Hybrid Model-Based Simulation Analysis on the Effects of Social Distancing Policy of the COVID-19 Epidemic
Abstract
:1. Introduction
2. Related Works
| Related Work | Modeling Approach | Modeling Method | Description |
|---|---|---|---|
| [14] | Analytical model | Equation-based model (SIR) |
|
| [15] | Analytical model | Equation-based mode (SEIR) |
|
| [16] | Analytical model | Equation-based model (SIRD) |
|
| [28] | Analytical model | Equation-based model (Modified SIR) |
|
| [17] | Simulation model | Agent-based model |
|
| [18] | Simulation model | Agent-based model |
|
3. Proposed Work
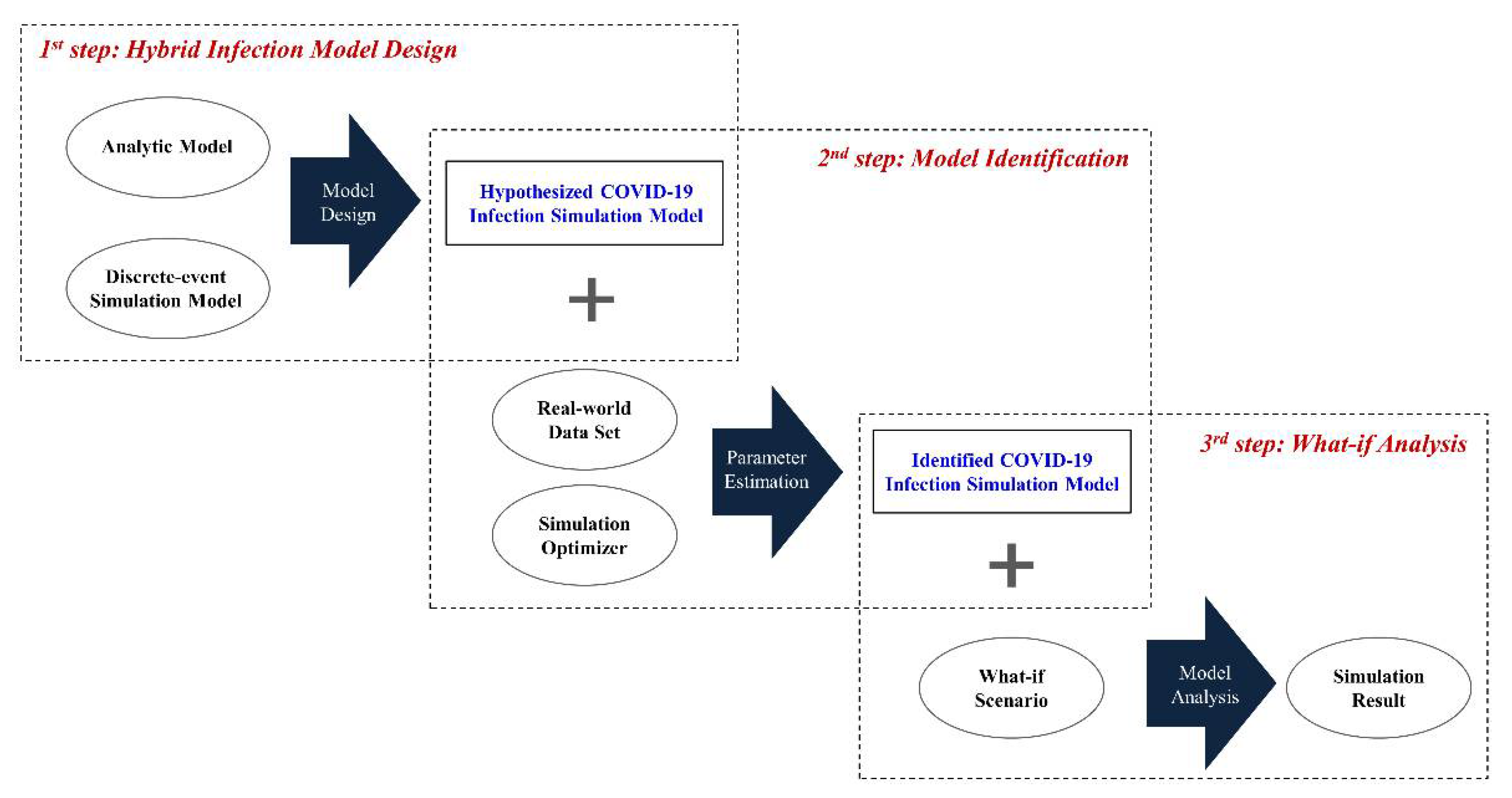
3.1. Overall Process Description
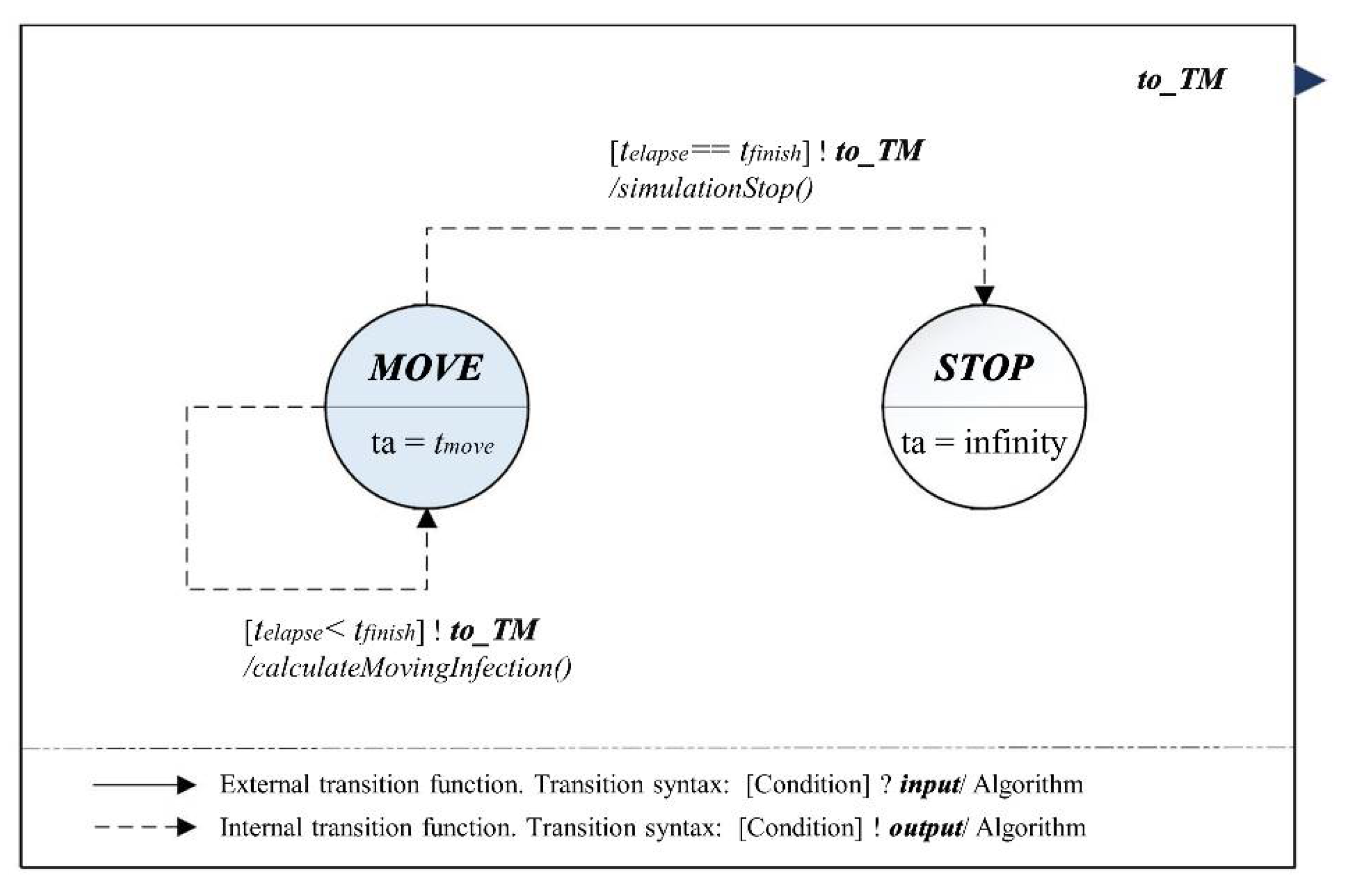
3.2. Hybrid Infection Simulation Model Design
3.3. Model Identification and What-If Analysis
4. Simulation Experiment
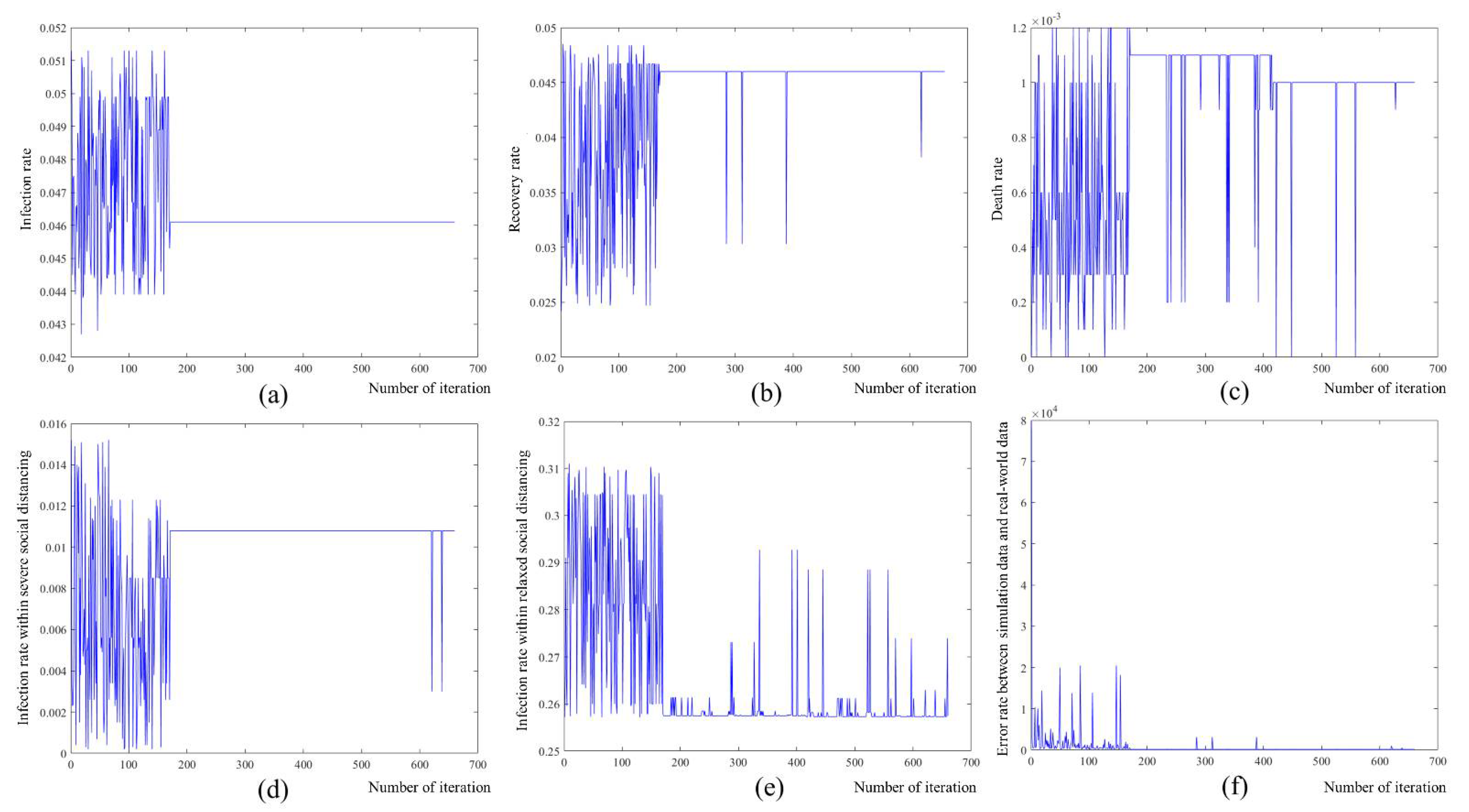
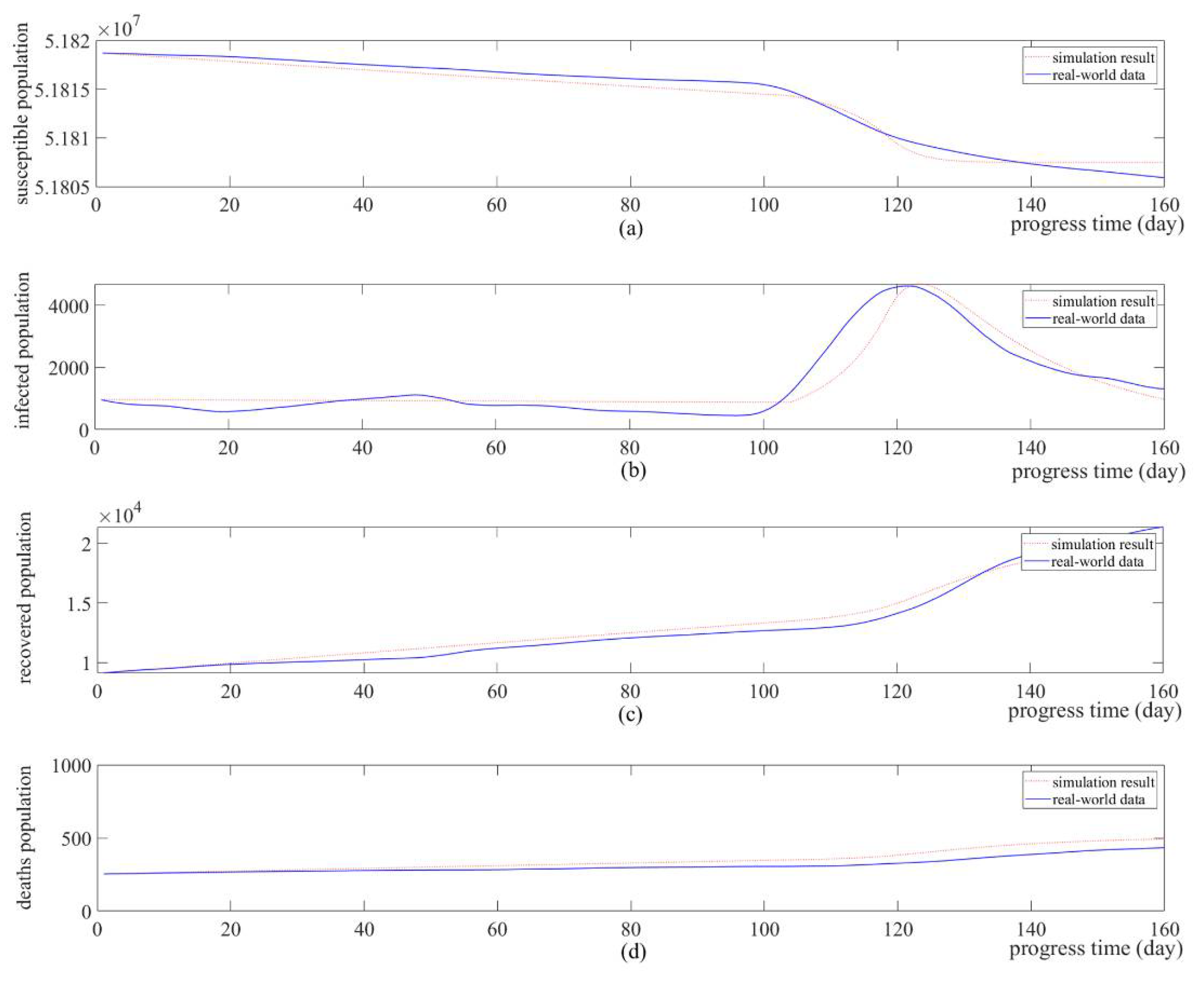
4.1. Simulation-Based Model Identification
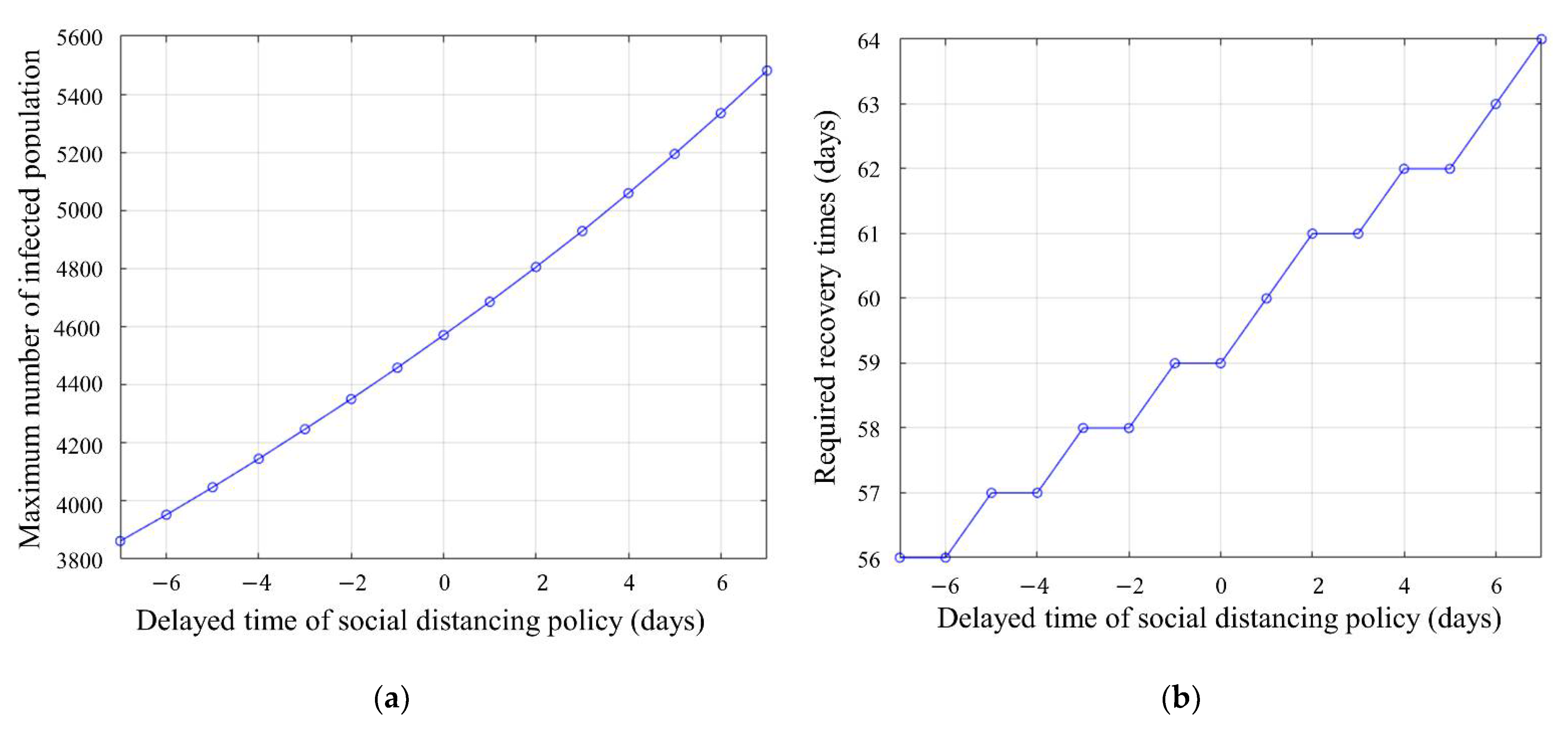
4.2. What-If Analysis: Simulation of Schedule Change in Social Distancing
4.3. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABM | Agent-Based Model |
| D | Dead |
| DEVS | Discrete Event System Specifications |
| EBM | Equation-Based Model |
| EIC | External Input Coupling Relation |
| EOC | External Output Coupling Relation |
| I | Infected |
| IC | Internal Coupling Relation |
| IIM | Inner Infection Model |
| ISM | Infection Simulation Model |
| MCM | Major City Model |
| OIM | Outward Infection Model |
| R | Recovered |
| RMSE | Root Mean Square Error |
| RSD | Relaxed Social Distancing |
| RSD_S | Relaxed Social Distancing Steady State |
| RSD_T | Relaxed Social Distancing Transient State |
| S | Susceptible |
| SEIR | Susceptible, Exposed, Infected, and Recovered |
| SIR | Susceptible, Infected, and Recovered |
| SIRD | Susceptible, Infected, Recovered, and Dead |
