The Association of Familial Hypertension and Risk of Gestational Hypertension and Preeclampsia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Inclusion Criteria
2.3. Method
2.4. Definitions of Dependent Variables
2.5. Independent Variables
2.6. Covariates
2.7. Statistical Analyses
3. Results
3.1. Basic Characteristics of the Partcipants
3.2. Odds Ratios of GH and PE for Chronic Hypertension in the Parents
3.3. GH and PE Risk after Cohort Dissection into BMI and Smoking Categories
4. Discussion
Summary—Advantages and Limitations
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| AOR | Adjusted odds ratio |
| BMI | Body mass index |
| CI | Confidence intervals |
| FIGO | International Federation of Gynecology and Obstetrics |
| GDM | Gestational diabetes mellitus |
| GH | Gestational hypertension |
| GWG | Gestational weight gain |
| OR | (Unadjusted) Odds ratio |
| PE | Preeclampsia |
| PIH | Pregnancy-induced hypertension |
| SOGC | Society of Obstetricians and Gynaecologists of Canada |
| WHO | World Health Organization |
References
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obs. Gynecol. 2020, 135, 1492–1495. [CrossRef]
- Poon, L.C.; Shennan, A.; Hyett, J.A.; Kapur, A.; Hadar, E.; Divakar, H.; McAuliffe, F.; da Silva Costa, F.; von Dadelszen, P.; McIntyre, H.D.; et al. The International Federation of Gynecology and Obstetrics (FIGO) Initiative on Pre-Eclampsia: A Pragmatic Guide for First-Trimester Screening and Prevention. Int. J. Gynaecol. Obs. 2019, 145 (Suppl. 1), 1–33. [Google Scholar] [CrossRef] [Green Version]
- Benschop, L.; Duvekot, J.J.; Roeters van Lennep, J.E. Future Risk of Cardiovascular Disease Risk Factors and Events in Women after a Hypertensive Disorder of Pregnancy. Heart 2019, 105, 1273–1278. [Google Scholar] [CrossRef]
- Fikadu, K.; G/Meskel, F.; Getahun, F.; Chufamo, N.; Misiker, D. Family History of Chronic Illness, Preterm Gestational Age and Smoking Exposure before Pregnancy Increases the Probability of Preeclampsia in Omo District in Southern Ethiopia: A Case-Control Study. Clin. Hypertens 2020, 26, 16. [Google Scholar] [CrossRef]
- Malik, A.; Jee, B.; Gupta, S.K. Preeclampsia: Disease Biology and Burden, Its Management Strategies with Reference to India. Pregnancy Hypertens 2019, 15, 23–31. [Google Scholar] [CrossRef]
- Bokslag, A.; van Weissenbruch, M.; Mol, B.W.; de Groot, C.J.M. Preeclampsia; Short and Long-Term Consequences for Mother and Neonate. Early Hum. Dev. 2016, 102, 47–50. [Google Scholar] [CrossRef]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-Eclampsia: Pathogenesis, Novel Diagnostics and Therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef]
