Effects of 16 Weeks of Taekwondo Training on the Cerebral Blood Flow Velocity, Circulating Neurotransmitters, and Subjective Well-Being of Obese Postmenopausal Women
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Participants
2.2. Study Procedure
2.3. Anthropometric Measurements
2.4. Blood Sampling and Analysis
2.5. CBF Velocity Monitoring
2.6. SWB Measurements
2.7. Taekwondo Training Protocol
2.8. Statistical Analyses
3. Results
3.1. Characteristics of the Participants
3.2. Changes in the Body Composition after the 16-Week Intervention
3.3. Changes in the Serum Lipid Profiles after the 16-Week Intervention
3.4. Changes in the CBF Velocities after the 16-Week Intervention
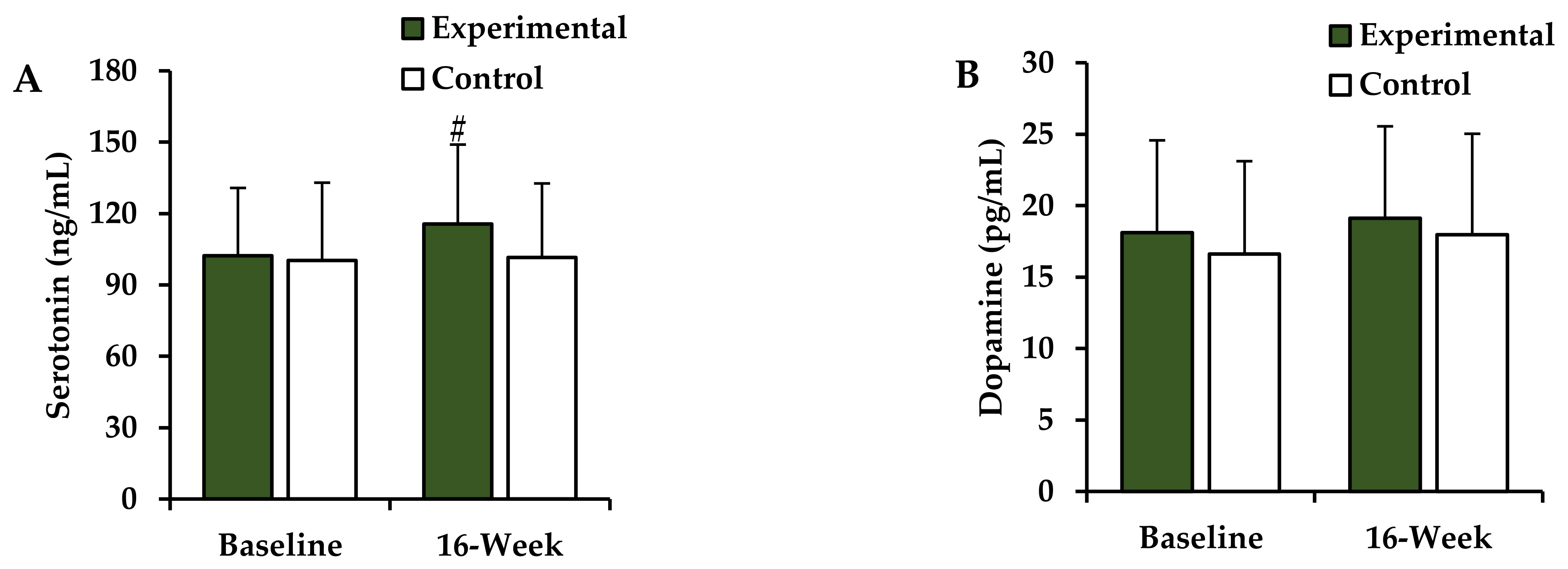
3.5. Changes in the Plasma Neurotransmitter Levels after the 16-Week Intervention
3.6. Changes in the SWB Indices after the 16-Week Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Schetz, M.; De Jong, A.; Deane, A.M.; Druml, W.; Hemelaar, P.; Pelosi, P.; Pickkers, P.; Reintam-Blaser, A.; Roberts, J.; Sakr, Y.; et al. Obesity in the critically ill: A narrative review. Intensive Care Med. 2019, 45, 757–769. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Drummond, P.D. Obesity and psychiatric disorders: Commonalities in dysregulated biological pathways and their implications for treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Keddie, A.M. Associations between severe obesity and depression: Results from the National Health and Nutrition Examination Survey, 2005–2006. Prev. Chronic Dis. 2011, 8, A57. [Google Scholar] [PubMed]
- Ko, S.H.; Kim, H.S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef] [Green Version]
- Stachowiak, G.; Pertyński, T.; Pertyńska-Marczewska, M. Metabolic disorders in menopause. Prz. Menopauzalny 2015, 14, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.; Jones, R.; Novak, P.; Zhao, P.; Novak, V. The effects of body mass index on cerebral blood flow velocity. Clin. Auton. Res. 2008, 18, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Knight, S.P.; Laird, E.; Williamson, W.; O’Connor, J.; Newman, L.; Carey, D.; De Looze, C.; Fagan, A.J.; Chappell, M.A.; Meaney, J.F.; et al. Obesity is associated with reduced cerebral blood flow—Modified by physical activity. Neurobiol. Aging 2021, 105, 35–47. [Google Scholar] [CrossRef]
- Williamson, W.; Lewandowski, A.J.; Forkert, N.D.; Griffanti, L.; Okell, T.W.; Betts, J.; Boardman, H.; Siepmann, T.; McKean, D.; Huckstep, O.; et al. Association of Cardiovascular Risk Factors with MRI Indices of Cerebrovascular Structure and Function and White Matter Hyperintensities in Young Adults. JAMA 2018, 320, 665–673. [Google Scholar] [CrossRef]
- Bray, G.A.; Frühbeck, G.; Ryan, D.H.; Wilding, J.P. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef] [Green Version]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metabolism 2019, 92, 163–169. [Google Scholar] [CrossRef] [Green Version]
