Insulin Withdrawal in Diabetic Kidney Disease: What Are We Waiting for?
Abstract
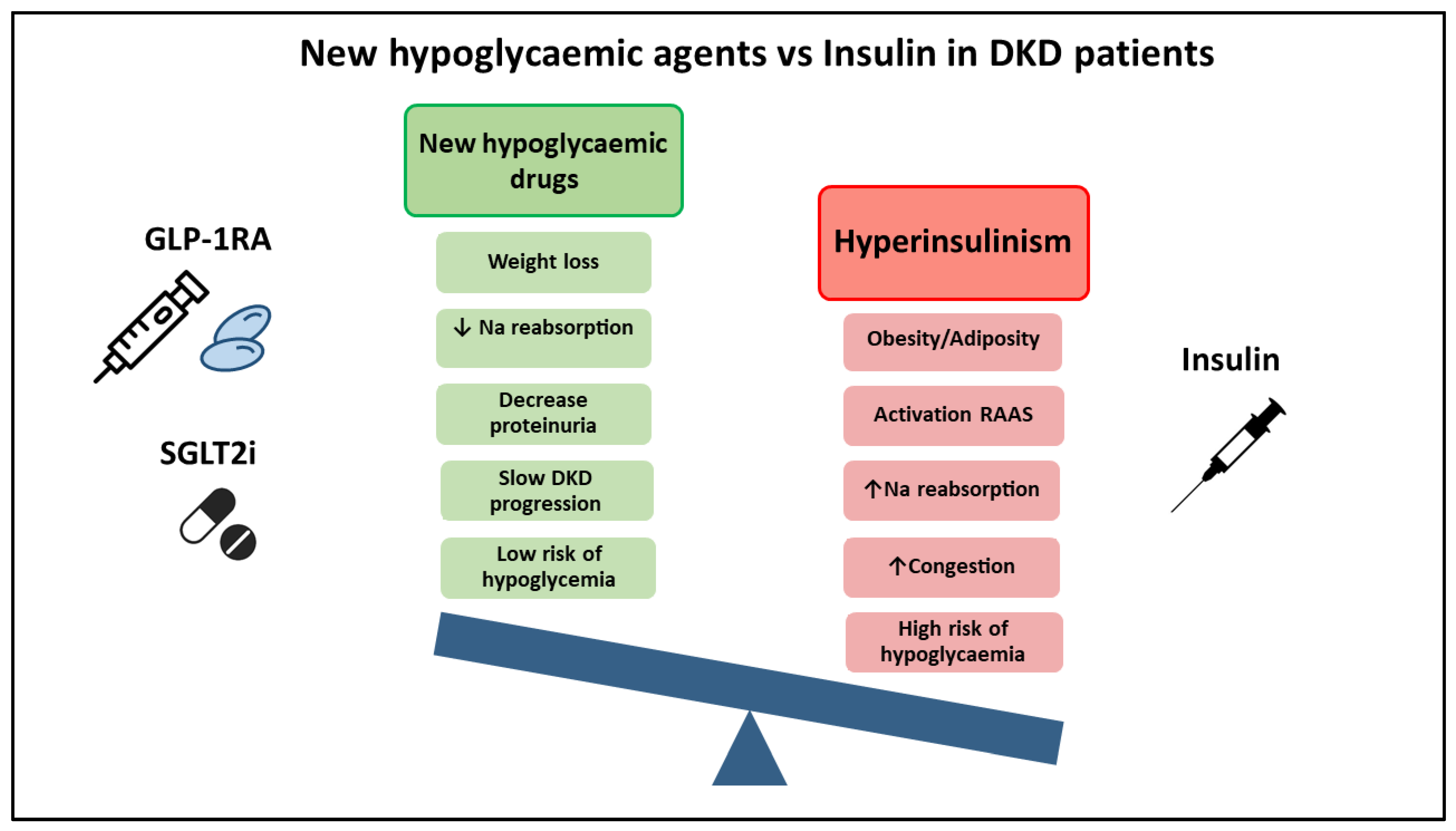
:1. Introduction
2. What We Know: Advantages of New Therapeutic Agents (SGLT2i and GLP1-RA)
3. What We don’t Know
4. What Do the Guidelines Say? Why Don’t the Guidelines Recommend Insulin Withdrawal?
5. Suggestions Considering the Current Evidence
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K. Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Sun, C.-Y.; Lee, C.-C.; Wu, I.-W.; Chen, Y.-C.; Lin, Y.-H.; Fang, W.-C.; Pan, H.-C. Ketoanalogue supplements reduce mortality in patients with pre-dialysis advanced diabetic kidney disease: A nationwide population-based study. Clin. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Bell, K.; Stanford, A.; Kern, D.M.; Tunceli, O.; Vupputuri, S.; Kalsekar, I.; Willey, V. Understanding CKD among patients with T2DM: Prevalence, temporal trends, and treatment patterns—NHANES 2007–2012. BMJ Open Diabetes Res. Care 2016, 4, e000154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Boer, I.H.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- Marbury, T.C.; Flint, A.; Jacobsen, J.B.; Karsbøl, J.D.; Lasseter, K. Pharmacokinetics and Tolerability of a Single Dose of Semaglutide, a Human Glucagon-Like Peptide-1 Analog, in Subjects With and Without Renal Impairment. Clin. Pharmacokinet. 2017, 56, 1381–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, L.V.; Flint, A.; Olsen, A.K.; Ingwersen, S.H. Liraglutide in Type 2 Diabetes Mellitus: Clinical Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2016, 55, 657–672. [Google Scholar] [CrossRef] [Green Version]
- Jacobsen, L.V.; Hindsberger, C.; Robson, R.; Zdravkovic, M. Effect of renal impairment on the pharmacokinetics of the GLP-1 analogue liraglutide. Br. J. Clin. Pharmacol. 2009, 68, 898–905. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Arganda, C. IQVIA Estima el Impacto de la OPR en Oficina de Farmacia. Diario Farma 2020. Available online: https://www.diariofarma.com/2020/12/03/iqvia-estima-el-impacto-de-la-opr-en-oficina-de-farmacia-en-34-millones-y-deja-al-mercado-sin-crecimiento (accessed on 17 May 2021).
- Cas, A.D.; Khan, S.S.; Butler, J.; Mentz, R.J.; Bonow, R.O.; Avogaro, A.; Tschoepe, D.; Doehner, W.; Greene, S.J.; Senni, M.; et al. Impact of Diabetes on Epidemiology, Treatment, and Outcomes of Patients With Heart Failure. JACC: Hear. Fail. 2015, 3, 136–145. [Google Scholar] [CrossRef]