- Serrano, N.C.; Quintero-Lesmes, D.C.; Dudbridge, F.; Leon, L.J.; Hingorani, A.D.; Williams, D.J.; Casas, J.P. Family History of Pre-Eclampsia and Cardiovascular Disease as Risk Factors for Pre-Eclampsia: The GenPE Case-Control Study. Hypertens Pregnancy 2020, 39, 56–63. [Google Scholar] [CrossRef]
- Gray, K.J.; Saxena, R.; Karumanchi, S.A. Genetic Predisposition to Preeclampsia Is Conferred by Fetal DNA Variants near FLT1, a Gene Involved in the Regulation of Angiogenesis. Am. J. Obs. Gynecol. 2018, 218, 211–218. [Google Scholar] [CrossRef]
- Smith, C.J.; Saftlas, A.F.; Spracklen, C.N.; Triche, E.W.; Bjonnes, A.; Keating, B.; Saxena, R.; Breheny, P.J.; Dewan, A.T.; Robinson, J.G.; et al. Genetic Risk Score for Essential Hypertension and Risk of Preeclampsia. Am. J. Hypertens 2016, 29, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Kay, V.R.; Wedel, N.; Smith, G.N. Family History of Hypertension, Cardiovascular Disease, or Diabetes and Risk of Developing Preeclampsia: A Systematic Review. J. Obs. Gynaecol. Can. 2021, 43, 227–236.e19. [Google Scholar] [CrossRef]
- Qiu, C.; Williams, M.A.; Leisenring, W.M.; Sorensen, T.K.; Frederick, I.O.; Dempsey, J.C.; Luthy, D.A. Family History of Hypertension and Type 2 Diabetes in Relation to Preeclampsia Risk. Hypertension 2003, 41, 408–413. [Google Scholar] [CrossRef] [Green Version]
- Rigó, J.; Boze, T.; Derzsy, Z.; Derzbach, L.; Treszl, A.; Lázár, L.; Sobel, G.; Vásárhelyi, B. Family History of Early-Onset Cardiovascular Disorders Is Associated with a Higher Risk of Severe Preeclampsia. Eur J. Obs. Gynecol. Reprod. Biol. 2006, 128, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Guerrier, G.; Oluyide, B.; Keramarou, M.; Grais, R.F. Factors Associated with Severe Preeclampsia and Eclampsia in Jahun, Nigeria. Int. J. Womens Health 2013, 5, 509–513. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Tan, H.; Huang, X.; Zhou, S.; Hu, S.; Wang, X.; Xu, X.; Liu, Q.; Wen, S.W. Similarities and Differences between the Risk Factors for Gestational Hypertension and Preeclampsia: A Population Based Cohort Study in South China. Pregnancy Hypertens 2016, 6, 66–71. [Google Scholar] [CrossRef]
- Martinez-Fierro, M.L.; Castruita-De La Rosa, C.; Garza-Veloz, I.; Cardiel-Hernandez, R.M.; Espinoza-Juarez, M.A.; Delgado-Enciso, I.; Castañeda-Lopez, M.E.; Cardenas-Vargas, E.; Trejo-Vázquez, F.; Sotelo-Ham, E.I.; et al. Early Pregnancy Protein Multiplex Screening Reflects Circulating and Urinary Divergences Associated with the Development of Preeclampsia. Hypertens Pregnancy 2018, 37, 37–50. [Google Scholar] [CrossRef]
- Sanchez, S.E.; Zhang, C.; Qiu, C.-F.; Williams, M.A. Family History of Hypertension and Diabetes in Relation to Preeclampsia Risk in Peruvian Women. Gynecol. Obs. Invest. 2003, 56, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Luealon, P.; Phupong, V. Risk Factors of Preeclampsia in Thai Women. J. Med. Assoc. Thai. 2010, 93, 661–666. [Google Scholar]
