Short-Term Aerobic Exercise Did Not Change Telomere Length While It Reduced Testosterone Levels and Obesity Indexes in PCOS: A Randomized Controlled Clinical Trial Study
Abstract
:1. Introduction
2. Participants, Materials and Methods
2.1. Study Design and Ethics Statement
2.2. Participants
2.3. Clinical and Biochemical Measurements
2.4. Anthropometry and DXA
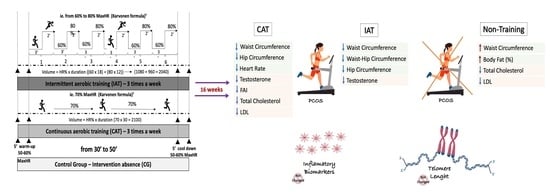
2.5. Aerobic Physical Training Protocols
2.6. Telomere Length Measurement
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Hardiman, P.J.; Petersen, I.; Wang, F.F.; Qu, F.; Baio, G. The prevalence of polycystic ovary syndrome in repro-ductive-aged women of different ethnicity: A systematic review and meta-analysis. Oncotarget 2017, 8, 96351–96358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teede, H.; Deeks, A.; Moran, L. Polycystic ovary syndrome: A complex condition with psychological, reproductive and met-abolic manifestations that impacts on health across the lifespan. BMC Med. 2010, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vesa, C.M.; Popa, L.; Popa, A.R.; Rus, M.; Zaha, A.A.; Bungau, S.; Tit, D.M.; Aron, R.A.C.; Zaha, D.C. Current Data Regarding the Relationship between Type 2 Diabetes Mellitus and Cardiovascular Risk Factors. Diagnostics 2020, 10, 314. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Vellanki, P.; Ata, B.; Yildiz, B.O. Metabolic syndrome, hypertension, and hyperlipidemia in mothers, fathers, sisters, and brothers of women with polycystic ovary syndrome: A systematic review and meta-analysis. Fertil. Steril. 2018, 109, 356–364.e32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crosignani, P.G.; Colombo, M.; Vegetti, W.; Somigliana, E.; Gessati, A.; Ragni, G. Overweight and obese anovulatory patients with polycystic ovaries: Parallel improvements in anthropometric indices, ovarian physiology and fertility rate induced by diet. Hum. Reprod. 2003, 18, 1928–1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda-Furtado, C.L.; Ramos, F.K.; Kogure, G.S.; Santana-Lemos, B.A.; Ferriani, R.A.; Calado, R.T.; Dos Reis, R.M. A Nonrandomized Trial of Progressive Resistance Training Intervention in Women With Polycystic Ovary Syndrome and Its Implications in Telomere Content. Reprod. Sci. 2016, 23, 644–654. [Google Scholar] [CrossRef]
- Kogure, G.S.; Miranda-Furtado, C.L.; Silva, R.C.; Melo, A.S.; Ferriani, R.A.; De Sá, M.F.; Dos Reis, R.M. Resistance Exercise Impacts Lean Muscle Mass in Women with Polycystic Ovary Syndrome. Med. Sci. Sports Exerc. 2016, 48, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Patten, R.K.; Boyle, R.A.; Moholdt, T.; Kiel, I.; Hopkins, W.G.; Harrison, C.L.; Stepto, N.K. Exercise Interventions in Poly-cystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Physiol. 2020, 11, 606. [Google Scholar] [CrossRef]
- Sagvekar, P.; Kumar, P.; Mangoli, V.; Desai, S.; Mukherjee, S. DNA methylome profiling of granulosa cells reveals altered methylation in genes regulating vital ovarian functions in polycystic ovary syndrome. Clin. Epigenetics 2019, 11, 61. [Google Scholar] [CrossRef] [Green Version]
