Mortality Associated with Idiopathic Pulmonary Fibrosis in Northeastern Italy, 2008–2020: A Multiple Cause of Death Analysis
Abstract
:1. Introduction
2. Methods
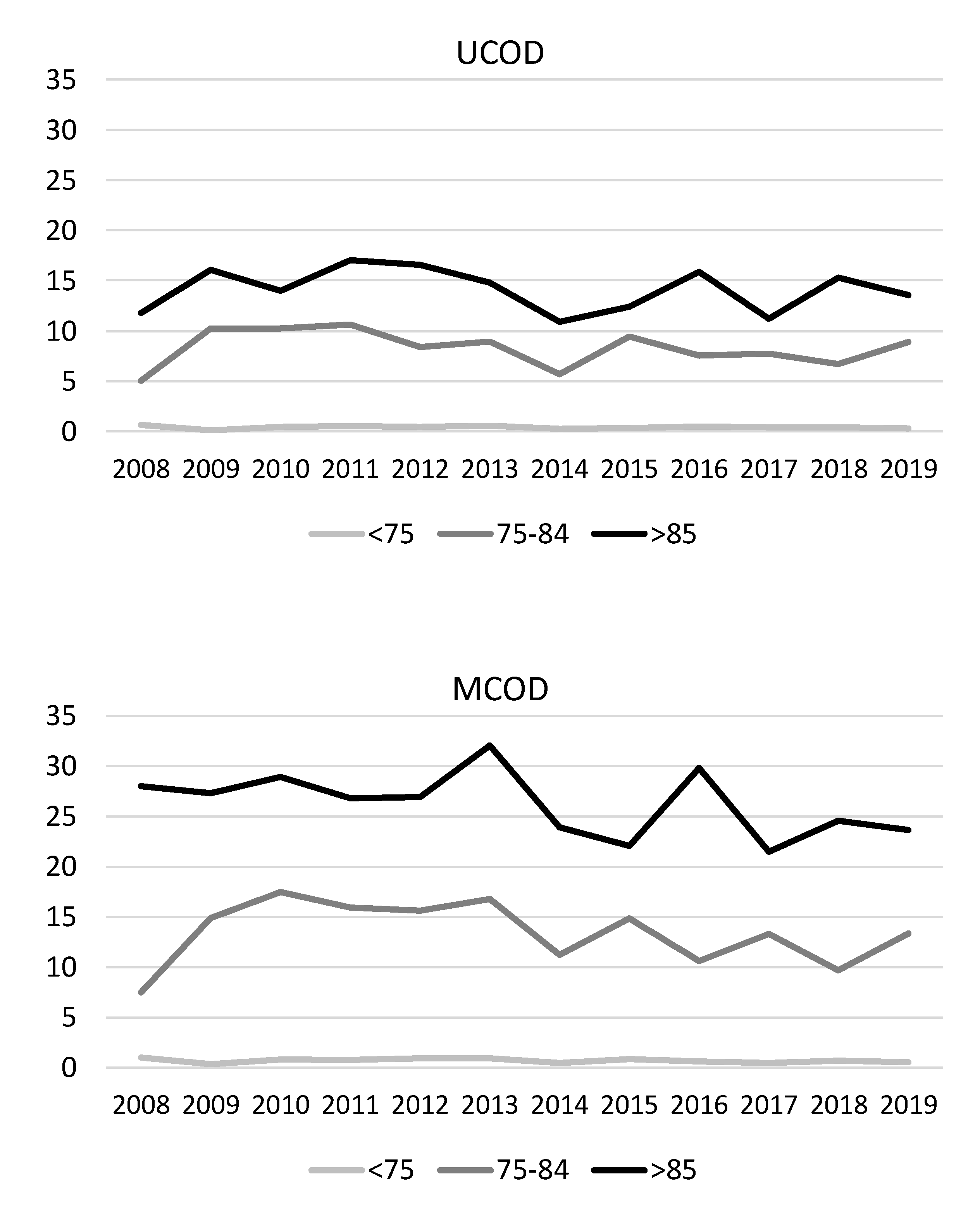
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lederer, D.J.; Martinez, F.J. Idiopathic Pulmonary Fibrosis. N. Engl. J. Med. 2018, 378, 1811–1823. [Google Scholar] [CrossRef] [PubMed]
- Olson, A.L.; Swigris, J.J.; Lezotte, D.C.; Norris, J.M.; Wilson, C.G.; Brown, K.K. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am. J. Respir. Crit. Care Med. 2007, 176, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Richeldi, L.; Collard, H.R.; Jones, M.G. Idiopathic pulmonary fibrosis. Lancet 2017, 389, 1941–1952. [Google Scholar] [CrossRef]
- Strongman, H.; Kausar, I.; Maher, T.M. Incidence, Prevalence, and Survival of Patients with Idiopathic Pulmonary Fibrosis in the UK. Adv. Ther. 2018, 35, 724–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, B.; Collard, H.R.; King, T.E., Jr. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 431440. [Google Scholar] [CrossRef]
- Marshall, D.C.; Salciccioli, J.D.; Shea, B.S.; Akuthota, P. Trends in mortality from idiopathic pulmonary fibrosis in the European Union: An observational study of the WHO mortality database from 2001–2013. Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, J.; Fogarty, A.; Hubbard, R.; McKeever, T. Global incidence and mortality of idiopathic pulmonary fibrosis: A systematic review. Eur. Respir. J. 2015, 46, 795–806. [Google Scholar] [CrossRef] [Green Version]
- Marcon, A.; Saugo, M.; Fedeli, U. COPD-Related Mortality and Co-morbidities in Northeastern Italy, 2008–2012: A Multiple Causes of Death Analysis. J. Chronic Obstr. Pulm. Dis. 2016, 13, 35–41. [Google Scholar] [CrossRef]
- Lee, H.; Choi, H.; Yang, B.; Lee, S.K.; Park, T.S.; Park, D.W.; Moon, J.Y.; Kim, T.H.; Sohn, J.W.; Yoon, H.J.; et al. Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur. Respir. J. 2021. [Google Scholar] [CrossRef]
- Hutchinson, J.P.; McKeever, T.M.; Fogarty, A.W.; Navaratnam, V.; Hubbard, R.B. Increasing global mortality from idiopathic pulmonary fibrosis in the twenty-first century. Ann. Am. Thorac. Soc. 2014, 11, 1176–1185. [Google Scholar] [CrossRef]
- Mannino, D.M.; Etzel, R.A.; Parrish, R.G. Pulmonary fibrosis deaths in the United States, 1979–1991. An analysis of multiple-cause mortality data. Am. J. Respir. Crit. Care Med. 1996, 153, 1548–1552. [Google Scholar] [CrossRef]
