Association between Adherence with Recommended Antenatal Care in Low-Risk, Uncomplicated Pregnancy, and Maternal and Neonatal Adverse Outcomes: Evidence from Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Cohort
2.3. Adherence with Recommendations
2.4. Outcomes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- Italian Ministry of Health, Dept of Health Planning: Silvia ARCÀ, Donata BELLENTANI, Velia BRUNO, Simona CARBONE, Carla CECCOLINI, Angela DE FEO, Lucia LISPI, Rosanna MARINIELLO, Maurizio MASULLO, Federica MEDICI, Paola PISANTI, Modesta VISCA, Rinaldo ZANINI; Dept of health prevention: Teresa DI FIANDRA, Natalia MAGLIOCCHETTI, Giovanna ROMANO
- University of Milano-Bicocca, Laboratory of Healthcare Research & Pharmacoepidemiology: Anna CANTARUTTI, Giovanni CORRAO, Pietro PUGNI, Federico REA
- Department of Epidemiology Lazio Region: Marina DAVOLI, Mirko DI MARTINO, Adele LALLO
- Aosta Valley Region: Patrizia VITTORI, Giuliana Vuillermin
- Campania Region: Alfonso Bernardo, Anna Fusciante
- Emilia Romagna Region: Laura BELOTTI, Rossana DE PALMA, Enza DI FELICE
- Friuli Venezia Giulia Region: Roberta CHIANDETTI, Elena CLAGNAN, Stefania DEL ZOTTO, Andrea DI LENARDA, Aldo MARIOTTO, Marisa PREZZA, Loris ZANIER
- Lazio Region: Marina DAVOLI, Danilo FUSCO, Mirko DI MARTINO, Adele LALLO, Chiara MARINACCI
- Lombardy Region: Antonio LORA, Luca MERLINO
- Marche Region: Liana SPAZZAFUMO, Simone PIZZI
- Molise Region: Maria SIMIELE, Giuseppe MASSARO
- Puglia Region: Ettore ATTOLINI, Vito LEPORE, Vito PETRAROLO
- Sicily Region: Giovanni DE LUCA, Giovanna FANTACI, Sebastiano POLLINA ADDARIO, Salvatore SCONDOTTO
- Tuscany Region: Francesco BELLOMO, Mario BRAGA, Valeria DI FABRIZIO, Silvia FORNI, Paolo FRANCESCONI, Francesco PROFILI
- Veneto Region: Francesco AVOSSA, Matteo CORRADIN, Silvia VIGNA
- Research and Health Foundation (Fondazione ReS -Ricerca e Salute-): Letizia DONDI, Nello MARTINI, Antonella PEDRINI, Carlo PICCINNI
- National Agency for Regional Health Services: Mimma COSENTINO, Maria Grazia MARVULLI
- ANMCO (National Association of Hospital Cardiologists) Study Center: Aldo MAGGIONI
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Recommendations on Antenatal Care for a Positive Pregnancy Experience. 2016. Available online: www.who.int/reproductivehealth/publications/maternal_perinatal_health/anc-positive-pregnancy-experience/en/ (accessed on 1 January 2016).
- Abalos, E.; Chamillard, M.; Diaz, V.; Tuncalp, Ӧ.; Gulmezoglu, A.M. Antenatal care for healthy pregnant women: A mapping of interventions from existing guidelines to inform the development of new WHO guidance on antenatal care. BJOG Int. J. Obstet. Gynaecol. 2015, 123, 519–528. [Google Scholar] [CrossRef]
- Yeoh, P.L.; Hornetz, K.; Dahlui, M. Antenatal Care Utilisation and Content between Low-Risk and High-Risk Pregnant Women. PLoS ONE 2016, 11, e0152167. [Google Scholar] [CrossRef] [Green Version]
- Coco, L.; Giannone, T.T.; Zarbo, G. Management of high-risk pregnancy. Minerva Ginecol. 2014, 66, 383–389. [Google Scholar]
- NICE. Antenatal Care for Uncomplicated Pregnancies. 2019. Available online: www.nice.org.uk/guidance/CG62 (accessed on 4 February 2019).
- Department of Health. Clinical Practice Guidelines: Pregnancy Care; Australian Government Department of Health: Canberra, Australian, 2019.