- Sun, F.; Qian, W.; Zhang, C.; Fan, J.-X.; Huang, H.-F. Correlation of Maternal Serum Homocysteine in the First Trimester with the Development of Gestational Hypertension and Preeclampsia. Med. Sci. Monit. 2017, 23, 5396–5401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewandowska, M.; Więckowska, B. The Influence of Various Smoking Categories on The Risk of Gestational Hypertension and Pre-Eclampsia. J. Clin. Med. 2020, 9, 1743. [Google Scholar] [CrossRef]
- Klonoff-Cohen, H.S.; Cross, J.L.; Pieper, C.F. Job Stress and Preeclampsia. Epidemiology 1996, 7, 245–249. [Google Scholar] [CrossRef]
- Voerman, E.; Santos, S.; Inskip, H.; Amiano, P.; Barros, H.; Charles, M.-A.; Chatzi, L.; Chrousos, G.P.; Corpeleijn, E.; Crozier, S.; et al. Association of Gestational Weight Gain with Adverse Maternal and Infant Outcomes. JAMA 2019, 321, 1702–1715. [Google Scholar] [CrossRef] [Green Version]
- Radoń-Pokracka, M.; Adrianowicz, B.; Płonka, M.; Danił, P.; Nowak, M.; Huras, H. Evaluation of Pregnancy Outcomes at Advanced Maternal Age. Open Access Maced. J. Med. Sci. 2019, 7, 1951–1956. [Google Scholar] [CrossRef] [Green Version]
- England, L.; Zhang, J. Smoking and Risk of Preeclampsia: A Systematic Review. Front. Biosci. 2007, 12, 2471–2483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laule, C.F.; Wing, C.R.; Odean, E.J.; Wilcox, J.A.; Gilbert, J.S.; Regal, J.F. Effect of Nicotine on Placental Ischemia-Induced Complement Activation and Hypertension in the Rat. J. Immunotoxicol. 2017, 14, 235–240. [Google Scholar] [CrossRef] [Green Version]
- Lewandowska, M.; Więckowska, B.; Sajdak, S.; Lubiński, J. Pre-Pregnancy Obesity vs. Other Risk Factors in Probability Models of Preeclampsia and Gestational Hypertension. Nutrients 2020, 12, 2681. [Google Scholar] [CrossRef]
- Shen, M.; Smith, G.N.; Rodger, M.; White, R.R.; Walker, M.C.; Wen, S.W. Comparison of Risk Factors and Outcomes of Gestational Hypertension and Pre-Eclampsia. PLoS ONE 2017, 12, e0175914. [Google Scholar] [CrossRef] [Green Version]
- Shiozaki, A.; Matsuda, Y.; Satoh, S.; Saito, S. Comparison of Risk Factors for Gestational Hypertension and Preeclampsia in Japanese Singleton Pregnancies. J. Obs. Gynaecol. Res. 2013, 39, 492–499. [Google Scholar] [CrossRef]
- Hromadnikova, I.; Dvorakova, L.; Kotlabova, K.; Krofta, L. The Prediction of Gestational Hypertension, Preeclampsia and Fetal Growth Restriction via the First Trimester Screening of Plasma Exosomal C19MC MicroRNAs. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Gray, K.J.; Kovacheva, V.P.; Mirzakhani, H.; Bjonnes, A.C.; Almoguera, B.; DeWan, A.T.; Triche, E.W.; Saftlas, A.F.; Hoh, J.; Bodian, D.L.; et al. Gene-Centric Analysis of Preeclampsia Identifies Maternal Association at PLEKHG1. Hypertension 2018, 72, 408–416. [Google Scholar] [CrossRef]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of Oxidative Stress in the Dysfunction of the Placental Endothelial Nitric Oxide Synthase in Preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef]
