Effects of Natural Ventilation and Saliva Standard Ejectors during the COVID-19 Pandemic: A Quantitative Analysis of Aerosol Produced during Dental Procedures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
- The effects of natural ventilation on the reduction of PM in the dental unit
- The effectiveness of low-volume suction (40 L/min air) in reducing PM concentrations during dental procedures
- The difference in terms of PM between the scaling procedures and the other procedures performed. This discriminant was selected following previous literature and guidelines that described ultrasonic scaling as the dental activity that produces the greatest amount of airborne contamination [24].
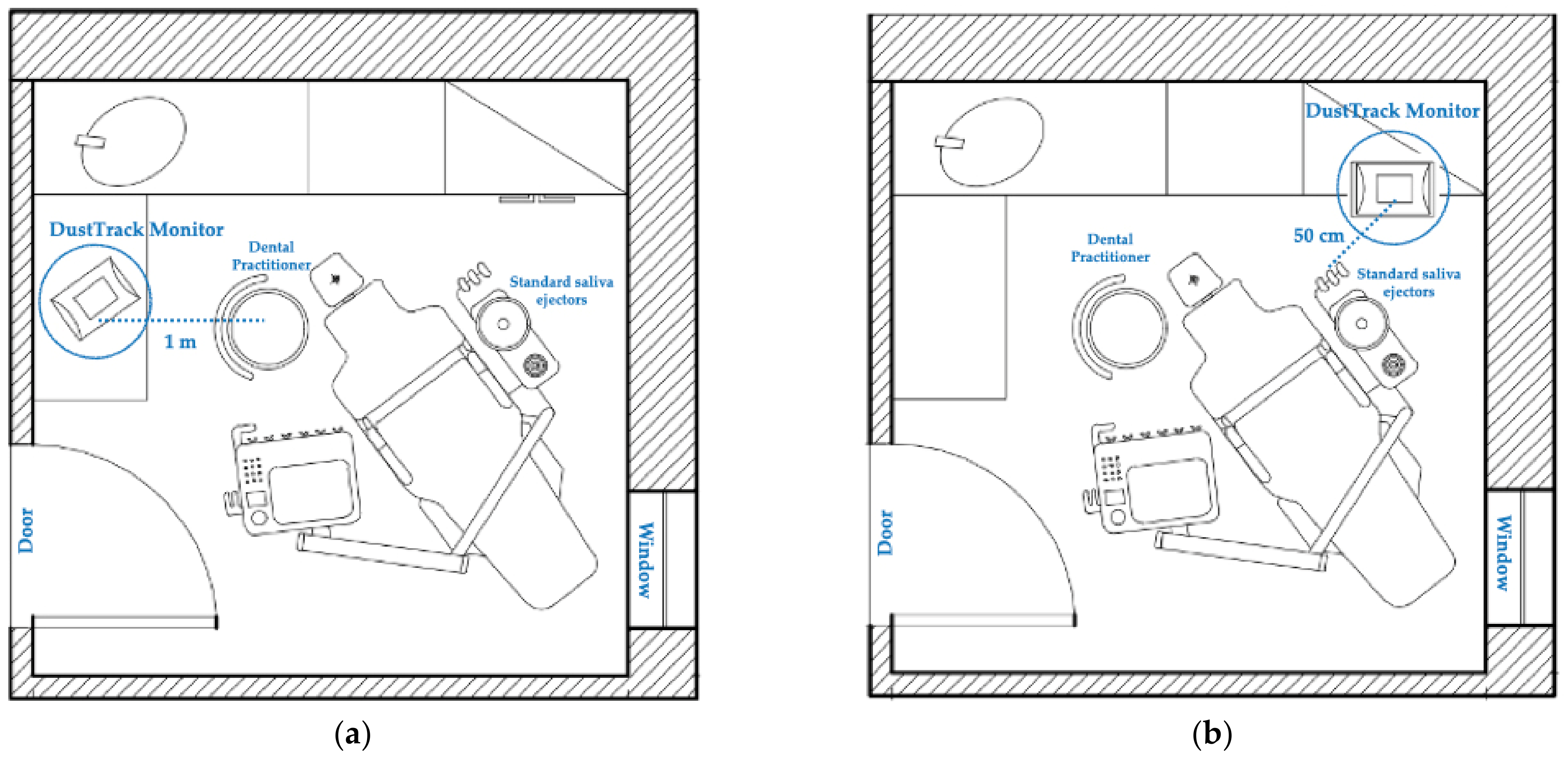
2.2. Study Setting
2.3. Working Distance
2.4. Validation and Background Level
2.5. Statistical Analyses
3. Results
3.1. Variations in Ventilation
3.2. Effectiveness of the Suction System
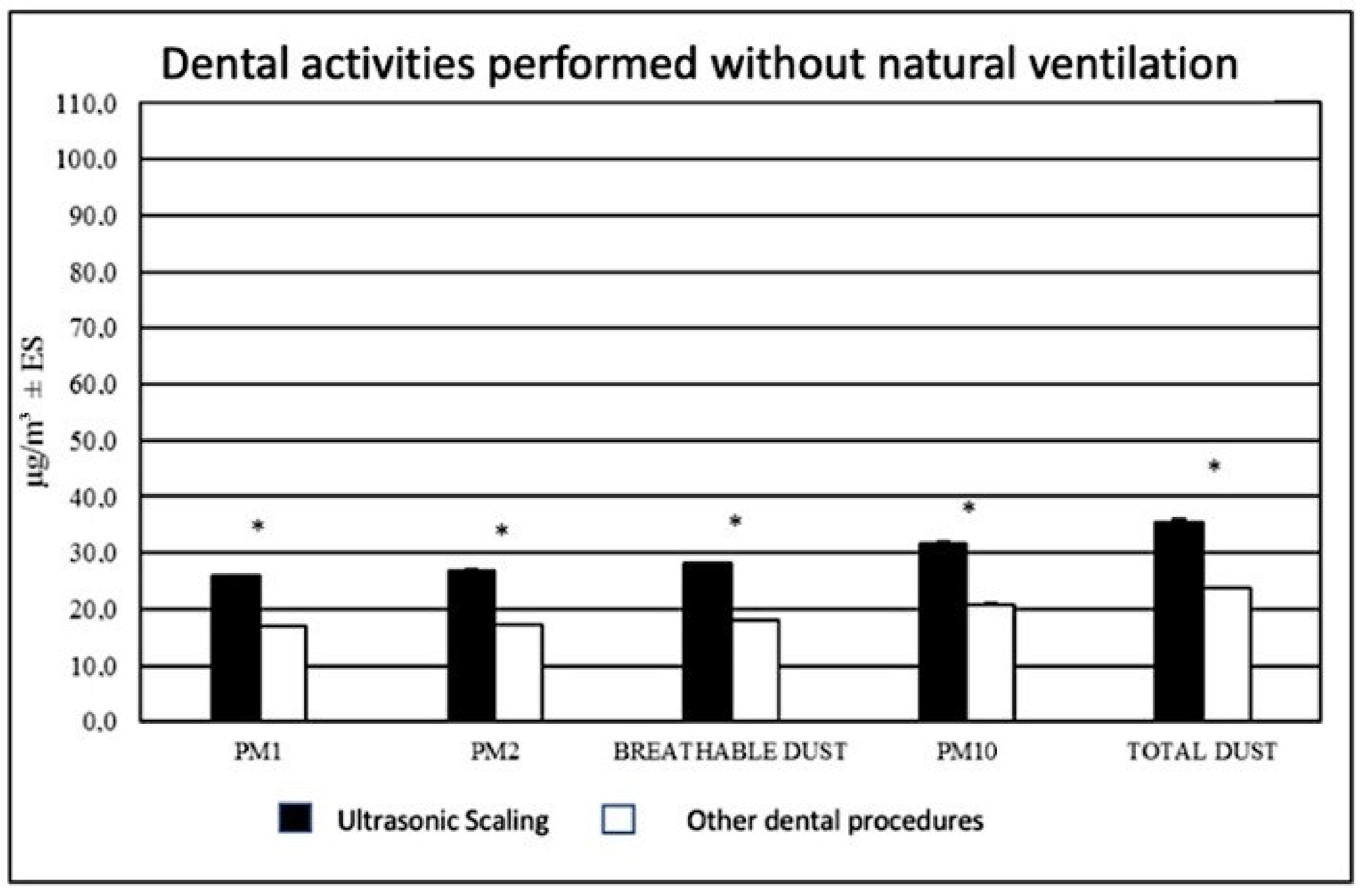
3.3. Differences between Dental Procedures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johns Hopkins University Coronavirus Resource Centre. COVID-19 Dashboard by the Center for Systems Science and Engineering. 2020. Available online: https://0-coronavirus-jhu-edu.brum.beds.ac.uk/map.html (accessed on 15 June 2021).
