Nakayama, Y.; Suzuki, Y.; Suzuki, T.; Nagasawa, H.; Kinosada, Y.; Nakamura, M. Evaluation of dabigatran- and warfarin-associated hemorrhagic events using the FDA-adverse event peporting system database stratified by age. Int. J. Med. Sci. 2015, 12, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Halvorsen, S.; Atar, D.; Yang, H.; De Caterina, R.; Erol, C.; Garcia, D.; Granger, C.B.; Hanna, M.; Held, C.; Husted, S.; et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: Observations from the ARISTOTLE trial. Eur. Heart J. 2014, 35, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J.L.; Hankey, G.J.; Wojdyla, D.M.; Piccini, J.P.; Lokhnygina, Y.; Patel, M.R.; Breithardt, G.; Singer, D.E.; Becker, R.C.; Hacke, W.; et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the rivaroxaban once daily, oral, direct factor xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET AF). Circulation 2014, 130, 138–146. [Google Scholar] [PubMed] [Green Version]
- Giugliano, R.P.; Ruff, C.T.; Braunwald, E.; Murphy, S.A.; Wiviott, S.D.; Halperin, J.L.; Waldo, A.L.; Ezekowitz, M.D.; Weitz, J.I.; Špinar, J.; et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013, 369, 2093–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easton, J.D.; Lopes, R.D.; Bahit, M.C.; Wojdyla, D.M.; Granger, C.B.; Wallentin, L.; Alings, M.; Goto, S.; Lewis, B.S.; Rosenqvist, M.; et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: A subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012, 11, 503–511. [Google Scholar] [CrossRef]
- Hankey, G.J.; Patel, M.R.; Stevens, S.R.; Becker, R.C.; Breithardt, G.; Carolei, A.; Diener, H.-C.; Donnan, G.A.; Halperin, J.L.; Mahaffey, K.W.; et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: A subgroup analysis of ROCKET AF. Lancet Neurol. 2012, 11, 315–322. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.V.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, T.; Koretsune, Y.; Yang, Y.; Chen, S.-A.; Chung, N.; Shimada, Y.J.; Kimura, T.; Miyazaki, K.; Abe, K.; Mercuri, M.; et al. Edoxaban vs. warfarin in East Asian patients with atrial fibrillation—An ENGAGE AF-TIMI 48 subanalysis. Circ. J. 2016, 80, 860–869. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Hu, D.; Stevens, S.; Lokhnygina, Y.; Becker, R.C.; Berkowitz, S.D.; Breithardt, G.; Hacke, W.; Halperin, J.L.; Hankey, G.J.; et al. Efficacy and safety of rivaroxaban versus warfarin in patients from mainland China with nonvalvular atrial fibrillation: A subgroup analysis from the ROCKET AF trial. Thromb. Res. 2017, 156, 184–190. [Google Scholar] [CrossRef]