- Kalmbach, K.H.; Antunes, D.M.; Kohlrausch, F.; Keefe, D.L. Telomeres and Female Reproductive Aging. Semin. Reprod. Med. 2015, 33, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Wu, M.; Bondy, S.C. Telomere shortening during aging: Attenuation by antioxidants and anti-inflammatory agents. Mech. Ageing Dev. 2017, 164, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T.; Yewdell, W.T.; Wilkerson, K.L.; Regal, J.A.; Kajigaya, S.; Stratakis, C.A.; Young, N.S. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 2009, 114, 2236–2243. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, D.C.; Miranda-Furtado, C.L.; Kogure, G.S.; Meola, J.; Okuka, M.; Silva, C.; Calado, R.T.; Ferriani, R.A.; Keefe, D.L.; dos Reis, R.M. Inflammatory biomarkers and telomere length in women with polycystic ovary syndrome. Fertil. Steril. 2015, 103, 542–547.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shen, F.; Zhu, Y.; Fang, Y.; Lu, S. Telomeric repeat-containing RNA (TERRA) related to polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2017, 86, 552–559. [Google Scholar] [CrossRef]
- Vasilopoulos, E.; Fragkiadaki, P.; Kalliora, C.; Fragou, D.; Docea, A.O.; Vakonaki, E.; Tsoukalas, D.; Calina, D.; Buga, A.M.; Georgiadis, G. The association of female and male in-fertility with telomere length (Review). Int. J. Mol. Med. 2019, 44, 375–389. [Google Scholar] [CrossRef]
- LaRocca, T.J.; Seals, D.R.; Pierce, G.L. Leukocyte telomere length is preserved with aging in endurance exercise-trained adults and related to maximal aerobic capacity. Mech. Ageing Dev. 2010, 131, 165–167. [Google Scholar] [CrossRef] [Green Version]
- Puterman, E.; Weiss, J.; Lin, J.; Schilf, S.; Slusher, A.L.; Johansen, K.L.; Epel, E.S. Aerobic exercise lengthens telomeres and reduces stress in family caregivers: A randomized controlled trial—Curt Richter Award Paper 2018. Psychoneuroendocrinology 2018, 98, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.; O’Brien, B.J.; Charchar, F.J. Telomere Length Maintenance and Cardio-Metabolic Disease Prevention Through Exercise Training. Sports Med. 2016, 46, 1213–1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dankel, S.J.; Loenneke, J.P.; Loprinzi, P.D. The impact of overweight/obesity duration and physical activity on telomere length: An application of the WATCH paradigm. Obes Res. Clin. Pr. 2017, 11, 247–252. [Google Scholar] [CrossRef]
- Hutchison, S.K.; Stepto, N.K.; Harrison, C.L.; Moran, L.J.; Strauss, B.J.; Teede, H.J. Effects of exercise on insulin resistance and body composition in overweight and obese women with and without polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E48–E56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwood, E.A.; Noel, M.W.; Kao, C.N.; Shinkai, K.; Pasch, L.A.; Cedars, M.I.; Huddleston, H.G. Vigorous exercise is associated with superior metabolic profiles in polycystic ovary syndrome independent of total exercise expenditure. Fertil. Steril. 2016, 105, 486–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, V.B.; Kogure, G.S.; Lopes, I.P.; Silva, R.C.; Pedroso, D.C.C.; de Melo, A.S.; de Souza, H.C.D.; Ferriani, R.A.; Miranda Furtado, C.L.; Dos Reis, R.M. Effects of continuous and intermittent aerobic physical training on hormonal and metabolic profile, and body composition in women with polycystic ovary syndrome: A randomized controlled trial. Clin. En-Docrinol. 2020, 11, 14194. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- International Society for the Advancement of Kinanthropometry. International Standards for Anthropometric Assessment; The University of South Australia: Adelaide, Australia, 2001. [Google Scholar]
- American College of Sports Medicine. Guidelines for Exercise Testing and Prescription, 9th ed.; Guanabara Koogan: Philadelphia, PA, USA, 2014. [Google Scholar]
- Calado, R.T.; Brudno, J.; Mehta, P.; Kovacs, J.J.; Wu, C.; Zago, M.A.; Chanock, S.J.; Boyer, T.D.; Young, N.S. Constitutional telomerase mutations are genetic risk factors for cirrhosis. Hepatology 2011, 53, 1600–1607. [Google Scholar] [CrossRef] [Green Version]