- Fernandez Perez, E.R. Changing trends in age-adjusted pulmonary fibrosis mortality in the USA: A joinpoint regression analysis. Eur. Respir. J. 2019, 54. [Google Scholar] [CrossRef]
- Jeganathan, N.; Smith, R.A.; Sathananthan, M. Mortality Trends of Idiopathic Pulmonary Fibrosis in the United States From 2004 Through 2017. Chest 2021, 159, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.H.; Anderson, R.N.; Kawachi, I. Trends in frequency of reporting improper diabetes-related cause-of-death statements on death certificates, 1985-2005: An algorithm to identify incorrect causal sequences. Am. J. Epidemiol. 2010, 171, 1069–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orsi, C.; Navarra, S.; Frova, L.; Grande, E.; Marchetti, S.; Pappagallo, M.; Grippo, F. Impact of the implementation of ICD-10 2016 version and Iris software on mortality statistics in Italy. Epidemiol. Prev. 2019, 43, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Pitter, G.; Da Re, F.; Tonon, M.; Avossa, F.; Bellio, S.; Fedeli, U.; Gubian, L.; Monetti, D.; Saia, M.; et al. Epidemiology and public health response in early phase of COVID-19 pandemic, Veneto Region, Italy, 21 February to 2 April 2020. Eurosurveillance 2020, 25. [Google Scholar] [CrossRef]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Navaratnam, V.; Fleming, K.M.; West, J.; Smith, C.J.; Jenkins, R.G.; Fogarty, A.; Hubbard, R.B. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax 2011, 66, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Agabiti, N.; Porretta, M.A.; Bauleo, L.; Coppola, A.; Sergiacomi, G.; Fusco, A.; Cavalli, F.; Zappa, M.C.; Vignarola, R.; Carlone, S.; et al. Idiopathic Pulmonary Fibrosis (IPF) incidence and prevalence in Italy. Sarcoidosis Vasc. Diffus. Lung Dis. 2014, 31, 191–197. [Google Scholar]
- Algranti, E.; Saito, C.A.; Silva, D.; Carneiro, A.P.S.; Bussacos, M.A. Mortality from idiopathic pulmonary fibrosis: A temporal trend analysis in Brazil, 1979–2014. J. Bras. Pneumol. 2017, 43, 445–450. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Collard, H.R.; Raghu, G.; Sweet, M.P.; Hays, S.R.; Campos, G.M.; Golden, J.A.; King, T.E., Jr. Does chronic microaspiration cause idiopathic pulmonary fibrosis? Am. J. Med. 2010, 123, 304–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harari, S.; Davi, M.; Biffi, A.; Caminati, A.; Ghirardini, A.; Lovato, V.; Cricelli, C.; Lapi, F. Epidemiology of idiopathic pulmonary fibrosis: A population-based study in primary care. Intern. Emerg. Med. 2020, 15, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Drake, T.M.; Docherty, A.B.; Harrison, E.M.; Quint, J.K.; Adamali, H.; Agnew, S.; Babu, S.; Barber, C.M.; Barratt, S.; Bendstrup, E.; et al. Outcome of Hospitalization for COVID-19 in Patients with Interstitial Lung Disease. An International Multicenter Study. Am. J. Respir. Crit. Care Med. 2020, 202, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Aveyard, P.; Gao, M.; Lindson, N.; Hartmann-Boyce, J.; Watkinson, P.; Young, D.; Coupland, C.A.C.; Tan, P.S.; Clift, A.K.; Harrison, D.; et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: A population cohort study. Lancet Respir. Med. 2021. [Google Scholar] [CrossRef]
- Mohamed, M.O.; Gale, C.P.; Kontopantelis, E.; Doran, T.; de Belder, M.; Asaria, M.; Luscher, T.; Wu, J.; Rashid, M.; Stephenson, C.; et al. Sex Differences in Mortality Rates and Underlying Conditions for COVID-19 Deaths in England and Wales. Mayo Clin. Proc. 2020, 95, 2110–2124. [Google Scholar] [CrossRef]
- Woolf, S.H.; Chapman, D.A.; Lee, J.H. COVID-19 as the Leading Cause of Death in the United States. JAMA 2021, 325, 123–124. [Google Scholar] [CrossRef]


| Number of Deaths | Proportional Mortality | Rate per 100,000/Year | |||||
|---|---|---|---|---|---|---|---|
| Age Class | UCOD | MCOD | UCOD | MCOD | UCOD | MCOD | |
| Males | <45 | 7 | 11 | 0.1% | 0.1% | 0.0 | 0.1 |
| 45–49 | 2 | 3 | 0.0% | 0.1% | 0.1 | 0.1 | |
| 50–54 | 7 | 12 | 0.1% | 0.2% | 0.3 | 0.5 | |
| 55–59 | 13 | 23 | 0.1% | 0.3% | 0.7 | 1.2 | |
| 60–64 | 42 | 64 | 0.3% | 0.5% | 2.4 | 3.7 | |
| 65–69 | 62 | 100 | 0.3% | 0.5% | 3.9 | 6.3 | |
| 70–74 | 123 | 202 | 0.4% | 0.7% | 9.0 | 14.7 | |
| 75–79 | 176 | 295 | 0.4% | 0.7% | 16.3 | 27.2 | |
| 80–84 | 177 | 331 | 0.4% | 0.7% | 24.7 | 46.2 | |
| 85–89 | 146 | 238 | 0.3% | 0.5% | 40.0 | 65.2 | |
| 90+ | 45 | 87 | 0.1% | 0.2% | 33.0 | 63.9 | |
| Overall | 800 | 1366 | 0.3% | 0.5% | 2.8 | 4.8 | |
| Females | <45 | 4 | 11 | 0.1% | 0.3% | 0.0 | 0.1 |
| 45–49 | 2 | 5 | 0.1% | 0.2% | 0.1 | 0.2 | |
| 50–54 | 9 | 12 | 0.2% | 0.3% | 0.4 | 0.5 | |
| 55–59 | 13 | 18 | 0.3% | 0.4% | 0.7 | 0.9 | |
| 60–64 | 11 | 25 | 0.1% | 0.3% | 0.6 | 1.4 | |
| 65–69 | 22 | 37 | 0.2% | 0.3% | 1.3 | 2.2 | |
| 70–74 | 54 | 92 | 0.3% | 0.5% | 3.4 | 5.8 | |
| 75–79 | 101 | 160 | 0.4% | 0.6% | 7.1 | 11.3 | |
| 80–84 | 117 | 194 | 0.2% | 0.4% | 10.1 | 16.7 | |
| 85–89 | 117 | 226 | 0.2% | 0.3% | 14.5 | 28.1 | |
| 90+ | 60 | 105 | 0.1% | 0.1% | 13.2 | 23.1 | |
| Overall | 510 | 885 | 0.2% | 0.3% | 1.7 | 3.0 | |
| Condition (ICD10) | Number of Deaths | % of All Deaths | ||||