- Sufriyana, H.; Wu, Y.-W.; Su, E.C.-Y. Prediction of Preeclampsia and Intrauterine Growth Restriction: Development of Machine Learning Models on a Prospective Cohort. JMIR Med. Inf. 2020, 8, e15411. [Google Scholar] [CrossRef] [PubMed]
- Kaartokallio, T.; Lokki, A.I.; Peterson, H.; Kivinen, K.; Hiltunen, L.; Salmela, E.; Lappalainen, T.; Maanselkä, P.; Heino, S.; Knuutila, S.; et al. Preeclampsia Does Not Share Common Risk Alleles in 9p21 with Coronary Artery Disease and Type 2 Diabetes. Ann. Med. 2016, 48, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Vanderlelie, J.J.; Perkins, A.V.; Redman, C.W.G.; Ahmadi, K.R.; Rayman, M.P. Genetic Polymorphisms That Affect Selenium Status and Response to Selenium Supplementation in United Kingdom Pregnant Women. Am. J. Clin. Nutr. 2016, 103, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhu, M.; Hu, R.; Yan, W. The Effects of Gene Polymorphisms in Angiotensin II Receptors on Pregnancy-Induced Hypertension and Preeclampsia: A Systematic Review and Meta-Analysis. Hypertens Pregnancy 2015, 34, 241–260. [Google Scholar] [CrossRef]
| Variates | Definitions and Categories | Description |
|---|---|---|
| Pre-pregnancy BMI | (the quotient of pre-pregnancy weight (kg) and height (meters) squared) BMI was assessed as a continuous variable (as a covariate); BMI was assessed in the 5 following categories (as subgroups): (1) underweight (<18.5); (2) normal weight (18.5–24.9); (3) overweight (25.0–29.9); (4) obesity (≥30); and (5) BMI ≥ 25 kg/m2. | Self-reported |
| GWG | (the difference between the weight before childbirth and the weight before pregnancy) GWG was assessed in the three following categories, regardless of BMI category (according to the Institute of Medicine recommendations from 2009): (1) GWG above the range; (2) GWG in the range; (3) GWG below the range. GWG out of the range was a covariate. | From medical reports |
| Maternal age | (defined as completed maternal age at conception, in years) Maternal age was assessed as a continuous variable (as a covariate) | From medical reports |
| Primiparity | Parity was assessed in the two following categories: (1) primiparity (zero prior delivery); (2) multiparity (i.e., ≥1 prior deliveries). Primiparity was a covariate. | From medical reports |
| Smoking | Smoking was assessed in the three following categories: (1) mothers who had never smoked; (2) smokers (smoked before pregnancy); (3) smokers in the first trimester. Smokers in the first trimester was a covariate. The three smoking categories was assessed as subgroups. | Self-reported |