- Sarah, J.; Kilpatrick, S.J.; Papile, L.-A.; Macones, G.A. Guidelines for Perinatal Care, 8th ed.; AAP Committee on Fetus and Newborn and ACOG Committee on Obstetric Practice. The American College of Obstetricians and Gynecologists: Washington, DC, USA, 2017. [Google Scholar]
- Carroli, G.; Villar, J.; Piaggio, G.; Khan-Neelofur, D.; Gülmezoglu, M.; Mugford, M.; Lumbiganon, P.; Farnot, U.; Bersgjø, P.; WHO Antenatal Care Trial Research Group. WHO systematic review of randomised controlled trials of routine antenatal care. Lancet 2001, 357, 1565–1570. [Google Scholar] [CrossRef]
- Symon, A.; Pringle, J.; Downe, S.; Hundley, V.; Lee, E.; Lynn, F.; McFadden, A.; McNeill, J.; Renfrew, M.J.; Ross-Davie, M.; et al. Antenatal care trial interventions: A systematic scoping review and taxonomy development of care models. BMC Pregnancy Childbirth 2017, 17, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Dowswell, T.; Carroli, G.; Duley, L.; Gates, S.; Gülmezoglu, A.M.; Khan-Neelofur, D.; Piaggio, G. Alternative versus standard packages of antenatal care for low-risk pregnancy. Cochrane Database Syst. Rev. 2015, 2015, CD000934. [Google Scholar] [CrossRef] [Green Version]
- Corrao, G.; Rea, F.; Di Martino, M.; Lallo, A.; Davoli, M.; PlALMA, R.D.; Belotti, L.; Merlino, L.; Pisanti, P.; Lispi, L.; et al. Effectiveness of adherence to recommended clinical examinations of diabetic patients in preventing diabetes-related hospitalizations. Int. J. Qual. Health Care 2019, 31, 464–472. [Google Scholar] [CrossRef]
- Cantarutti, A.; Rea, F.; Locatelli, A.; Merlino, L.; Lundin, R.; Perseghin, G.; Corrao, G. Adherence to clinical evaluations in women with pre-existing diabetes during pregnancy: A call to action from an Italian real-life investigation. Diabetes Res. Clin. Pract. 2019, 154, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cantarutti, A.; Franchi, M.; Rea, F.; Merlino, L.; Corrao, G. Use of Nimesulide during Early Pregnancy and the Risk of Congenital Malformations: A Population-Based Study from Italy. Adv. Ther. 2018, 35, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Cantarutti, A.; Franchi, M.; Compagnoni, M.M.; Merlino, L.; Giovanni, G. Mother’s education and the risk of several neonatal outcomes: An evidence from an Italian population-based study. BMC Pregnancy Childbirth 2017, 17, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantarutti, A.; Merlino, L.; Giaquinto, C.; Corrao, G. Use of antidepressant medication in pregnancy and adverse neonatal out-comes: A population-based investigation. Pharmacoepidemiol. Drug Saf. 2017, 26, 1100–1108. [Google Scholar] [CrossRef] [PubMed]
- Cantarutti, A.; Merlino, L.; Monzani, E.; Giaquinto, C.; Corrao, G. Is the Risk of Preterm Birth and Low Birth Weight Affected by the Use of Antidepressant Agents during Pregnancy? A Population-Based Investigation. PLoS ONE 2016, 11, e0168115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SNLG—ISS Linea Guida 20. Gravidanza Fisiologica. 2011, Last Update in 2014. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_1436_allegato.pdf (accessed on 23 November 2011).