- Cawthon, R.M. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Du, J.; Feng, R.; Xu, Y.; Wang, H.; Sang, Q.; Xing, Q.; Zhao, X.; Jin, L.; He, L.; et al. A possible new mechanism in the pathophysiology of polycystic ovary syndrome (PCOS): The discovery that leukocyte telomere length is strongly associated with PCOS. J. Clin. Endocrinol. Metab. 2014, 99, E234–E240. [Google Scholar] [CrossRef] [Green Version]
- Wei, D.; Xie, J.; Yin, B.; Hao, H.; Song, X.; Liu, Q.; Zhang, C.; Sun, Y. Significantly lengthened telomere in granulosa cells from women with polycystic ovarian syndrome (PCOS). J. Assist. Reprod. Genet. 2017, 34, 861–866. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Sng, E. Mode-specific physical activity and leukocyte telomere length among U.S. adults: Implications of running on cellular aging. Prev Med. 2016, 85, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.M.; Buxton, J.L.; Kantomaa, M.T.; Tammelin, T.H.; Blakemore, A.I.F.; Järvelin, M.R. Associations of Leukocyte Telomere Length With Aerobic and Muscular Fitness in Young Adults. Am. J. Epidemiol. 2017, 185, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Tucker, L.A. Physical activity and telomere length in U.S. men and women: An NHANES investigation. Prev. Med. 2017, 100, 145–151. [Google Scholar] [CrossRef]
- Borghini, A.; Giardini, G.; Tonacci, A.; Mastorci, F.; Mercuri, A.; Mrakic-Sposta, S.; Sposta, S.M.; Moretti, S.; Andreassi, M.G.; Pratali, L. Chronic and acute effects of endurance training on telomere length. Mutagenesis 2015, 30, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Almenning, I.; Rieber-Mohn, A.; Lundgren, K.M.; Shetelig Løvvik, T.; Garnæs, K.K.; Moholdt, T. Effects of High Intensity Interval Training and Strength Training on Metabolic, Cardiovascular and Hormonal Outcomes in Women with Polycystic Ovary Syndrome: A Pilot Study. PLoS ONE 2015, 10, e0138793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mario, F.M.; Graff, S.K.; Spritzer, P.M. Habitual physical activity is associated with improved anthropometric and androgenic profile in PCOS: A cross-sectional study. J. Endocrinol. Investig. 2017, 40, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.; Broom, D.; Harrop, D.; Lahart, I.; Carter, A.; Dalton, C.; Metwally, M.; Klonizakis, M. The effects of physical exercise on cardiometabolic outcomes in women with polycystic ovary syndrome not taking the oral contraceptive pill: A sys-tematic review and meta-analysis. J. Diabetes Metab. Dis. 2019, 18, 597–612. [Google Scholar] [CrossRef] [Green Version]
- Salehi, M.; Bravo-Vera, R.; Sheikh, A.; Gouller, A.; Poretsky, L. Pathogenesis of polycystic ovary syndrome: What is the role of obesity? Metabolism 2004, 53, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Armanios, M. Telomeres and age-related disease: How telomere biology informs clinical paradigms. J. Clin. Investig. 2013, 123, 996–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandao, C.F.C.; Nonino, C.B.; de Carvalho, F.G.; Nicoletti, C.F.; Noronha, N.Y.; San Martin, R.; de Freitas, E.C.; Jun-queira-Franco, M.V.M.; Marchini, J.S. The effects of short-term combined exercise training on telomere length in obese women: A prospective, interventional study. Sports Med. Open 2020, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Mason, C.; Risques, R.A.; Xiao, L.; Duggan, C.R.; Imayama, I.; Campbell, K.L.; Kong, A.; Foster-Schubert, K.E.; Wang, C.Y.; Alfano, C.M.; et al. Independent and combined effects of dietary weight loss and exercise on leukocyte telomere length in postmenopausal women. Obesity 2013, 21, E549–E554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calado, R.T.; Cooper, J.N.; Padilla-Nash, H.M.; Sloand, E.M.; Wu, C.O.; Scheinberg, P.; Ried, T.; Young, N.S. Short