| SEIR | Suspected, Exposed, Infected, and Recovered |
| SSD | Severe Social Distancing |
| SSD_S | Severe Social Distancing Steady State |
| SSD_T | Severe Social Distancing Transient State |
| TM | Transfer Model |
References
- Kim, Y.; Jiang, X. Evolving transmission network dynamics of COVID-19 cluster infections in South Korea: A descriptive study. MedRxiv 2020. [Google Scholar] [CrossRef]
- Shukla, P.; Kundu, R.; Arivarasi, A.; Alagiri, G. A Social Distance Monitoring System to ensure Social Distancing in Public Areas. In Proceedings of the 2021 International Conference on Computational Intelligence and Knowledge Economy (ICCIKE), Dubai, United Arab Emirates, 17–18 March 2021; pp. 96–101. [Google Scholar]
- Roncone, R.; Giusti, L.; Mammarella, S.; Salza, A.; Bianchini, V.; Lombardi, A.; Prosperocco, M.; Ursini, E.; Scaletta, V.; Casacchia, M.; et al. “Hang in There!”: Mental Health in a Sample of the Italian Civil Protection Volunteers during the COVID-19 Health Emergency. Int. J. Environ. Res. Public Health 2021, 18, 8587. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Ki, M. Analyzing the effects of social distancing on the COVID-19 pandemic in Korea using mathematical modeling. Epidemiol. Health 2020, 42, e2020064. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.E.F.; Anderson, D.E.; Wei, W.E.; Pang, J.; Chia, W.N.; Tan, C.W.; Teoh, Y.L.; Rajendram, P.; Toh, M.P.H.S.; Poh, C.; et al. Connecting clusters of COVID-19: An epidemiological and serological investigation. Lancet Infect. Dis. 2020, 10, 809–815. [Google Scholar] [CrossRef]
- McGrail, D.J.; Dai, J.; McAndrews, K.M.; Kalluri, R. Enacting national social distancing policies corresponds with dramatic reduction in COVID19 infection rates. PLoS ONE 2020, 15, e0236619. [Google Scholar] [CrossRef]
- Kim, B.S.; Nam, S.; Jin, Y.; Seo, K.-M. Simulation Framework for Cyber-Physical Production System: Applying Concept of LVC Interoperation. Complexity 2020, 2020, 4321873. [Google Scholar] [CrossRef]