- Riehle, C.; Abel, E.D. Insulin Signaling and Heart Failure. Circ. Res. 2016, 118, 1151–1169. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Cui, X.; Yang, J.; Zheng, M.; Jia, J.; Han, F.; Yang, X.; Wang, J.; Guo, Z.; et al. Hyperinsulinemia Can Cause Kidney Disease in the IGT Stage of OLETF Rats via the INS/IRS-1/PI3-K/Akt Signaling Pathway. J. Diabetes Res. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Naing, S.; Ramesh, G.; Garcha, J.; Poliyedath, A.; Khandelwal, S.; Mills, P. SUN-LB115 Is the Stepping-Down Approach a Better Option Than Multiple Daily Injections in Patients With Chronic Poorly-Controlled Diabetes on Advanced Insulin Therapy? J. Endocr. Soc. 2020, 4. [Google Scholar] [CrossRef]
- Tofé, S.; Argüelles, I.; Mena, E.; Serra, G.; Codina, M.; Urgeles, J.R.; García, H.; Pereg, V. Real-world GLP-1 RA therapy in type 2 diabetes: A long-term effectiveness observational study. Endocrinol. Diabetes Metab. 2018, 2, e00051. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, T.; Awad, M.; Moorman, J.M.; Gothard, M.D. Evaluating the Impact of Glucagon-Like Peptide-1 Receptor Agonists on Metabolic Changes in Patients With Type 2 Diabetes on High-Dose Insulin. Am. J. Ther. 2020, Publish Ah, 29. [Google Scholar] [CrossRef]
- Goto, A.; Takaichi, M.; Kishimoto, M.; Takahashi, Y.; Kajio, H.; Shimbo, T.; Noda, M. Body Mass Index, Fasting Plasma Glucose Levels, and C-peptide Levels as Predictors of the Future Insulin Use in Japanese Type 2 Diabetic Patients. Endocr. J. 2010, 57, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, K.R.; Lakshmanan, M.C.; Rayner, B.; Busch, R.S.; Zimmermann, A.G.; Woodward, D.B.; Botros, F.T. Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): A multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol. 2018, 6, 605–617. [Google Scholar] [CrossRef]
- Jones, A.G.; Hattersley, A.T. The clinical utility of C-peptide measurement in the care of patients with diabetes. Diabet. Med. 2013, 30, 803–817. [Google Scholar] [CrossRef] [Green Version]