| Prior PIH | Mothers with pregnancy-induced hypertension (gestational hypertension or preeclampsia, GH/PE) in previous pregnancies. Prior PIH was assessed in the two following categories: (1) prior PIH; (2) no prior PIH. Prior PIH was a covariate. | From medical reports |
| Infertility treatment | (Different methods of infertility treatment, covering in vitro fertilization and other methods of assisting reproduction) Infertility treatment was assessed in the two following categories: (1) infertility treatment; (2) no infertility treatment. Infertility treatment was a covariate. | From medical reports |
| Education | Education level was assessed in the following categories: (1) ≥12 years of education (secondary education and tertiary education); (2) <12 years of education (primary and vocational education) | From medical reports |
| Financial status | Financial status was assessed on the 5-point Likert scale. The basis of assessment was the question ‘Is your household’s financial status sufficient for your needs?’ and the 5 following answers: ‘1—definitely No’; ‘2—rather No’; ‘3—hard to say’; ‘4—rather Yes’; ‘5—definitely Yes’. Financial status was assessed in the following categories: (1) Lower financial status (the answers/levels 1–3); and (2) Higher financial status (the answers/levels 4–5). | Self-reported |
| Maternal Characteristics ** | Normotensive Group (n = 775) | PIH Group (n = 137) | p * |
|---|---|---|---|
| Mean (SD), n (%) | Mean (SD), n (%) | ||
| Basic characteristics | |||
| Maternal age (years) | 33.5 (4.8) | 34.8 (4.4) | 0.005 |
| Pre-pregnancy BMI (kg/m2) | 23.3 (4.1) | 26.7 (5.4) | <0.001 |
| GWG (kg) | 13.4 (5.3) | 14.7 (8.0) | 0.107 |
| Primiparous women | 318 (41.0%) | 65 (47.5%) | 0.161 |
| Infertility treatment | 29 (3.7%) | 8 (7.1%) | 0.097 |
| Smokers in the 1st tr. | 37 (4.8%) | 20 (14.6%) | <0.001 |
| Prior GH/PE | 4 (0.5%) | 15 (11.0%) | <0.001 |
| GH/PE in the mother or sister | 4 (0.5%) | 6 (4.4%) | <0.001 |
| Chronic diseases in family | |||
| Hypertension (H): | |||
| In the mother | 137 (17.8%) | 42 (30.9%) | <0.001 |
| In the father | 135 (17.5%) | 38 (27.9%) | 0.004 |
| In the grandmother(s) | 47 (6.1%) | 5 (3.7%) | 0.263 |
| In the grandfather(s) | 20 (2.6%) | 5 (3.7%) | 0.407 |
| - Absence of H in the parents | 541 (70.2%) | 72 (52.9%) | <0.001 |
| - Absence of H in the family | 496 (64.3%) | 69 (50.7%) | 0.003 |
| Diabetes: | |||
| In the mother | 55 (7.1%) | 15 (11.0%) | 0.117 |
| In the father | 90 (11.7%) | 20 (14.7%) | 0.318 |
| Pregnancy outcomes | |||
| Fetal sex—male | 405 (52.3%) | 68 (49.6%) | 0.571 |
| Gestational age (weeks) | 38.8 (1.6) | 37.7 (2.8) | <0.001 |