- Chan, Y.H.; Lee, H.F.; See, L.C.; Tu, H.T.; Chao, T.F.; Yeh, Y.H.; Wu, L.S.; Kuo, C.T.; Chang, S.H.; Lip, G.Y.H. Effectiveness and safety of four direct oral anticoagulants in Asian patients with nonvalvular atrial fibrillation. Chest 2019, 156, 529–543. [Google Scholar] [CrossRef]
- Lau, W.C.Y.; Li, X.; Wong, I.C.K.; Man, K.K.C.; Lip, G.Y.H.; Leung, W.K.; Siu, C.W.; Chan, E.W. Bleeding-related hospital admissions and 30-day readmissions in patients with non-valvular atrial fibrillation treated with dabigatran versus warfarin. J. Thromb. Haemost. 2017, 15, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Koretsune, Y.; Yamashita, T.; Yasaka, M.; Ono, Y.; Hirakawa, T.; Ishida, K.; Kuroki, D.; Sumida, T.; Urushihara, H. Comparative effectiveness and safety of warfarin and dabigatran in patients with non-valvular atrial fibrillation in Japan: A claims database analysis. J. Cardiol. 2019, 73, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.R.; Choi, E.K.; Kwon, S.; Han, K.D.; Jung, J.H.; Cha, M.-J.; Oh, S.; Lip, G.Y.H. Effectiveness and safety of contemporary oral anticoagulants among Asians with nonvalvular atrial fibrillation. Stroke 2019, 50, 2245–2249. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.S.; Dorreen, A.; Martel, M.; Huynh, T.; Barkun, A.N. Risk of gastrointestinal bleeding in patients taking non-vitamin K antagonist oral anticoagulants: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 1674–1683. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.C.; Wei, A.H.; Zhang, C.; Wang, X.H.; Zhang, L.; Shen, L.; Li, Z.; Pan, M.M.; Liu, X.Y.; Pu, J.; et al. Risk of major gastrointestinal bleeding with new vs conventional oral anticoagulants: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2019, 18, P792–799. [Google Scholar] [CrossRef]
- Guo, W.Q.; Chen, X.H.; Tian, X.Y.; Li, L. Differences in gastrointestinal safety profiles among novel oral anticoagulants: Evidence from a network meta-analysis. Clin. Epidemiol. 2019, 11, 911–921. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Wong, I.C.K.; Li, X.; Anand, S.; Leung, W.K.; Siu, C.W.; Chan, E.W. The association between non-vitamin K antagonist oral anticoagulants and gastrointestinal bleeding: A meta-analysis of observational studies. Br. J. Clin. Pharmacol. 2016, 82, 285–300. [Google Scholar] [CrossRef] [Green Version]