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate—A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B-Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Verret-Chalifour, J.; Giguère, Y.; Forest, J.C.; Croteau, J.; Zhang, P.; Marc, I. Breastfeeding initiation: Impact of obesity in a large Ca-nadian perinatal cohort study. PLoS ONE 2015, 10, e0117512. [Google Scholar] [CrossRef] [Green Version]
- Schneeweiss, S.; Rassen, J.A.; Glynn, R.J.; Avorn, J.; Mogun, H.; Brookhart, M.A. High-dimensional Propensity Score Adjustment in Studies of Treatment Effects Using Health Care Claims Data. Epidemiology 2009, 20, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Desai, R.J.; Rothman, K.J.; Bateman, B.T.; Hernandez-Diaz, S.; Huybrechts, K.F. A propensity-score-based fine stratification ap-proach for confounding adjustment when exposure is infrequent. Epidemiology 2017, 28, 249–257. [Google Scholar] [CrossRef]
- Corrao, G.; Rea, F.; Franchi, M.; Beccalli, B.; Locatelli, A.; Cantarutti, A. Warning of Immortal Time Bias When Studying Drug Safe-ty in Pregnancy: Application to Late Use of Antibiotics and Preterm Delivery. Int. J. Environ. Res. Public Health 2020, 17, 6465. [Google Scholar] [CrossRef]
- Rodrigues, P.B.; Zambaldi, C.F.; Cantilino, A.; Sougey, E.B. Special features of high-risk pregnancies as factors in development of mental distress: A review. Trends Psychiatry Psychother. 2016, 38, 136–140. [Google Scholar] [CrossRef]
- Rodrigues, A.R.M.; Rodrigues, D.P.; Viana, A.B.; Cabral, L.S.; Silveira, M.A.M. Nursing care in high-risk pregnancies: An integrative review. Online Braz. J. Nurs. 2016, 15, 472–483. [Google Scholar] [CrossRef] [Green Version]