- Kang, B.G.; Seo, K.-M.; Kim, T.G. Model-based Design of Defense Cyber-Physical Systems to Analyze Mission Effectiveness and Network Performance. IEEE Access 2019, 7, 42063–42080. [Google Scholar] [CrossRef]
- Seo, K.-M.; Hong, W.; Kim, T.G. Enhancing model composability and reusability for entity-level combat simulation: A conceptual modeling approach. Simulation 2017, 93, 825–840. [Google Scholar] [CrossRef] [Green Version]
- Fan, R.; Wang, Y.; Lin, J. Study on Multi-Agent Evolutionary Game of Emergency Management of Public Health Emergencies Based on Dynamic Rewards and Punishments. Int. J. Environ. Res. Public Health 2021, 18, 8278. [Google Scholar] [CrossRef]
- Hussein, T.; Löndahl, J.; Thuresson, S.; Alsved, M.; Al-Hunaiti, A.; Saksela, K.; Aqel, H.; Junninen, H.; Mahura, A.; Kulmala, M. Indoor model simulation for covid-19 transport and exposure. Int. J. Environ. Res. Public Health 2020, 17, 2927. [Google Scholar]
- Qi, B.; Tan, J.; Zhang, Q.; Cao, M.; Wang, X.; Zou, Y. Unfixed Movement Route Model, Non-Overcrowding and Social Distancing Reduce the Spread of COVID-19 in Sporting Facilities. Int. J. Environ. Res. Public Health 2021, 18, 8212. [Google Scholar] [CrossRef]
- Chen, Z.; Shu, Z.; Huang, X.; Peng, K.; Pan, J. Modelling Analysis of COVID-19 Transmission and the State of Emergency in Japan. Int. J. Environ. Res. Public Health 2021, 18, 6858. [Google Scholar] [CrossRef] [PubMed]
- Nesteruk, I. Estimations of the coronavirus epidemic dynamics in South Korea with the use of SIR model. Preprint 2020. [Google Scholar] [CrossRef]
- Mulder, L.J. A set of operational parameters for the SEIR model of the coronavirus for the world and some of the more heavily affected countries. J. Infect. Disease Model. 2020. [Google Scholar] [CrossRef]