- Loffredo, L.; Perri, L.; Violi, F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: A meta-analysis of interventional trials. Dig. Liver Dis. 2015, 47, 429–431. [Google Scholar] [CrossRef]
- Apostolakis, S.; Sullivan, R.M.; Olshansky, B.; Lip, G.Y.H. Factors affecting quality of anticoagulation control among patients with atrial fibrillation on warfarin: The SAMe-TT₂R₂ score. Chest 2013, 144, 1555–1563. [Google Scholar] [CrossRef]
- Chan, P.H.; Hai, J.J.; Chan, E.W.; Li, W.H.; Tse, H.F.; Wong, I.C.K.; Lip, G.Y.H.; Siu, C.W. Use of the SAMe-TT2R2 score to predict good anticoagulation control with Warfarin in Chinese patients with atrial fibrillation: Relationship to ischemic stroke incidence. PLoS ONE 2016, 11, e0150674. [Google Scholar] [CrossRef]
- Llewellyn-Bennett, R.; Bowman, L.; Bulbulia, R. Post-trial follow-up methodology in large randomized controlled trials: A systematic review protocol. Syst. Rev. 2016, 5, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S Food & Drug Administration FDA Drug Safety Communication: FDA Study of Medicare Patients Finds Risks Lower for Stroke and Death but Higher for Gastrointestinal Bleeding with Pradaxa (Dabigatran) Compared to Warfarin. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-study-medicare-patients-finds-risks-lower-stroke-and-death-higher (accessed on 10 January 2020).
- Graham, D.J.; Reichman, M.E.; Wernecke, M.; Zhang, R.; Southworth, M.R.; Levenson, M.; Sheu, T.-C.; Mott, K.; Goulding, M.R.; Houstoun, M.; et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015, 131, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, Y.; Hirayama, C.; Hotoda, H.; Nishikawa, Y.; Nishiwaki, A. Postmarketing safety experience with edoxaban in Japan for thromboprophylaxis following major orthopedic surgery. Vasc. Health Risk Manag. 