- Liu, Y.; Ning, Z.; Chen, Y.; Guo, M.; Liu, Y.; Gali, N.K.; Sun, L.; Duan, Y.; Cai, J.; Westerdahl, D.; et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature 2020, 582, 557–560. [Google Scholar] [CrossRef]
- Hadei, M.; Hopke, P.K.; Jonidi, A.; Shahsavani, A. A Letter about the Airborne Transmission of SARS-CoV-2 Based on the Current Evidence. Aerosol Air Qual. Res. 2020, 20, 911–914. [Google Scholar] [CrossRef] [Green Version]
- Di Carlo, P.; Chiacchiaretta, P.; Sinjari, B.; Aruffo, E.; Stuppia, L.; De Laurenzi, V.; Di Tomo, P.; Pelusi, L.; Potenza, F.; Veronese, A.; et al. Air and surface measurements of SARS-CoV-2 inside a bus during normal operation. PLoS ONE 2020, 15, e0235943. [Google Scholar] [CrossRef]
- Buonanno, G.; Stabile, L.; Morawska, L. Estimation of airborne viral emission: Quanta emission rate of SARS-CoV-2 for infection risk assessment. Environ. Int. 2020, 141, 105794. [Google Scholar] [CrossRef] [PubMed]
- Nagraj, S.K.; Eachempati, P.; Paisi, M.; Nasser, M.; Sivaramakrishnan, G.; Verbeek, J.H. Interventions to reduce contaminated aerosols produced during dental procedures for preventing infectious diseases. Cochrane Database Syst. Rev. 2020, 10, CD013686. [Google Scholar]
- Gallagher, J.E.; Sukriti, K.C.; Johnson, I.G.; Al-Yaseen, W.; Jones, R.; McGregor, S.; Robertson, M.; Harris, R.; Innes, N.; Wade, W.G. A systematic review of contamination (aerosol, splatter and droplet generation) associated with oral surgery and its relevance to COVID-19. BDJ Open 2020, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Eugen, C.; Carl, E.; Cook, M.S. Characterization of infectious aerosols in health care facilities: An aid to effective engineering controls and preventive strategies. Am. J. Infect. Control 1998, 26, 453–464. [Google Scholar]
- Cao, C.; Jiang, W.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T.F. Inhalable microorganisms in Beijing’s PM2.5 and PM10 pollutants during a severe smog event. Environ. Sci. Technol. 2014, 48, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Xie, J.; Huang, F.; Cao, L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci. Total Environ. 2020, 727, 138704. [Google Scholar] [CrossRef] [PubMed]
- Paital, B.; Agrawal, P.K. Air pollution by NO2 and PM2.5 explains COVID-19 infection severity by overexpression of angiotensin-converting enzyme 2 in respiratory cells: A review. Environ. Chem. Lett. 2020, 18, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Allison, J.R.; Currie, C.C.; Edwards, D.C.; Bowes, C.; Coulter, J.; Pickering, K.; Kozhevnikova, E.; Durham, J.; Nile, C.J.; Jakubovics, N.; et al. Evaluating aerosol and splatter following dental procedures: Addressing new challenges for oral health care and rehabilitation. J. Oral Rehabil. 2021, 48, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Guidance for Dental Settings—Interim Infection Prevention and Control Guidance for Dental Settings during the COVID-19 Response; CDC: Atlanta, GA, USA, 2020.