- Calafiore, G.C.; Novara, C.; Possieri, C. A Modified SIR Model for the COVID-19 Contagion in Italy. In Proceedings of the 2020 59th IEEE Conference on Decision and Control (CDC), Jeju, Korea, 14–18 December 2020; Volume 59, pp. 3889–3894. [Google Scholar] [CrossRef]
- Alagoz, O.; Sethi, A.K.; Patterson, B.W.; Churpek, M.; Safdar, N. Effect of Timing of and Adherence to Social Distancing Measures on COVID-19 Burden in the United States. Ann. Intern. Med. 2021, 174, 50–57. [Google Scholar] [CrossRef]
- D’Orazio, M.; Bernardini, G.; Quagliarini, E. How to restart? An agent-based simulation model towards the definition of strategies for COVID-19 “second phase” in public buildings. arXiv 2020, arXiv:2004.12927. [Google Scholar]
- Cooper, I.; Mondal, A.; Antonopoulos, C.G. A SIR model assumption for the spread of COVID-19 in different communities. Chaos Solitons Fractals 2020, 139, 110057. [Google Scholar] [CrossRef] [PubMed]
- Malavika, B.; Marimuthu, S.; Joy, M.; Nadaraj, A.; Asirvatham, E.S.; Jeyaseelan, L. Forecasting COVID-19 epidemic in India and high incidence states using SIR and logistic growth models. Clin. Epidemiol. Glob. Heal. 2021, 9, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Xiao, C.; Yang, P. Numerical solution and parameter estimation for uncertain SIR model with application to COVID-19. Fuzzy Optim. Decis. Mak. 2020, 20, 189–208. [Google Scholar] [CrossRef]
- Piovella, N. Analytical solution of SEIR model describing the free spread of the COVID-19 pandemic. Chaos Solitons Fractals 2020, 140, 110243. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, X.; Ma, X.; Hong, N.; Su, L.; Ma, Y.; He, J.; Jiang, H.; Liu, C.; Shan, G.; et al. Forecasting the Worldwide Spread of COVID-19 based on Logistic Model and SEIR Model. MedRxiv 2020. [Google Scholar] [CrossRef]
- Mahmud, A.; Lim, P.Y. Applying the SEIR model in forecasting the COVID-19 trend in malaysia: A preliminary study. MedRxiv 2020. [Google Scholar] [CrossRef]