2013, 9, 593–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Southworth, M.R.; Reichman, M.E.; Unger, E.F. Dabigatran and postmarketing reports of bleeding. N. Engl. J. Med. 2013, 368, 1272–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, C.J.; Kalisch Ellett, L.M.; Barratt, J.D.; Caughey, G.E. A cross-country comparison of rivaroxaban spontaneous adverse event reports and concomitant medicine use with the potential to increase the risk of harm. Drug Saf. 2014, 37, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.J.; Kalisch Ellett, L.M.; Barratt, J.D.; Caughey, G.E. An international comparison of spontaneous adverse event reports and potentially inappropriate medicine use associated with dabigatran. Pharm. Drug Saf. 2015, 24, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Bahit, M.C.; Lopes, R.D.; Wojdyla, D.M.; Held, C.; Hanna, M.; Vinereanu, D.; Hylek, E.M.; Verheugt, F.; Goto, S.; Alexander, J.H.; et al. Non-major bleeding with apixaban versus warfarin in patients with atrial fibrillation. Heart 2017, 103, 623–628. [Google Scholar] [CrossRef]
- Lee, S.R.; Choi, E.K.; Han, K.D.; Jung, J.H.; Oh, S.; Lip, G.Y.H. Comparison of once-daily administration of Edoxaban and Rivaroxaban in Asian patients with atrial fibrillation. Sci. Rep. 2019, 9, 6690. [Google Scholar] [CrossRef] [Green Version]




| Author, Year | Intervention | Design | Number of Patients (NOAC/VKA) | Average Age | Data Source | Country/Region | Outcome |
|---|---|---|---|---|---|---|---|
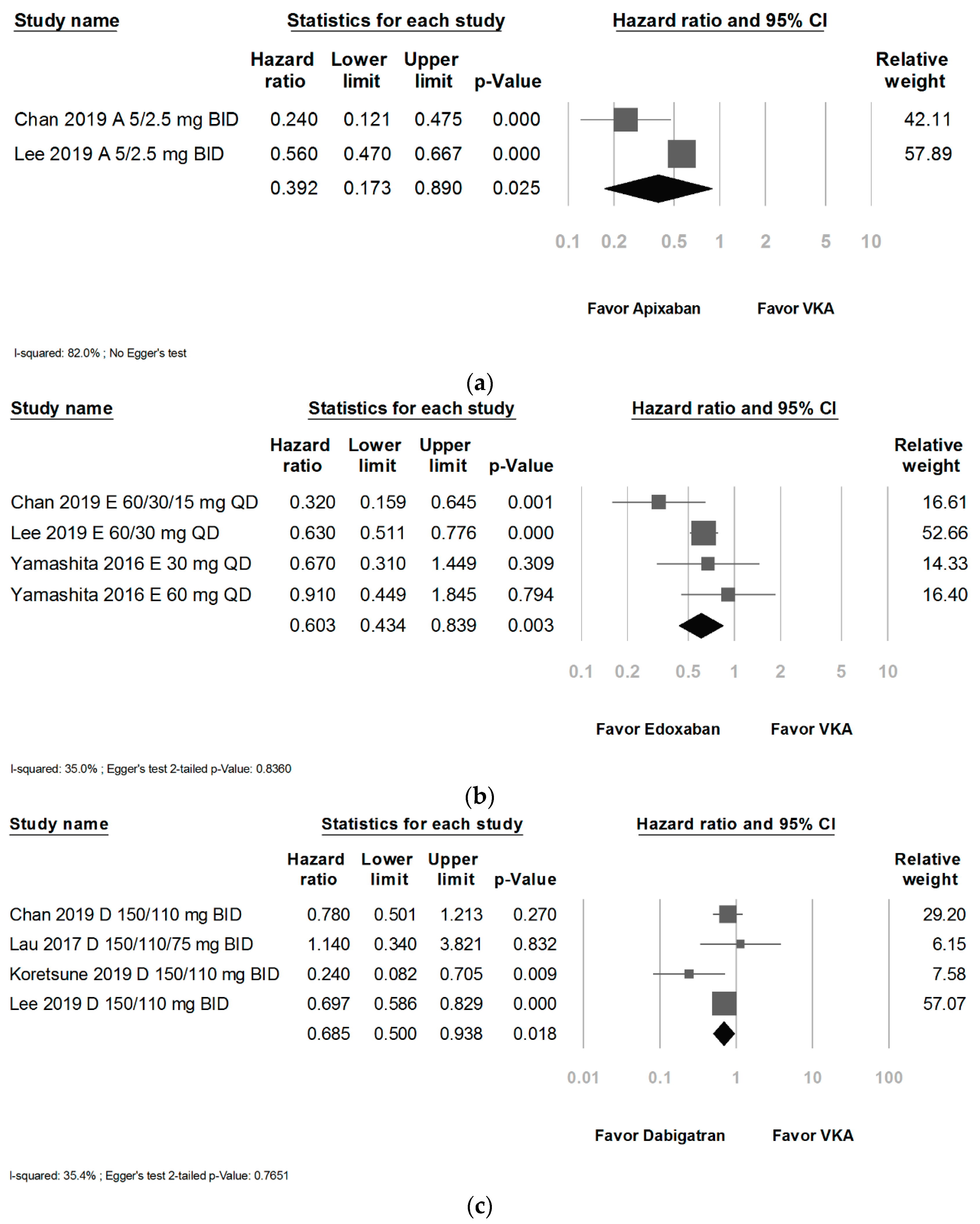
| Chan, 2019 [51] | R: 20/15/10 mg QD D: 150/110 mg BID A: 5/2.5 mg BID E: 60/30/15 mg QD | Retro | 69922/19761 | R: 75; D: 75 A: 75; E: 75 VKA: 75 | Taiwan National Health Insurance Database | Taiwan | Favor apixaban and edoxaban for less GIB and a trend toward rivaroxaban and dabigatran for less GIB than VKAs |
| Lau, 2017 [52] | D: 150/110/75 mg BID | Retro | 2580/2580 | D: 74 VKA: 74 | CDARS * of the HongKong Hospital Authority | China/Hong Kong | A trend toward VKAs for less GIB than dabigatran |
| Korestune, 2019 [53] | D: 150/110 mg BID | Retro | 4606/4606 | D: 74 VKA: 73 | Medical Data Vision (MDV, Tokyo, Japan) | Japan | Favor dabigatran for less GIB than VKAs |
| Lee, 2019 [54] | R: 20/15/10 mg QD D: 150/110 mg BID A: 5/2.5 mg BID E: 60/30 mg QD | Retro | 91383/25420 | R: 71; D: 71 A: 71; E: 71 VKA: 71 | Korean Health Insurance Review service | Korea | Favor overall NOACs for less GIB than VKAs |
| Yamashita, 2016 [49] | E: 60/30 mg QD | RCT | 1221/592 | East Asian: 70 | ENGAGE AF-TIMI 48 † subanalysis | China, Japan, Taiwan, South Korea | A trend toward edoxaban for less GIB than VKAs |