| Birth weight (grams) | 3416.5 (511.7) | 3020.0 (838.0) | <0.001 |
| GDM cases | 121 (15.6%) | 25 (18.3%) | 0.438 |
| SABP (mmHg) *** | 107.9 (10.8) | 159.5 (18.2) | <0.001 |
| DABP (mmHg) *** | 66.8 (8.8) | 100.9 (11.0) | <0.001 |
| Basic Risk Factors | GH Risk | PE Risk |
|---|---|---|
| AOR-a (95% CI); p * | AOR-a (95% CI); p * | |
| Pre-pregnancy BMI (kg/m2): | ||
| Obesity (≥30) | 4.72 (2.73–8.14); <0.001 | 8.68 (3.30–23.20); <0.001 |
| Overweight (25.0–29.9) | 2.04 (1.23–3.37); 0.005 | 1.68 (0.50–5.63); 0.397 |
| Underweight (<18.5) | 0.23 (0.03–1.69); 0.148 | 2.56 (0.52–12.62); 0.248 |
| Normal BMI (18.5–24.9) | 1 | 1 |
| Smoking in the 1st tr. | 3.51 (1.79–6.89); <0.001 | 2.79 (0.76–10.27); 0.124 |
| Smoking (ever) | 1.64 (1.01–2.66); 0.044 | 0.88 (0.29–2.65); 0.817 |
| Never smoked | 1 | 1 |
| GWG above the range | 1.90 (1.15–3.12); 0.012 | 1.15 (0.44–3.00); 0.770 |
| GWG below the range | 1.09 (0.59–2.01); 0.779 | 0.84 (0.26–2.67); 0.768 |
| GWG in the range | 1 | 1 |
| Maternal age (years): | ||
| ≥40 | 2.97 (1.2–7.32) 0.018 | 0.94 (0.08–11.6); 0.963 |
| 18–24 | 0.51 (0.1–2.53) 0.413 | 3.26 (0.42–25.0); 0.256 |
| 25–29 | 1 | 1 |
| Prior GH/PE | ||
| Yes | 37.98 (11.16–129); <0.001 | 31.11 (5.83–166.0); <0.001 |
| No | 1 | 1 |
| Primiparity | 1.90 (1.22–2.96); 0.005 | 1.55 (0.64–3.77); 0.332 |
| Multiparity | 1 | 1 |
| Education <12 years | 2.76 (1.48–5.15); 0.001 | 5.44 (2.01–14.71); 0.001 |
| Education ≥12 years | 1 | 1 |
| Lower financial status ** | 2.53 (1.56–4.12); <0.001 | 3.64 (1.51–8.76); 0.004 |
| Higher financial status ** | 1 | 1 |
| Infertility treatment | ||
| Yes | 1.65 (0.68–4.01); 0.269 | 4.29 (1.06–17.39); 0.041 |
| No | 1 | 1 |
| Risk Factors/Hypertension in the Family | Cases/Controls | OR (95% CI); p | AOR-a (95% CI); p * | AOR-b (95% CI); p * |
|---|---|---|---|---|
| GH Risk | ||||
| In the mother | 31/137 | 1.90 (1.18–3.06); 0.008 | 1.51 (0.90–2.52); 0.117 | 1.34 (0.79–2.29); 0.279 |
| In the father | 33/135 | 2.06 (1.29–3.28); 0.002 | 1.98 (1.20–3.28); 0.008 | 1.88 (1.12–3.17); 0.017 |
| In the mother or father | 51/230 | 1.86 (1.24–2.8); 0.003 | 1.66 (1.07–2.55); 0.022 | 1.53 (0.98–2.39); 0.063 |
| In the mother and father ** | 13/42 | 2.60 (1.32–5.13); 0.006 | 2.18 (1.04–4.58); 0.039 | 1.98 (0.91–4.30); 0.084 |
| Ref *** | 59/496 | 1 | 1 | 1 |
| PE Risk | ||||
| In the mother | 11/137 | 3.98 (1.66–9.57); 0.002 | 3.26 (1.30–8.16); 0.012 | 3.09 (1.20–7.98); 0.020 |
| In the father | 5/135 | 1.84 (0.62–5.47); 0.274 | 1.60 (0.52–4.90); 0.412 | 1.40 (0.41–4.74); 0.592 |
| In the mother or father | 13/230 | 2.80 (1.21–6.49); 0.016 | 2.34 (0.99–5.55); 0.054 | 2.14 (0.87–5.29); 0.098 |
| In the mother and father ** | 3/42 | 3.54 (0.94–13.37); 0.062 | 2.81 (0.68–11.53); 0.152 | 2.59 (0.58–11.56); 0.212 |