- Ferrari, L.; Gerardi, G.; Manzi, G.; Micheletti, A.; Nicolussi, F.; Biganzoli, E.; Salini, S. Modeling provincial Covid-19 epidemic data in Italy using an adjusted time-dependent SIRD model. arXiv 2020, arXiv:2005.12170v2. [Google Scholar]
- Chatterjee, S.; Sarkar, A.; Chatterjee, S.; Karmakar, M.; Paul, R. Studying the progress of COVID-19 outbreak in India using SIRD model. Indian J. Phys. 2020. [Google Scholar] [CrossRef]
- Fanelli, D.; Piazza, F. Analysis and forecast of COVID-19 spreading in China, Italy and France. Chaos Solitons Fractals 2020, 134, 109761. [Google Scholar] [CrossRef]
- Osemwinyen, A.C. Mathematical Modelling of the Transmission Dynamics of Ebola Virus. Appl. Comput. Math. 2015, 4, 313–320. [Google Scholar] [CrossRef]
- Basti, B.; Hammami, N.; Berrabah, I.; Nouioua, F.; Djemiat, R.; Benhamidouche, N. Stability Analysis and Existence of Solutions for a Modified SIRD Model of COVID-19 with Fractional Derivatives. Symmetry 2021, 13, 1431. [Google Scholar] [CrossRef]
- Shringi, S.; Sharma, H.; Rathie, P.; Bansal, J.; Nagar, A. Modified SIRD Model for COVID-19 Spread Prediction for Northern and Southern States of India. Chaos Solitons Fractals 2021, 148, 111039. [Google Scholar] [CrossRef] [PubMed]
- Devosmita, S.; Debasis, S. Use of Modified SIRD Model to Analyze COVID-19 Data. Ind. Eng. Chem. Res. 2021, 60, 4251–4260. [Google Scholar] [CrossRef]
- Kemeny, A.; Özmen, Ö.; Nutaro, J.J.; Pullum, L.L.; Ramanathan, A. Analyzing the impact of modeling choices and assumptions in compartmental epidemiological models. Simulation 2016, 92, 459–472. [Google Scholar]
- Zeigler, B.P.; Muzy, A.; Kofman, E. Theory of Modeling and Simulation: Discrete Event & Iterative System Computational Foundations; Academic Press: Cambridge, CA, USA, 2018. [Google Scholar]
- Kim, T.G.; Sung, C.H.; Hong, S.Y.; Hong, J.H.; Choi, C.B.; Kim, J.H.; Seo, K.M.; Bae, J.W. DEVSim++ Toolset for Defense Modeling and Simulation and Interoperation. J. Def. Model. Simul. 2011, 8, 129–142. [Google Scholar] [CrossRef]