- Meeusen, R. Exercise and the brain: Insight in new therapeutic modalities. Ann. Transplant. 2005, 10, 49–51. [Google Scholar] [PubMed]
- Chaouloff, F. Physical exercise and brain monoamines: A review. Acta. Physiol. Scand. 1989, 137, 1–13. [Google Scholar] [CrossRef]
- Robertson, A.D.; Marzolini, S.; Middleton, L.E.; Basile, V.S.; Oh, P.I.; MacIntosh, B.J. Exercise Training Increases Parietal Lobe Cerebral Blood Flow in Chronic Stroke: An Observational Study. Front. Aging Neurosci. 2017, 9, 318. [Google Scholar] [CrossRef]
- Fong, S.S.; Ng, G.Y. Does Taekwondo training improve physical fitness? Phys. Ther. Sport 2011, 12, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Roh, H.T.; Cho, S.Y.; So, W.Y. Effects of Regular Taekwondo Intervention on Oxidative Stress Biomarkers and Myokines in Overweight and Obese Adolescents. Int. J. Environ. Res. Public Health 2020, 17, 2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roh, H.T.; Cho, S.Y.; So, W.Y. Taekwondo Training Improves Mood and Sociability in Children from Multicultural Families in South Korea: A Randomized Controlled Pilot Study. Int. J. Environ. Res. Public Health 2018, 15, 757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.Y.; Roh, H.T. Regular Taekwondo Training Affects Mood State and Sociality but Not Cognitive Function among International Students in South Korea. Healthcare 2021, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Cha, E.J.; Lee, T.S. Analysis of physical activities in Taekwondo Pumsae. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2008, 2008, 5164–5167. [Google Scholar] [PubMed]
- Lakes, K.D.; Bryars, T.; Sirisinahal, S.; Salim, N.; Arastoo, S.; Emmerson, N.; Kang, D.; Shim, L.; Wong, D.; Kang, C.J. The Healthy for Life Taekwondo Pilot Study: A Preliminary Evaluation of Effects on Executive Function and BMI, Feasibility, and Acceptability. Ment. Health Phys. Act. 2013, 6, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Roh, H.T.; Cho, S.Y.; So, W.Y. A Cross-Sectional Study Evaluating the Effects of Resistance Exercise on Inflammation and Neurotrophic Factors in Elderly Women with Obesity. J. Clin. Med. 2020, 9, 842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.W.; Kim, D.Y. Effects of aerobic exercise training on serum sex hormone binding globulin, body fat index, and metabolic syndrome factors in obese postmenopausal women. Metab. Syndr. Relat. Disord. 2012, 10, 452–457. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Aaslid, R.; Markwalder, T.M.; Nornes, H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J. Neurosurg. 