- Zadeh, M.A.; Khajehei, M.; Sharif, F.; Hadzic, M. High-risk pregnancy: Effects on postpartum depression and anxiety. Br. J. Midwifery 2012, 20, 104–113. [Google Scholar] [CrossRef]
- Leinonen, E.; Gissler, M.; Haataja, L.; Rahkonen, P.; Andersson, S.; Metsäranta, M.; Rahkonen, L. Low Apgar scores at both one and five minutes are associated with long-term neurological morbidity. Acta Paediatr. 2018, 107, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Chang, Y.S.; Ahn, S.Y.; Sung, S.I.; Park, W.S. Predicting mortality in extremely low birth weight infants: Comparison be-tween gestational age, birth weight, Apgar score, CRIB II score, initial and lowest serum albumin levels. PLoS ONE 2018, 13, e0192232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OECD. Health at a Glance: Europe 2018. Available online: https://0-doi-org.brum.beds.ac.uk/10.1787/health_glance_eur-2018-en (accessed on 22 November 2018).
- Johnson, C.D.; Jones, S.; Paranjothy, S. Reducing low birth weight: Prioritizing action to address modifiable risk factors. J. Public Health 2016, 39, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Poppel, M.; Jelsma, J.J.; Simmons, D.; Devlieger, R.; Jans, G.; Galjaard, S.; Corcoy, R.; Adelantado, J.M.; Dunne, F.; Harreiter, J.; et al. Mediators of Lifestyle Behaviour Changes in Obese Pregnant Women. Secondary Analyses from the DALI Lifestyle Randomised Controlled Trial. Nutrients 2019, 11, 311. [Google Scholar] [CrossRef] [Green Version]