- Venkatramanan, S.; Lewis, B.; Chen, J.; Higdon, D.; Vullikanti, A.; Marathe, M. Using data-driven agent-based models for forecasting emerging infectious diseases. Epidemics 2018, 22, 43–49. [Google Scholar] [CrossRef]
- Hong, J.H.; Seo, K.M.; Kim, T.G. Simulation-based optimization for design parameter exploration in hybrid system: A defense system example. Simulation 2013, 89, 362–380. [Google Scholar] [CrossRef]
- Kang, B.G.; Choi, S.H.; Kwon, S.J.; Lee, J.H.; Kim, T.G. Simulation-based optimization on the system-of-systems model via model transformation and genetic algorithm: A case study of network-centric warfare. Complexity 2018. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Bae, J.W. An Effective Adjustment to the Integration of Optimal Computing Budget Allocation for Particle Swarm Optimization in Stochastic Environments. IEEE Access 2020, 8, 173654–173665. [Google Scholar] [CrossRef]
- Shim, E.; Tariq, A.; Choi, W.; Lee, Y.; Chowell, G. Transmission potential and severity of COVID-19 in South Korea. Int. J. Infect. Dis. 2020, 93, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, J.; Song, W.; Xiu, C.; Ma, L.; Pei, T. Spread of COVID-19 in China: Analysis from a city-based epidemic and mobility model. Cities 2021, 110, 103010. [Google Scholar] [CrossRef]
- Courtemanche, C.; Garuccio, J.; Le, A.; Pinkston, J.; Yelowitz, A. Strong social distancing measures in the united states reduced the covid-19 growth rate. Health Aff. 2020, 39, 1237–1246. [Google Scholar] [CrossRef]
- Moosa, I.A. The effectiveness of social distancing in containing Covid-19. Appl. Econ. 2020, 52, 6292–6305. [Google Scholar] [CrossRef]