- Rodbard, H.W.; Lingvay, I.; Reed, J.; De La Rosa, R.; Rose, L.; Sugimoto, D.; Araki, E.; Chu, P.-L.; Wijayasinghe, N.; Norwood, P. Semaglutide Added to Basal Insulin in Type 2 Diabetes (SUSTAIN 5): A Randomized, Controlled Trial. J. Clin. Endocrinol. Metab. 2018, 103, 2291–2301. [Google Scholar] [CrossRef] [Green Version]
- E Brown, R.; Bech, P.G.; Aronson, R. Semaglutide once weekly in people with type 2 diabetes: Real-world analysis of the Canadian LMC diabetes registry ( SPARE study). Diabetes Obes. Metab. 2020, 22, 2013–2020. [Google Scholar] [CrossRef]
- Bolli, G.B.; Porcellati, F.; Meier, J.J. Switching From Insulin Bolus Treatment to GLP-1 RAs Added to Continued Basal Insulin in People With Type 2 Diabetes on Basal-Bolus Insulin. Diabetes Care 2020, 43, 2333–2335. [Google Scholar] [CrossRef]
- Naing, S.; Ramesh, G.; Garcha, J.; Poliyedath, A.; Khandelwal, S.; Mills, P.K. Is the stepping-down approach a better option than multiple daily injections in obese patients with poorly controlled Type 2 diabetes on advanced insulin therapy? Endocrinol. Diabetes Metab. 2021, 4. [Google Scholar] [CrossRef]
| Adverse Effects | Insulin | SGLT2i | GLP1-RA |
|---|---|---|---|
| Hypoglycaemic risk | +++ | + | + |
| Weight gain | +++ | - | - |
| GI discomfort | - | + | +++ |
| Ketoacidosis | ++ | +++ | + |
| Volume depletion | - | ++ | + |
| Orthostatic hypotension | - | ++ | + |
| Genital infection | - | ++ | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morillas, C.; D’Marco, L.; Puchades, M.J.; Solá-Izquierdo, E.; Gorriz-Zambrano, C.; Bermúdez, V.; Gorriz, J.L. Insulin Withdrawal in Diabetic Kidney Disease: What Are We Waiting for? Int. J. Environ. Res. Public Health 2021, 18, 5388. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18105388
Morillas C, D’Marco L, Puchades MJ, Solá-Izquierdo E, Gorriz-Zambrano C, Bermúdez V, Gorriz JL. Insulin Withdrawal in Diabetic Kidney Disease: What Are We Waiting for? International Journal of Environmental Research and Public Health. 2021; 18(10):5388. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18105388
Chicago/Turabian StyleMorillas, Carlos, Luis D’Marco, María Jesús Puchades, Eva Solá-Izquierdo, Carmen Gorriz-Zambrano, Valmore Bermúdez, and José Luis Gorriz. 2021. "Insulin Withdrawal in Diabetic Kidney Disease: What Are We Waiting for?" International Journal of Environmental Research and Public Health 18, no. 10: 5388. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18105388