- Henrichs, J.; Heuvel, M.I.V.D.; Witteveen, A.B.; Wilschut, J.; Bergh, B.R.H.V.D. Does Mindful Parenting Mediate the Association between Maternal Anxiety during Pregnancy and Child Behavioral/Emotional Problems? Mindfulness 2019, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Weir, Z.; Bush, J.; Robson, S.; McParlin, C.; Rankin, J.; Bell, R. Physical activity in pregnancy: A qualitative study of the beliefs of overweight and obese pregnant women. BMC Pregnancy Childbirth 2010, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Olander, E.K.; Smith, D.; Darwin, Z. Health behaviour and pregnancy: A time for change. J. Reprod. Infant Psychol. 2018, 36, 1–3. [Google Scholar] [CrossRef]
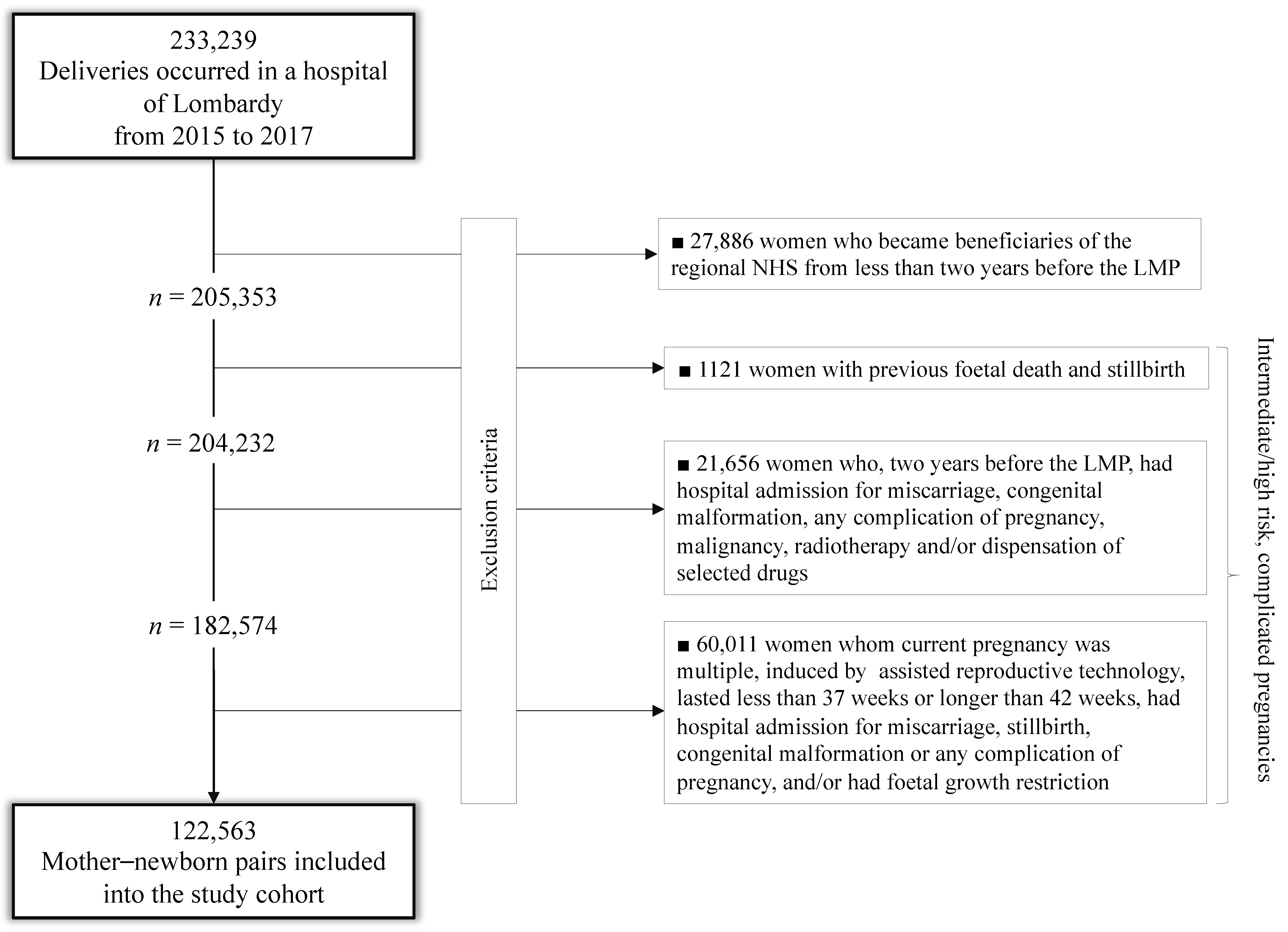

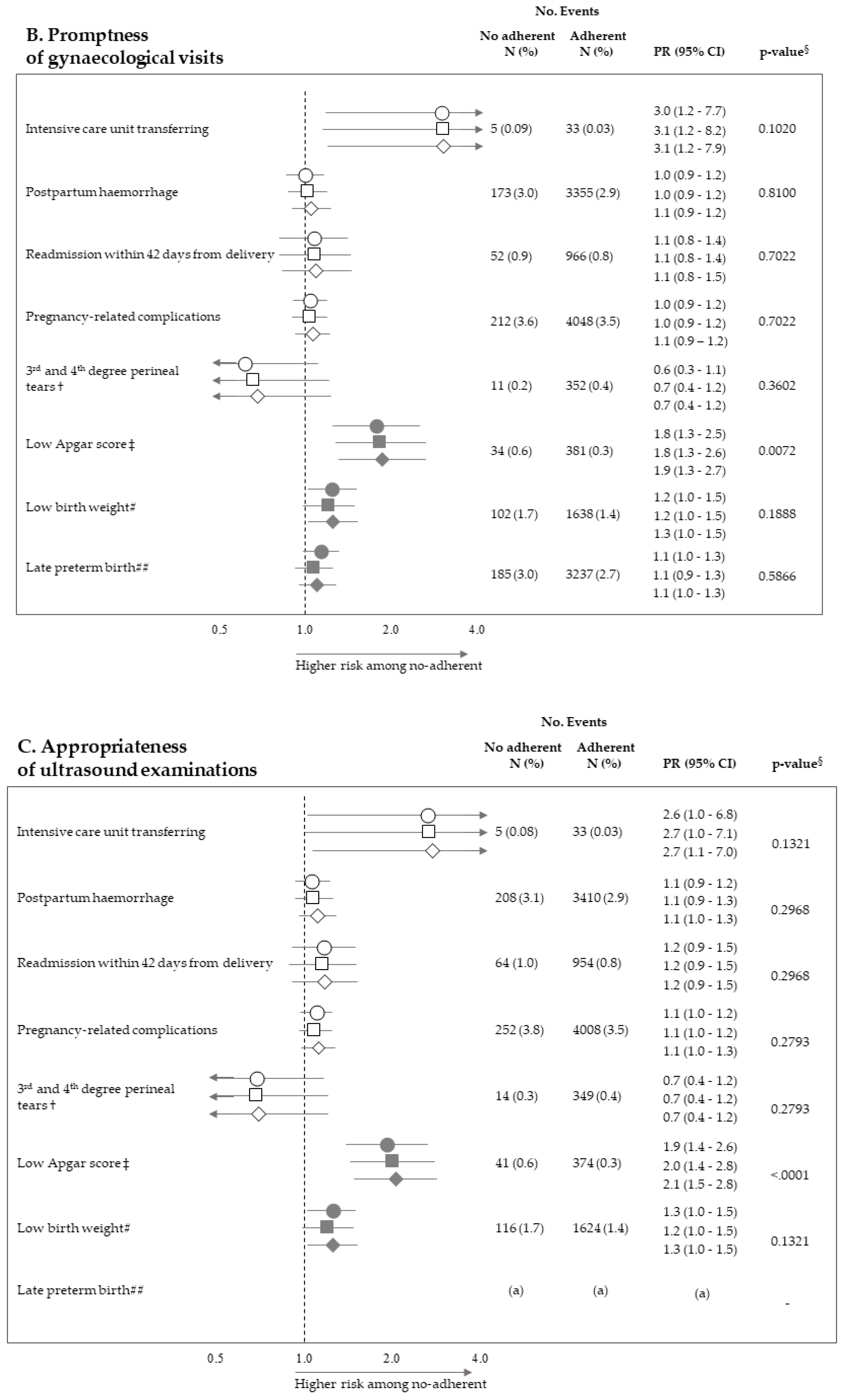
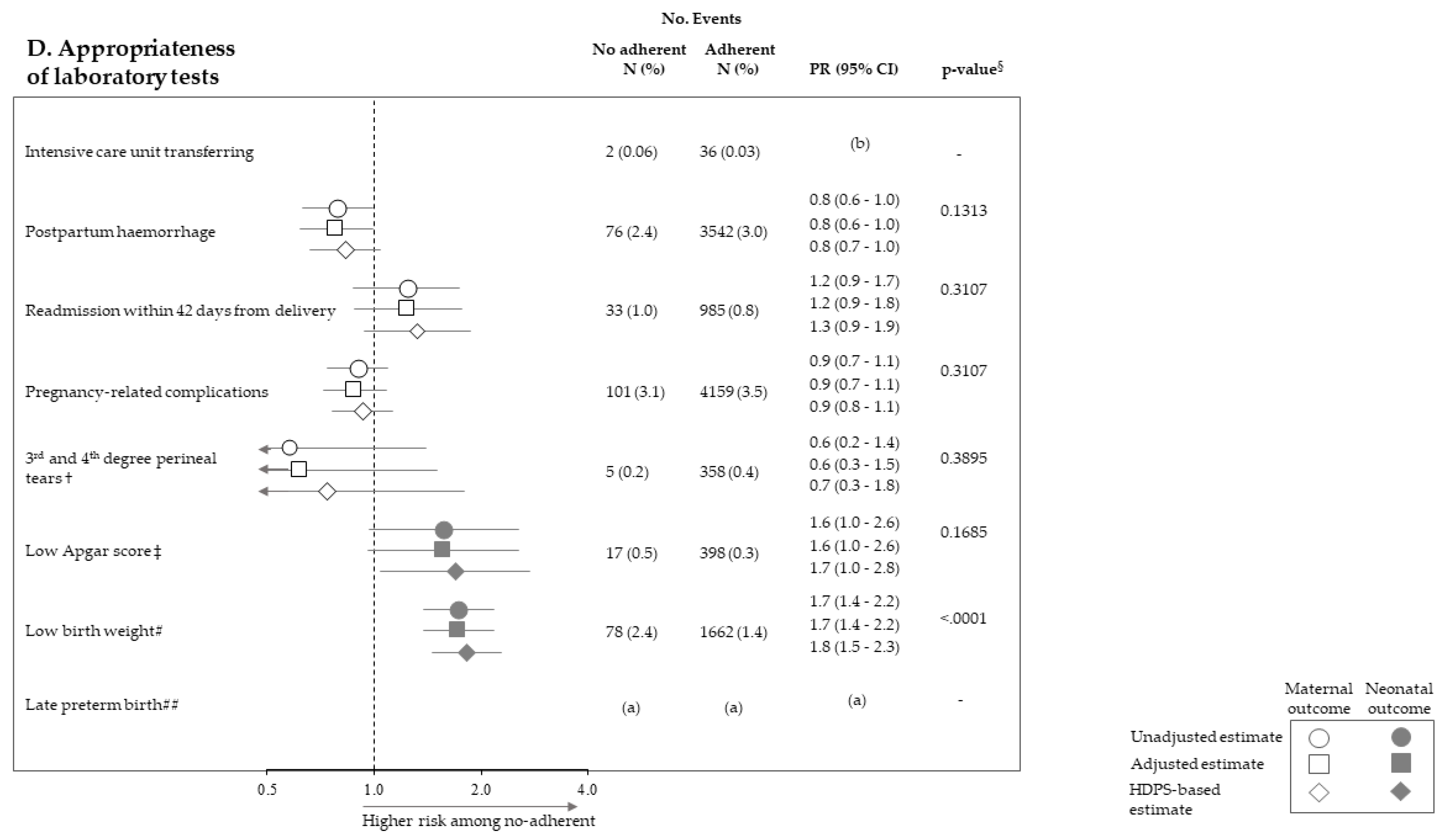
| Socio-Demographic Traits | No. (%) |
|---|---|
| Age at delivery | |
| 16 to 25 years | 12,314 (10.1) |
| 26 to 35 years | 68,365 (55.8) |
| 36 to 45 years | 41,884 (34.2) |
| Birth place | |
| Italian-born | 90,676 (74.0) |
| Foreign-born | 29,981 (24.5) |
| Missing | 1906 (1.6) |
| Marital status | |
| Married | 76,667 (62.6) |
| Unmarried | 45,856 (37.4) |
| Missing | 40 (0.0) |
| Employment | |
| Employed | 87,196 (71.1) |
| Unemployed | 35,262 (28.8) |
| Missing | 105 (0.1) |
| Education | |
| Low | 26,380 (21.5) |
| Intermediate | 53,554 (43.7) |
| High | 42,567 (34.7) |
| Missing | 62 (0.1) |
| Parity | |
| Null parity | 16,505 (13.5) |
| Multi parity | 65,493 (53.4) |
| Missing | 40,565 (33.1) |
| Non-adherence to selected recommendations | |
| Appropriateness of gynaecological visits | 7262 (5.9) |
| Promptness of gynaecological visits | 5868 (4.8) |