1982, 57, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.Y.; So, W.Y.; Roh, H.T. The Effects of Taekwondo Training on Peripheral Neuroplasticity-Related Growth Factors, Cerebral Blood Flow Velocity, and Cognitive Functions in Healthy Children: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2017, 14, 454. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, V. A bifactor model of subjective well-being: A re-examination of the structure of subjective well-being. Pers. Individ. Differ. 2015, 87, 45–49. [Google Scholar] [CrossRef]
- Lim, Y.J.; Yu, B.H.; Kim, D.K.; Kim, J.H. The positive and negative affect schedule: Psychometric properties of the korean version. Psychiatry Investig. 2010, 7, 163–169. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Diener, E.; Emmons, R.A.; Larsen, R.J.; Griffin, S. The satisfaction with life scale. J. Pers. Assess. 1985, 49, 71–75. [Google Scholar] [CrossRef]
- Emerson, S.D.; Guhn, M.; Gadermann, A.M. Measurement invariance of the Satisfaction with Life Scale: Reviewing three decades of research. Qual. Life Res. 2017, 26, 2251–2264. [Google Scholar] [CrossRef]
- Cho, S.Y.; Roh, H.T. Taekwondo Enhances Cognitive Function as a Result of Increased Neurotrophic Growth Factors in Elderly Women. Int. J. Environ. Res. Public Health 2019, 16, 962. [Google Scholar] [CrossRef] [Green Version]
- Pieter, W.; Fife, G.P.; O’Sullivan, D.M. Competition injuries in taekwondo: A literature review and suggestions for prevention and surveillance. Br. J. Sports Med. 2012, 46, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.S.; Lim, K. Effects of Taekwondo training on physical fitness factors in Korean elementary students: A systematic review and meta-analysis. J. Exerc. Nutr. Biochem. 2019, 23, 36–47. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cha, E.J.; Kim, S.M.; Kang, K.D.; Han, D.H. The Effects of Taekwondo Training on Brain Connectivity and Body Intelligence. Psychiatry Investig. 2015, 12, 335–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2018. [Google Scholar]
- Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; National Lipid Association Expert Panel on Familial Hypercholesterolemia. Familial hypercholesterolemias: Prevalence, genetics, diagnosis and screening recommendations from the National Lipid Association Expert Panel on Familial Hypercholesterolemia. J. Clin. Lipidol. 2011, 5, S9–S17. [Google Scholar] [CrossRef]
- De Lorenzo, A.; Gratteri, S.; Gualtieri, P.; Cammarano, A.; Bertucci, P.; Di Renzo, L. Why primary obesity is a disease? J. Transl. Med. 2019, 17, 169. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Nakasuga, K.; Ohshima, A.; Sakai, Y.; Maruyama, T.; Kaji, Y.; Harada, M.; Jingu, S.; Sakamoto, M. Excess accumulation of body fat is related to dyslipidemia in normal-weight subjects. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Hertelyova, Z.; Salaj, R.; Chmelarova, A.; Dombrovsky, P.; Dvorakova, M.C.; Kruzliak, P. The association between lipid parameters and obesity in university students. J. Endocrinol. Investig. 2016, 39, 769–778. [Google Scholar] [CrossRef]