- McKibbin, W.J.; Fernando, R. The Global Macroeconomic Impacts of COVID-19: Seven Scenarios. SSRN Electron. J. 2020, 19, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Brotherhood, L.; Kircher, P.; Santos, C.; Tertilt, M. An Economic Model of the Covid-19 Epidemic: The Importance of Testing and Age-Specific Policies. IZA Inst. Lab. Econ. Discuss. 2020, 13265, 1–71. [Google Scholar]
- Zhao, S.Z.; Wong, J.Y.H.; Wu, Y.; Choi, E.P.H.; Wang, M.P.; Lam, T.H. Social distancing compliance under covid-19 pandemic and mental health impacts: A population-based study. Int. J. Environ. Res. Public Health 2020, 17, 6692. [Google Scholar] [CrossRef]
- Banks, J. Institute for Fiscal Studies: The mental health effects of the first two months of lockdown and social distancing during the Covid-19 pandemic in the UK. Inst. Fisc. Stud. 2020. [Google Scholar] [CrossRef]
- Morato, M.M.; Pataro, I.M.L.; Americano da Costa, M.V.; Normey-Rico, J.E. A parametrized nonlinear predictive control strategy for relaxing COVID-19 social distancing measures in Brazil. ISA Trans. 2020. [Google Scholar] [CrossRef] [PubMed]
- Armando, C.; Luigi, D.F.; Maria, M. How mobility habits influenced the spread of the COVID-19 pandemic: Results from the Italian case study. Sci. Total Environ. 2020, 741, 140489. [Google Scholar] [CrossRef]
- Badr, H.S.; Du, H.; Marshall, M.; Dong, E.; Squire, M.M.; Gardner, L.M. Association between mobility patterns and COVID-19 transmission in the USA: A mathematical modelling study. Lancet Infect. Dis. 2020, 20, 1247–1254. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, R.; Hu, D.; Yue, Y.; Li, Q.; Xia, J. Effects of human mobility restrictions on the spread of COVID-19 in Shenzhen, China: A modelling study using mobile phone data. Lancet Digit. Heal. 2020, 2, 417–424. [Google Scholar] [CrossRef]
- Chang, S.; Pierson, E.; Koh, P.W.; Gerardin, J.; Redbird, B.; Grusky, D.; Leskovec, J. Mobility network models of COVID-19 explain inequities and inform reopening. Nature 2021, 589, 82–87. [Google Scholar] [CrossRef] [PubMed]