| Appropriateness of ultrasounds | 6649 (5.4) |
| Appropriateness of laboratory tests | 3237 (2.6) |
| Number of complied recommendations | |
| 0 | 176 (0.1) |
| 1 | 1251 (1.0) |
| 2 | 4854 (4.0) |
| 3 | 8851 (7.2) |
| 4 | 107,431 (87.7) |
| No. (%) | |
|---|---|
| Maternal adverse outcomes | |
| Hysterectomy | 12 (0.0%) |
| Transferring to the intensive care unit | 38 (0.0%) |
| Postpartum haemorrhage | 3618 (3.0%) |
| Readmission within 42 days from delivery | 1018 (0.8%) |
| Pregnancy-related complications | 4260 (3.5%) |
| 3rd and 4th degree perineal tears † | 363 (0.4%) |
| Neonatal adverse outcomes | |
| Low Apgar score at five minutes ‡ | 415 (0.3%) |
| Low birth weight # | 1740 (1.4%) |
| Late preterm birth ## | 3422 (2.7%) |
| Number of Complied Recommendations | p-Trend | ||||
|---|---|---|---|---|---|
| 4 | 3 | 2 | 0 or 1 | ||
| Maternal adverse outcomes | |||||
| Transferring to the intensive care unit | 1.0 (ref.) | 1.8 (0.6 to 5.0) | 3.8 (1.4 -10.1) | 0.0976 | |
| Postpartum haemorrhage | 1.0 (ref.) | 1.2 (1.1–1.3) | 1.2 (1.0–1.4) | 0.7 (0.5–1.0) | 0.2734 |
| Readmission within 42 days from delivery | 1.0 (ref.) | 1.1 (0.8–1.4) | 1.2 (0.9–1.6) | 1.1 (0.6–1.9) | 0.2478 |
| Pregnancy-related complications | 1.0 (ref.) | 1.1 (1.0–1.3) | 1.2 (1.0–1.3) | 0.9 (0.7–1.2) | 0.1227 |
| Neonatal adverse outcomes | |||||
| Low Apgar score at five minutes † | 1.0 (ref.) | 1.4 (1.0–2.0) | 1.6 (1.1–2.5) | 3.2 (1.8–5.6) | <0.0001 |