- D’Amico, C.; Bocchieri, S.; Stefano, R.; Gorassini, F.; Surace, G.; Amoroso, G.; Scoglio, C.; Mastroieni, R.; Gambino, D.; Amantia, E.; et al. Dental Office Prevention of Coronavirus Infection. Eur. J. Dent. 2020, 14, S146–S151. [Google Scholar]
- Lang, A.; Ovsenik, M.; Verdenik, I.; Remskar, M.; Oblak, C. Nanoparticle concentrations and composition in a dental office and dental laboratory: A pilot study on the influence of working procedures. J. Occup. Environ. Hyg. 2018, 15, 441–447. [Google Scholar] [CrossRef]
- Rupf, S.; Berger, H.; Buchter, A.; Harth, V.; Ong, M.F.; Hannig, M. Exposure of patient and dental staff to fine and ultrafine particles from scanning spray. Clin. Oral Investig. 2015, 19, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Sotiriou, M.; Ferguson, S.F.; Davey, M.; Wolfson, J.M.; Demokritou, P.; Lawrence, J.; Sax, S.N.; Koutrakis, P. Measurement of particle concentrations in a dental office. Environ. Monit. Assess. 2008, 137, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Polednik, B. Aerosol and bioaerosol particles in a dental office. Environ. Res. 2014, 134, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Chow, K.; Mathias, R. Dental procedure aerosols and COVID-19. Lancet Infect. Dis. 2021, 21, e73. [Google Scholar] [CrossRef]
- Graziani, F.; Izzetti, R.; Lardani, L.; Totaro, M.; Baggiani, A. Experimental Evaluation of Aerosol Production after Dental Ultrasonic Instrumentation: An Analysis on Fine Particulate Matter Perturbation. Int. J. Environ. Res. Public Health 2021, 18, 3357. [Google Scholar] [CrossRef]
- Zemouri, C.; De Soet, H.; Crielaard, W.; Laheij, A. A scoping review on bio-aerosols in healthcare and the dental environment. PLoS ONE 2017, 12, e0178007. [Google Scholar] [CrossRef]
- ISS: Istituto Superiore di Sanità. Indicazioni ad Interim per un Utilizzo Razionale delle Protezioni per Infezione da SARS-COV-2 Nelle Attività Sanitarie e Sociosanitarie (Assistenza a Soggetti Affetti da COVID-19) Nell’Attuale Scenario Emergenziale SARS-COV-2; Istituto Superiore di Sanità: Rome, Italy, 2020. Available online: https://www.iss.it/documents/20126/0/Rapporto+ISS+COVID+2_+Protezioni_REV.V6.pdf/740f7d89-6a28-0ca1-8f76-368ade332dae?t=1585569978473 (accessed on 25 March 2020).
- Kim, J.Y.; Magari, S.R.; Herrick, R.F.; Smith, T.J.; Christiani, D.C. Comparison of fine particle measurements from a direct-reading instrument and a gravimetric sampling method. J. Occup. Environ. Hyg. 2004, 1, 707–715. [Google Scholar] [CrossRef]
- Clementini, M.; Raspini, M.; Barbato, L.; Bernardelli, F.; Braga, G.; Di Gioia, C.; Littarru, C.; Oreglia, F.; Brambilla, E.; Iavicoli, I.; et al. Aerosol transmission for SARS-CoV-2 in the dental practice. A review by SIdP Covid-19 task-force. Oral Dis. 2020, 1–6. [Google Scholar] [CrossRef]
- Innes, N.; Johnson, I.G.; Al-Yaseen, W.; Harris, R.; Jones, R.; Kc, S.; McGregor, S.; Robertson, M.; Wade, W.G.; Gallagher, J.E. A Systematic Review of Droplet and Aerosol Generation in Dentistry. J. Dent. 2020, 105, 103556. [Google Scholar]
- Ahmed, M.A.; Jouhar, R. Dissemination of Aerosol and Splatter in Clinical Environment during Cavity Preparation: An in vitro Study. Int. J. Environ. Res. Public Health 2021, 18, 3773. [Google Scholar] [CrossRef] [PubMed]
- Holliday, R.; Allison, J.R.; Currie, C.C.; Edwards, D.C.; Bowes, C.; Pickering, K.; Reay, S.; Durham, J.; Lumb, J.; Rostami, N.; et al. Evaluating contaminated dental aerosol and splatter in an open plan clinic environment: Implications for the COVID-19 pandemic. J. Dent. 2021, 105, 103565. [Google Scholar] [CrossRef]