| Ref *** | 10/496 | 1 | 1 | 1 |
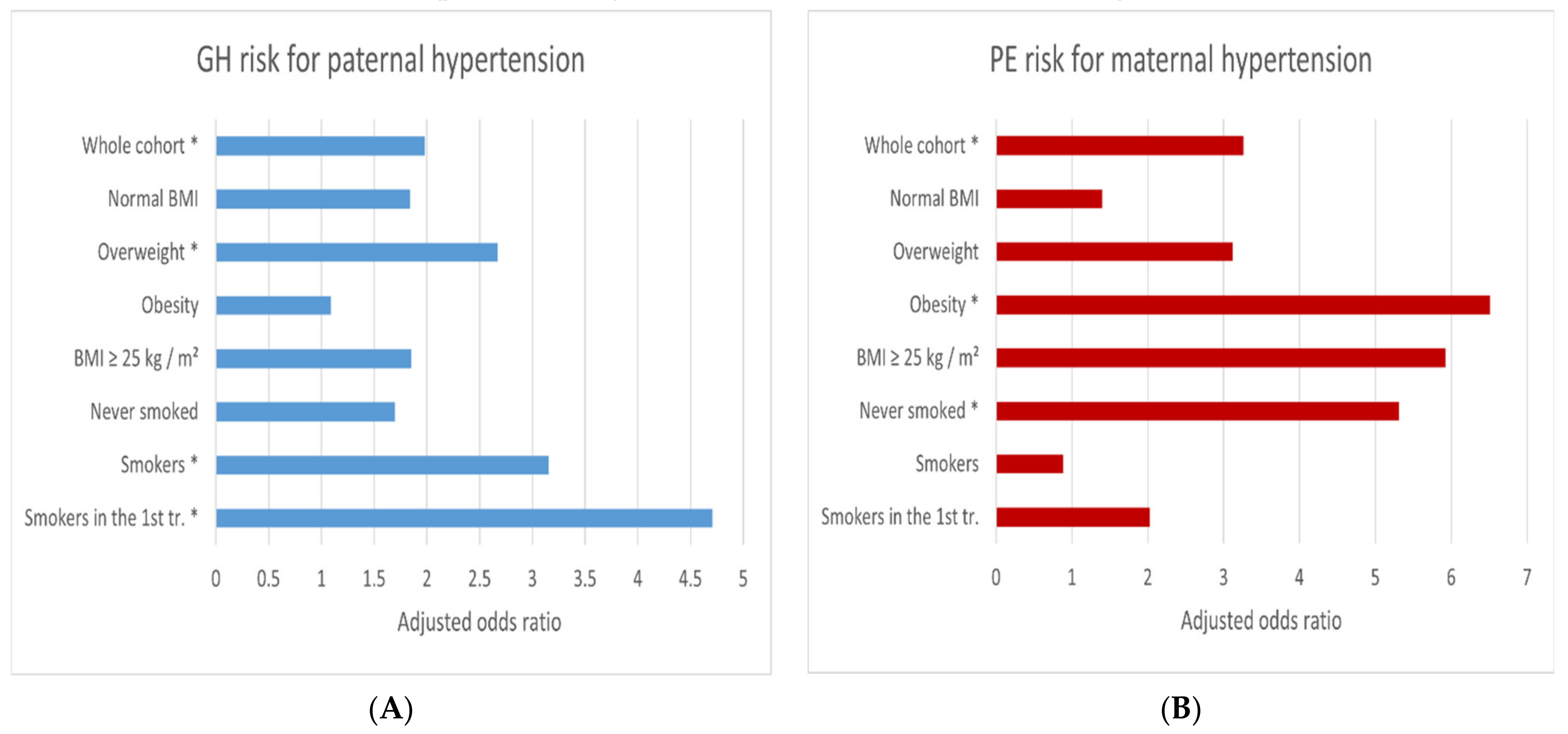
| Risk Factors/Hypertension in the Parents | Cases/Controls | GH Risk | Cases/Controls | PE Risk |
|---|---|---|---|---|
| AOR-a (95% CI); p * | AOR-a (95% CI); p * | |||
| Whole cohort | ||||
| In the mother | 31/137 | 1.51 (0.90–2.52); 0.117 | 11/137 | 3.26 (1.30–8.16); 0.012 |
| In the father | 33/135 | 1.98 (1.20–3.28); 0.008 | 5/135 | 1.60 (0.52–4.9); 0.412 |
| Ref ** | 59/496 | 1 | 10/496 | 1 |
| Normal BMI | ||||
| In the mother | 10/90 | 1.23 (0.56–2.69); 0.612 | 2/90 | 1.40 (0.26–7.60); 0.699 |
| In the father | 15/92 | 1.84 (0.92–3.67); 0.083 | 1/92 | 0.70 (0.08–6.34); 0.753 |
| Ref ** | 29/351 | 1 | 5/351 | 1 |
| Underweight | ||||
| In the mother | 0/6 | - | 1/6 | - |
| In the father | 1/6 | - | 1/6 | - |
| Ref ** | 0/30 | 1 | 1/30 | 1 |
| Overweight | ||||
| In the mother | 8/25 | 1.87 (0.69–5.11); 0.221 | 2/25 | 3.12 (0.37–26.42); 0.296 |
| In the father | 11/26 | 2.67 (1.02–7.02); 0.046 | 0/26 | - |
| Ref ** | 14/87 | 1 | 2/87 | 1 |
| Obesity | ||||
| In the mother | 13/16 | 1.60 (0.59–4.35); 0.359 | 6/16 | 6.51 (1.05–40.25); 0.044 |
| In the father | 6/11 | 1.09 (0.31–3.83); 0.894 | 3/11 | 3.57 (0.48–26.5); 0.214 |
| Ref ** | 16/28 | 1 | 2/28 | 1 |
| Never smoked | ||||
| In the mother | 23/104 | 1.87 (1.05–3.34); 0.034 | 10/104 | 5.31 (1.91–14.8); 0.001 |
| In the father | 21/114 | 1.70 (0.94–3.08); 0.079 | 4/114 | 1.99 (0.56–7.07); 0.289 |
| Ref ** | 46/421 | 1 | 7/421 | 1 |
| Smokers | ||||
| In the mother | 8/33 | 0.60 (0.19–1.96); 0.400 | 1/33 | 0.88 (0.07–11.38); 0.921 |
| In the father | 12/21 | 3.15 (1.16–8.54); 0.024 | 1/21 | 1.73 (0.13–23.57); 0.682 |
| Ref ** | 13/75 | 1 | 3/75 | 1 |
| Smokers in the 1st tr. | ||||
| In the mother | 2/8 | 0.39 (0.05–3.43); 0.398 | 1/8 | 2.03 (0.09–44.27); 0.653 |
| In the father | 7/5 | 4.71 (1.01–21.96); 0.048 | 1/5 | 6.32 (0.14–276.9); 0.339 |
| Ref ** | 8/27 | 1 | 2/27 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, M. The Association of Familial Hypertension and Risk of Gestational Hypertension and Preeclampsia. Int. J. Environ. Res. Public Health 2021, 18, 7045. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18137045
Lewandowska M. The Association of Familial Hypertension and Risk of Gestational Hypertension and Preeclampsia. International Journal of Environmental Research and Public Health. 2021; 18(13):7045. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18137045
Chicago/Turabian StyleLewandowska, Małgorzata. 2021. "The Association of Familial Hypertension and Risk of Gestational Hypertension and Preeclampsia" International Journal of Environmental Research and Public Health 18, no. 13: 7045. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18137045