|---|---|---|---|---|---|---|
| Overall | Males | Females | Overall | Males | Females | |
| IPF (J84.1) | 1310 | 800 | 510 | 58.2% | 58.6% | 57.6% |
| COPD (J40–J44, J47) | 87 | 62 | 25 | 3.9% | 4.5% | 2.8% |
| Other respiratory diseases (J00–J99) | 50 | 28 | 22 | 2.2% | 2.0% | 2.5% |
| Lung cancer (C33–C34) | 87 | 71 | 16 | 3.9% | 5.2% | 1.8% |
| Other neoplasms (C00–D48) | 103 | 67 | 36 | 4.6% | 4.9% | 4.1% |
| Ischemic heart diseases (I20–I25) | 149 | 106 | 43 | 6.6% | 7.8% | 4.9% |
| Other circulatory diseases (I00–I99) | 187 | 110 | 77 | 8.3% | 8.1% | 8.7% |
| Digestive diseases (K00–K99) | 30 | 14 | 16 | 1.3% | 1.0% | 1.8% |
| Conditions causally related to pulmonary fibrosis (M05–M08, M32–M36, D86, J60–J65, J67, J70.1) | 104 | 35 | 69 | 4.6% | 2.6% | 7.8% |
| Other diseases | 144 | 73 | 71 | 6.4% | 5.3% | 8.0% |
| Overall | 2251 | 1366 | 885 | 100% | 100% | 100% |
| Condition (ICD10) | Deaths with IPF (n = 2251) | Deaths without IPF (n = 555,681) | Odds Ratio (95% Confidence Interval) |
|---|---|---|---|
| Pulmonary hypertension (I27) | 12.5% | 0.8% | 15.7 (13.8–17.9) |
| Congestive heart failure (I50.0) | 2.5% | 1.8% | 1.5 (1.2–2.0) |
| Influenza, pneumonia (J09–J18) | 15.3% | 10.2% | 1.6 (1.5–1.8) |
| Pulmonary embolism (I26.x) | 2.8% | 2.0% | 1.4 (1.0–1.7) |
| COPD (J40–J44, J47) | 12.9% | 7.2% | 1.8 (1.6–2.0) |
| Esophagitis/reflux (K20, K21) | 0.3% | 0.1% | 2.9 (1.3–6.5) |
| Conditions causally related to pulmonary fibrosis (M05–M08, M32–M36, D86, J60–J65, J67, J70.1) | 8.0% | 0.8% | 9.8 (8.3–11.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcon, A.; Schievano, E.; Fedeli, U. Mortality Associated with Idiopathic Pulmonary Fibrosis in Northeastern Italy, 2008–2020: A Multiple Cause of Death Analysis. Int. J. Environ. Res. Public Health 2021, 18, 7249. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18147249
Marcon A, Schievano E, Fedeli U. Mortality Associated with Idiopathic Pulmonary Fibrosis in Northeastern Italy, 2008–2020: A Multiple Cause of Death Analysis. International Journal of Environmental Research and Public Health. 2021; 18(14):7249. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18147249
Chicago/Turabian StyleMarcon, Alessandro, Elena Schievano, and Ugo Fedeli. 2021. "Mortality Associated with Idiopathic Pulmonary Fibrosis in Northeastern Italy, 2008–2020: A Multiple Cause of Death Analysis" International Journal of Environmental Research and Public Health 18, no. 14: 7249. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18147249