- Narayana, T.V.; Mohanty, L.; Sreenath, G.; Vidhyadhari, P. Role of preprocedural rinse and high-volume evacuator in reducing bacterial contamination in bioaerosols. J. Oral Maxillofac. Pathol. 2016, 20, 59–65. [Google Scholar] [PubMed] [Green Version]
- Ge, Z.Y.; Yang, L.M.; Xia, J.J.; Fu, X.H.; Zhang, Y.Z. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J. Zhejiang Univ. Sci. B 2020, 5, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Chuaybamroong, P.; Chotigawin, R.; Supothina, S.; Sribenjalux, P.; Larpkiattaworn, S.; Wu, C.Y. Efficacy of photocatalytic HEPA filter on microorganism removal. Indoor Air 2010, 20, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Day, D.B.; Xiang, J.; Mo, J.; Clyde, M.A.; Weschler, C.J.; Li, F.; Gong, J.; Chung, M.; Zhang, Y.; Zhang, J. Combined use of an electrostatic precipitator and a high-efficiency particulate air filter in building ventilation systems: Effects on cardiorespiratory health indicators in healthy adults. Indoor Air 2018, 28, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Sinjari, B.; D’Ardes, D.; Santilli, M.; Rexhepi, I.; D’Addazio, G.; Di Carlo, P.; Chiacchiaretta, P.; Caputi, S.; Cipollone, F. SARS-CoV-2 and Oral Manifestation: An Observational, Human Study. J. Clin. Med. 2020, 9, 3218. [Google Scholar] [CrossRef]
- Cochrane Oral Health. Recommendations for the Re-Opening of Dental Services: A Rapid Review of International Sources. 2020. Available online: https://oralhealth.cochrane.org/sites/oralhealth.cochrane.org/files/public/uploads/covid19_dental_review_16_may_2020_update.pdf (accessed on 16 May 2020).
- Sinjari, B.; Rexhepi, I.; Santilli, M.; D’Addazio, G.; Chiacchiaretta, P.; Di Carlo, P.; Caputi, S. The Impact of COVID-19 Related Lockdown on Dental Practice in Central Italy-Outcomes of A Survey. Int. J. Environ. Res. Public Health 2020, 17, 5780. [Google Scholar] [CrossRef]
- Papi, P.; Di Murro, B.; Penna, D.; Pompa, G. Digital prosthetic workflow during COVID-19 pandemic to limit infection risk in dental practice. Oral Dis. 2020, 27, 723–726. [Google Scholar] [CrossRef]
- Maspero, C.; Abate, A.; Cavagnetto, D.; El Morsi, M.; Fama, A.; Farronato, M. Available Technologies, Applications and Benefits of Teleorthodontics. A Literature Review and Possible Applications during the COVID-19 Pandemic. J. Clin. Med. 2020, 9, 1891. [Google Scholar] [CrossRef]
- WHO Guideline. Natural Ventilation for Infection Control in Health-Care Settings. Available online: https://apps.who.int/iris/bitstream/handle/10665/44167/9789241547857_eng.pdf?sequence=1 (accessed on 20 May 2020).
- Banakar, M.; Lankarani, K.B.; Jafarpour, D.; Moayedi, S.; Banakar, M.H.; MohammadSadeghi, A. COVID-19 transmission risk and protective protocols in dentistry: A systematic review. BMC Oral Health 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Helmis, C.G.; Tzoutzas, J.; Flocas, H.A.; Halios, C.H.; Stathopoulou, O.I.; Assimakopoulos, V.D.; Panis, V.; Apostolatou, M.; Sgouros, G.; Adam, E. Indoor air quality in a dentistry clinic. Sci. Total Environ. 2007, 377, 349–365. [Google Scholar] [CrossRef]
- Sinjari, B.; D’Addazio, G.; Bozzi, M.; Celletti, R.; Traini, T.; Mavriqi, L.; Caputi, S. Comparison of a Novel Ultrasonic Scaler Tip vs. Conventional Design on a Titanium Surface. Materials 2018, 11, 2345. [Google Scholar] [CrossRef] [Green Version]