- Fikenzer, K.; Fikenzer, S.; Laufs, U.; Werner, C. Effects of endurance training on serum lipids. Vascul. Pharmacol. 2018, 101, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef]
- Kim, M.; Bae, S.; Lim, K.M. Impact of High Fat Diet-induced Obesity on the Plasma Levels of Monoamine Neurotransmitters in C57BL/6 Mice. Biomol. Ther. 2013, 21, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, H.; Tang, S.; Liu, X.; O′Neil, A.; Turner, A.; Chai, F.; Chen, F.; Berk, M. Assessing regional cerebral blood flow in depression using 320-slice computed tomography. PLoS ONE 2014, 9, e107735. [Google Scholar] [CrossRef] [Green Version]
- Toda, N.; Okamura, T. Cerebral blood flow regulation by nitric oxide in Alzheimer’s disease. J. Alzheimers Dis. 2012, 32, 569–578. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Cotter, J.D.; George, K.P.; Lucas, S.; Murrell, C.; Shave, R.; Thomas, K.N.; Williams, M.J.; Atkinson, G. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J. Physiol. 2008, 586, 4005–4010. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, N.; Choi, Y.; Miyaki, A.; Sugawara, J.; Ajisaka, R.; Maeda, S. Aerobic exercise training increases cerebral blood flow in postmenopausal women. Artery Res. 2012, 6, 124–129. [Google Scholar] [CrossRef]
- Petrovic, K. The Benefits of Taekwondo Training for Undergraduate Students: A Phenomenological Study. Societies 2017, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Ahn, D.; Shin, D.H. Is the social use of media for seeking connectedness or for avoiding social isolation? Mechanisms underlying media use and subjective well-being. Comput. Hum. Behav. 2013, 29, 2453–2462. [Google Scholar] [CrossRef]
- Depue, R.A.; Luciana, M.; Arbisi, P.; Collins, P.; Leon, A. Dopamine and the structure of personality: Relation of agonist-induced dopamine activity to positive emotionality. J. Pers. Soc. Psychol. 1994, 67, 485–498. [Google Scholar] [CrossRef]
- Knutson, B.; Wolkowitz, O.M.; Cole, S.W.; Chan, T.; Moore, E.A.; Johnson, R.C.; Terpstra, J.; Turner, R.A.; Reus, V.I. Selective alteration of personality and social behavior by serotonergic intervention. Am. J. Psychiatry 1998, 155, 373–379. [Google Scholar] [CrossRef] [PubMed]


| Variable | Experimental (n = 12) | Control (n = 12) | p |
|---|---|---|---|
| Age (years) | 56.0 ± 2.9 | 57.5 ± 2.9 | 0.223 |
| Height (cm) | 157.4 ± 4.7 | 156.6 ± 3.3 | 0.620 |
| Weight (kg) | 64.0 ± 5.8 | 62.6 ± 6.2 | 0.576 |
| BMI (kg/m2) | 25.8 ± 2.0 | 25.5 ± 1.7 | 0.674 |
| SMM (kg) | 22.0 ± 2.7 | 20.8 ± 2.5 | 0.288 |
| BFP (%) | 37.0 ± 3.1 | 36.3 ± 2.6 | 0.630 |
| TC (mg/dL) | 183.6 ± 42.8 | 184.9 ± 42.7 | 0.939 |