| Low birth weight ‡ | 1.0 (ref.) | 1.0 (0.9–1.2) | 1.2 (1.0–2.2) | 1.6 (1.1–2.3) | 0.0087 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrao, G.; Cantarutti, A.; Locatelli, A.; Porcu, G.; Merlino, L.; Carbone, S.; Carle, F.; Zanini, R., on behalf of the Monitoring and Assessing Diagnostic-Therapeutic Paths (MAP) Working Group of the Italian Heath Ministry. Association between Adherence with Recommended Antenatal Care in Low-Risk, Uncomplicated Pregnancy, and Maternal and Neonatal Adverse Outcomes: Evidence from Italy. Int. J. Environ. Res. Public Health 2021, 18, 173. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18010173
Corrao G, Cantarutti A, Locatelli A, Porcu G, Merlino L, Carbone S, Carle F, Zanini R on behalf of the Monitoring and Assessing Diagnostic-Therapeutic Paths (MAP) Working Group of the Italian Heath Ministry. Association between Adherence with Recommended Antenatal Care in Low-Risk, Uncomplicated Pregnancy, and Maternal and Neonatal Adverse Outcomes: Evidence from Italy. International Journal of Environmental Research and Public Health. 2021; 18(1):173. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18010173
Chicago/Turabian StyleCorrao, Giovanni, Anna Cantarutti, Anna Locatelli, Gloria Porcu, Luca Merlino, Simona Carbone, Flavia Carle, and Rinaldo Zanini on behalf of the Monitoring and Assessing Diagnostic-Therapeutic Paths (MAP) Working Group of the Italian Heath Ministry. 2021. "Association between Adherence with Recommended Antenatal Care in Low-Risk, Uncomplicated Pregnancy, and Maternal and Neonatal Adverse Outcomes: Evidence from Italy" International Journal of Environmental Research and Public Health 18, no. 1: 173. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18010173