- Tunkel, J.; Heinecke, A.; Flemmig, T.F. A systematic review of efficacy of machine-driven and manual subgingival debridement in the treatment of chronic periodontitis. J. Clin. Periodontol. 2002, 29, 72–81. [Google Scholar] [CrossRef]
- Bennett, A.M.; Fulford, M.R.; Walker, J.T.; Bradshaw, D.J.; Martin, M.V.; Marsh, P.D. Microbial aerosols in general dental practice. Br. Dent. J. 2000, 189, 664–667. [Google Scholar] [CrossRef] [PubMed]
- Gralton, J.; Tovey, E.; McLaws, M.-L.; Rawlinson, W.D. The role of particle size in aerosolised pathogen transmission: A review. J. Infect. 2011, 62, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aluko, O.; Noll, K.E. Deposition and Suspension of Large, Airborne Particles. Aerosol Sci. Technol. 2006, 40, 503–513. [Google Scholar] [CrossRef]
| Parameter | Real Time Monitor | p-Value a | |
|---|---|---|---|
| Dental Unit Window Open Mean ± SE (µg/m3) | Dental Unit Window Closed Mean ± SE (µg/m3) | ||
| PM1 | 34.49 ± 0.72 | 20.73 ± 0.08 | <0.001 * |
| PM2.5 | 35.34 ± 0.76 | 21.26 ± 0.08 | <0.001 * |
| BREATHABLE DUST | 37.1 ± 0.86 | 22.14 ± 0.09 | <0.001 * |
| PM10 | 44.66 ± 1.45 | 25.01 ± 0.1 | <0.001 * |
| TOTAL DUST | 53.95 ± 2.29 | 27.85 ± 0.14 | <0.001 * |
| Parameter | Real Time Monitor | p-Value a | |
|---|---|---|---|
| Dental Unit Door Closed Mean ± SE (µg/m3) | Dental Unit Door Open Mean ± SE (µg/m3) | ||
| PM1 | 34.49 ± 0.72 | 45.98 ± 0.1 | <0.001 * |
| PM2.5 | 35.34 ± 0.76 | 47.15 ± 0.12 | <0.001 * |
| BREATHABLE DUST | 37.1 ± 0.86 | 50.34 ± 0.14 | <0.001 * |
| PM10 | 44.66 ± 1.45 | 64.32 ± 0.09 | <0.001 * |
| TOTAL DUST | 53.95 ± 2.29 | 82.37 ± 0.12 | <0.001 * |
| Parameter | Real Time Monitor | p-Value a | % ∆ | |
|---|---|---|---|---|
| Position A (Near Practitioner Chair) Mean ± SE (µg/m3) | Position B (Near Standard Saliva Ejectors) Mean ± SE (µg/m3) | |||
| PM1 | 52.51 ± 4.81 | 40.71 ± 0.16 | 0.007 | −22.6 |
| PM2.5 | 54.34 ± 5.07 | 41.44 ± 0.16 | 0.005 | −23.7 |
| BREATHABLE DUST | 58.49 ± 5.70 | 43.37 ± 0.16 | 0.004 | −25.8 |
| PM10 | 82.40 ± 9.65 | 50.52 ± 0.23 | <0.001 * | −38.7 |
| TOTAL DUST | 117.17 ± 15.20 | 58.54 ± 0.38 | <0.001 * | −50.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rexhepi, I.; Mangifesta, R.; Santilli, M.; Guri, S.; Di Carlo, P.; D’Addazio, G.; Caputi, S.; Sinjari, B. Effects of Natural Ventilation and Saliva Standard Ejectors during the COVID-19 Pandemic: A Quantitative Analysis of Aerosol Produced during Dental Procedures. Int. J. Environ. Res. Public Health 2021, 18, 7472. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18147472
Rexhepi I, Mangifesta R, Santilli M, Guri S, Di Carlo P, D’Addazio G, Caputi S, Sinjari B. Effects of Natural Ventilation and Saliva Standard Ejectors during the COVID-19 Pandemic: A Quantitative Analysis of Aerosol Produced during Dental Procedures. International Journal of Environmental Research and Public Health. 2021; 18(14):7472. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18147472
Chicago/Turabian StyleRexhepi, Imena, Rocco Mangifesta, Manlio Santilli, Silvia Guri, Piero Di Carlo, Gianmaria D’Addazio, Sergio Caputi, and Bruna Sinjari. 2021. "Effects of Natural Ventilation and Saliva Standard Ejectors during the COVID-19 Pandemic: A Quantitative Analysis of Aerosol Produced during Dental Procedures" International Journal of Environmental Research and Public Health 18, no. 14: 7472. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18147472