| Sun, 2017 [50] | R: 20/15 mg QD | RCT | 249/246 | China: 71 | ROCKET AF trial # | China | A trend toward VKAs for less GIB than rivaroxaban |
| Group | HR [95% CI] | I2% | Egger’s Test p-Value | Quality of the Evidence (GRADE) |
|---|---|---|---|---|
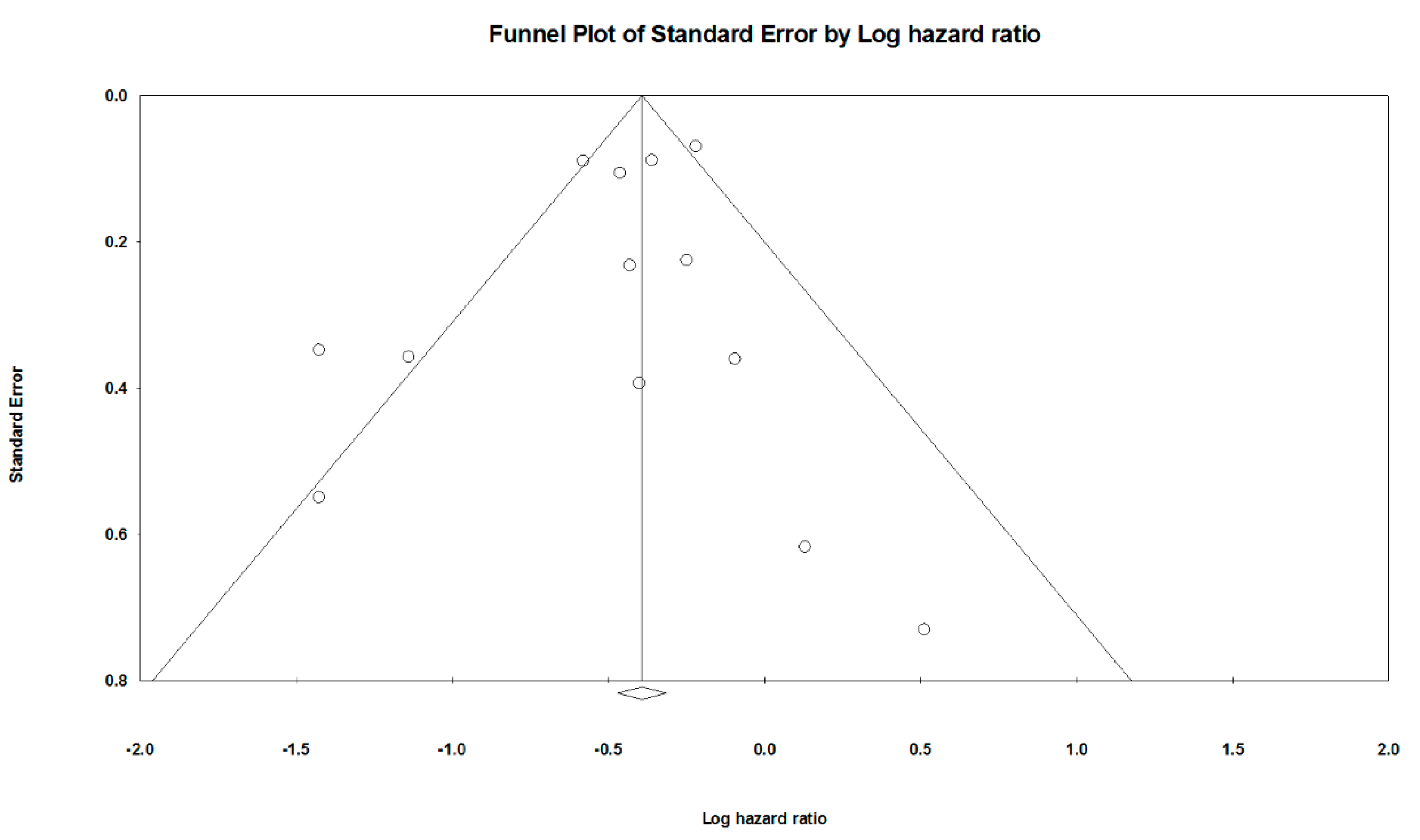
| Overall | 0.63 [0.54 to 0.75] | 61.6 | 0.3584 | Very low (a, b, g) |
| RCT subgroup | 0.86 [0.53 to 1.41] | 0 | 0.4281 | High (a, c, g, h) |
| Retrospective subgroup | 0.61 [0.51 to 0.73] | 68.9 | 0.1189 | Very low (a, b, g) |
| Apixaban subgroup | 0.39 [0.17 to 0.89] | 82.0 | NA | Very low (a, b, e, f, g) |
| Edoxaban subgroups | 0.60 [0.43 to 0.84] | 35.0 | 0.8360 | Low (a, g) |
| Dabigatran subgroup | 0.69 [0.50 to 0.94] | 35.4 | 0.7651 | Low (a, g) |
| Rivaroxaban subgroup | 0.79 [0.70 to 0.90] | 0 | 0.8630 | Low (a, g) |
| Covariate | Coefficient | Standard Error | 2-Sided p-Value | Tau Squared | Tau | Q | df |
|---|---|---|---|---|---|---|---|
| Age | −0.0789 | 0.0457 | 0.0842 | 0.0353 | 0.1880 | 27.39 | 11 |
| Female ratio | 0.1498 | 1.8083 | 0.9340 | 0.0425 | 0.2061 | 31.22 | 11 |
| Publication year | −0.1309 | 0.0985 | 0.1835 | 0.0397 | 0.1994 | 30.05 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.-T.; Sun, W.-C.; Tsai, T.-J.; Tsay, F.-W.; Chen, W.-C.; Cheng, J.-S. The Risk of Gastrointestinal Bleeding between Non-Vitamin K Antagonist Oral Anticoagulants and Vitamin K Antagonists in the Asian Atrial Fibrillation Patients: A Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 137. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18010137
Yang K-T, Sun W-C, Tsai T-J, Tsay F-W, Chen W-C, Cheng J-S. The Risk of Gastrointestinal Bleeding between Non-Vitamin K Antagonist Oral Anticoagulants and Vitamin K Antagonists in the Asian Atrial Fibrillation Patients: A Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(1):137. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18010137
Chicago/Turabian StyleYang, Kuang-Tsu, Wei-Chih Sun, Tzung-Jiun Tsai, Feng-Woei Tsay, Wen-Chi Chen, and Jin-Shiung Cheng. 2021. "The Risk of Gastrointestinal Bleeding between Non-Vitamin K Antagonist Oral Anticoagulants and Vitamin K Antagonists in the Asian Atrial Fibrillation Patients: A Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 1: 137. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18010137