| TG (mg/dL) | 127.6 ± 53.1 | 132.5 ± 42.4 | 0.805 |
| LDL-C (mg/dL) | 100.8 ± 38.8 | 102.8 ± 41.0 | 0.903 |
| HDL-C (mg/dL) | 53.6 ± 15.2 | 53.8 ± 15.2 | 0.979 |
| sFV (cm/s) | 81.7 ± 19.6 | 79.6 ± 19.3 | 0.796 |
| dFV (cm/s) | 39.0 ± 11.3 | 36.8 ± 8.8 | 0.605 |
| mFV (cm/s) | 52.9 ± 11.3 | 52.0 ± 11.4 | 0.845 |
| PI (unit) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.836 |
| Serotonin (ng/mL) | 102.2 ± 28.6 | 100.3 ± 32.7 | 0.883 |
| Dopamine (pg/mL) | 18.1 ± 6.5 | 16.6 ± 6.5 | 0.574 |
| PANAS-PA (score) | 18.3 ± 3.8 | 19.9 ± 4.2 | 0.340 |
| PANAS-NA (score) | 29.5 ± 4.2 | 28.7 ± 4.3 | 0.321 |
| SWLS (score) | 18.2 ± 3.3 | 17.7 ± 3.8 | 0.732 |
| Variables | Experimental (n = 12) | Control (n = 12) | Interaction (Group × Time) | |||
|---|---|---|---|---|---|---|
| Baseline | 16-Week | Baseline | 16-Week | F | p | |
| Weight (kg) | 64.0 ± 5.8 | 60.1 ± 4.3 # | 62.6 ± 6.2 | 62.4 ± 5.1 | 8.108 | 0.009 ** |
| BMI (kg/m2) | 25.8 ± 2.0 | 24.3 ± 1.7 # | 25.5 ± 1.7 | 25.4 ± 1.3 | 8.374 | 0.008 ** |
| SMM (kg) | 22.0 ± 2.7 | 21.2 ± 2.2 | 20.8 ± 2.5 | 20.5 ± 2.1 | 2.117 | 0.160 |
| BFP (%) | 37.0 ± 3.1 | 33.8 ± 4.9 # | 36.3 ± 2.6 | 36.5 ± 2.2 | 13.226 | 0.001 ** |
| Variables | Experimental (n = 12) | Control (n = 12) | Interaction (Group × Time) | |||
|---|---|---|---|---|---|---|
| Baseline | 16-Week | Baseline | 16-Week | F | p | |
| TC (mg/dL) | 183.6 ± 42.8 | 169.8 ± 42.2 # | 184.9 ± 42.7 | 190.7 ± 42.5 | 4.922 | 0.037 * |
| TG (mg/dL) | 127.6 ± 53.1 | 118.3 ± 42.5 | 132.5 ± 42.4 | 127.8 ± 32.6 | 0.280 | 0.602 |
| LDL-C (mg/dL) | 100.8 ± 38.8 | 93.5 ± 35.7 # | 102.8 ± 41.0 | 110.2 ± 40.6 | 4.761 | 0.040 * |
| HDL-C (mg/dL) | 53.6 ± 15.2 | 57.8 ± 12.0 | 53.8 ± 15.2 | 55.9 ± 12.8 | 0.250 | 0.622 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-K.; Cho, S.-Y.; Roh, H.-T. Effects of 16 Weeks of Taekwondo Training on the Cerebral Blood Flow Velocity, Circulating Neurotransmitters, and Subjective Well-Being of Obese Postmenopausal Women. Int. J. Environ. Res. Public Health 2021, 18, 10789. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph182010789
Lee Y-K, Cho S-Y, Roh H-T. Effects of 16 Weeks of Taekwondo Training on the Cerebral Blood Flow Velocity, Circulating Neurotransmitters, and Subjective Well-Being of Obese Postmenopausal Women. International Journal of Environmental Research and Public Health. 2021; 18(20):10789. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph182010789
Chicago/Turabian StyleLee, Yong-Kuk, Su-Youn Cho, and Hee-Tae Roh. 2021. "Effects of 16 Weeks of Taekwondo Training on the Cerebral Blood Flow Velocity, Circulating Neurotransmitters, and Subjective Well-Being of Obese Postmenopausal Women" International Journal of Environmental Research and Public Health 18, no. 20: 10789. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph182010789