| Input Parameter | Notation | Description |
|---|---|---|
| Infection parameter set | The rate of infection | |
| The rate of recovery | ||
| The rate of death | ||
| The attenuation coefficient of increase in the rate of infection | ||
| The attenuation coefficient of decrease in the rate of infection | ||
| Policy parameter set | The duration time of the relaxed social distancing policy | |
| The duration time of the severe social distancing policy |
| Output Parameter | Notation | Description |
|---|---|---|
| Variables for SIRD model | The number of susceptible population in the major city/country at time t | |
| The number of the infected population in the major city/country at time t | ||
| The number of recovered population in the major city/country at time t | ||
| The number of deaths population in the major city/country at time t |
| Parameter | Optimized Coefficient |
|---|---|
| 0.0461 | |
| 0.0460 | |
| 0.0010 | |
| 0.0108 | |
| 0.2573 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, B.G.; Park, H.-M.; Jang, M.; Seo, K.-M. Hybrid Model-Based Simulation Analysis on the Effects of Social Distancing Policy of the COVID-19 Epidemic. Int. J. Environ. Res. Public Health 2021, 18, 11264. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph182111264
Kang BG, Park H-M, Jang M, Seo K-M. Hybrid Model-Based Simulation Analysis on the Effects of Social Distancing Policy of the COVID-19 Epidemic. International Journal of Environmental Research and Public Health. 2021; 18(21):11264. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph182111264
Chicago/Turabian StyleKang, Bong Gu, Hee-Mun Park, Mi Jang, and Kyung-Min Seo. 2021. "Hybrid Model-Based Simulation Analysis on the Effects of Social Distancing Policy of the COVID-19 Epidemic" International Journal of Environmental Research and Public Health 18, no. 21: 11264. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph182111264