telo-meres result in chromosomal instability in hematopoietic cells and precede malignant evolution in human aplastic anemia. Leukemia 2012, 26, 700–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saßenroth, D.; Meyer, A.; Salewsky, B.; Kroh, M.; Norman, K.; Steinhagen-Thiessen, E.; Demuth, I. Sports and Exercise at Different Ages and Leukocyte Telomere Length in Later Life--Data from the Berlin Aging Study II (BASE-II). PLoS ONE 2015, 10, e0142131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gielen, M.; Hageman, G.J.; Antoniou, E.E.; Nordfjall, K.; Mangino, M.; Balasubramanyam, M.; de Meyer, T.; Hendricks, A.E.; Giltay, E.J.; Hunt, S.C.; et al. Body mass index is negatively associated with telomere length: A collaborative cross-sectional meta-analysis of 87 observational studies. Am. J. Clin. Nutr. 2018, 108, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Sabuncu, T.; Vural, H.; Harma, M.; Harma, M. Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease. Clin. Biochem. 2001, 34, 407–413. [Google Scholar] [CrossRef]
- Votruba, S.B.; Horvitz, M.A.; Schoeller, D.A. The role of exercise in the treatment of obesity. Nutrition 2000, 16, 179–188. [Google Scholar] [CrossRef]
- Sohail, M.U.; Yassine, H.M.; Sohail, A.; Al Thani, A.A. Impact of Physical Exercise on Gut Microbiome, Inflammation, and the Pathobiology of Metabolic Disorders. Rev. Diabet Stud. 2019, 15, 35–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codella, R.; Luzi, L.; Terruzzi, I. Exercise has the guts: How physical activity may positively modulate gut microbiota in chronic and immune-based diseases. Dig. Liver Dis. 2018, 50, 331–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| CG (n = 30) | CAT (n = 28) | IAT (n = 29) | ||||
|---|---|---|---|---|---|---|
| Variables | Before Mean (SD) | After Mean (SD) | Before Mean (SD) | After Mean (SD) | Before Mean (SD) | After Mean (SD) |
| Age (years) | 28.50 (5.76) | – | 29.14 (5.26) | – | 28.97 (4.32) | – |
| Height (m) | 1.61 (0.07) | – | 1.62 (0.06) | – | 1.64 (0.07) | – |
| Weight (kg) | 75.37 (14.33) | 76.05 (15.09) | 74.4 (16.5) | 73.74 (16.78) | 77.36 (16.91) | 77.00 (16.81) |
| BMI (kg/m2) | 29.09 (5.25) | 29.33 (5.43) | 28.43 (5.62) | 28.17 (5.67) | 28.67 (4.76) | 28.53 (4.82) |
| WC (cm) | 89.52 (12.61) | 90.98 (13.14) | 88.12 (13.60) | 86.58 (13.12) | 90.54 (11.33) | 88.67 (12.43) |
| HC (cm) | 106.34 (10.15) | 107.23 (9.75) | 105.88 (9.58) | 104.55 (10.27) | 107.31 (9.50) | 107.17 (10.98) |
| WHR (cm) | 0.84 (0.08) | 0.85 (0.07) | 0.83 (0.08) | 0.82 (0.07) | 0.84 (0.06) | 0.83 (0.07) |
| SBP (mm Hg) | 105.07 (11.29) | 108.13 (10.42) | 104.36 (9.39) | 100.39 (9.88) | 104.69 (12.12) | 103.93 (14.19) |
| DBP (mm Hg) | 71.40 (10.11) | 72.00 (8.89) | 69.64 (9.85) | 68.25 (9.02) | 68.52 (9.38) | 68.52 (11.38) |
| Heart Rate (bpm) | 74.93 (11.17) | 76.90 (10.00) | 76.82 (12.44) | 71.86 (9.52) | 73.90 (9.98) | 73.86 (12.34) |
| Biochemical parameters | ||||||
| Testosterone (ng/dL) | 86.2 (37) a | 99.67 (46.40) | 116.7 (49.5) a | 92.7 (37.8) | 107.69 (51.53) | 87.8 (54.2) |
| Androstenedione (ng/dL) | 87.2 (56) | 78.63 (45.60) | 86.64 (44.61) | 82.0 (28) | 77.69 (59.75) | 74.52 (49.42) |
| SHBG (nmol/L) | 50.56 (34.21) | 62.26 (45.29) | 54.31 (40.89) | 58 (61) | 47.82 (28.19) | 53.49 (31.00) |
| FAI | 7.52 (4.21) | 7.90 (5.98) | 11.33 (9.58) | 10.3 (10) | 9.87 (7.20) | 7.84 (7.72) |
| E2 (pg/mL) | 48.12 (23.73) | 48.31 (22.07) | 55.83 (42.84) | 67 (69) | 63.44 (55.42) | 61.41 (48.73) |
| LH (μUI/mL) | 9.25 (8.40) | 6.78 (4.81) | 7.79 (4.76) | 8.32 (8.56) | 7.77 (4.87) | 7.63 (4.74) |
| FSH (μIU/mL) | 5.63 (1.99) | 5.16 (1.78) | 5.66 (2.14) | 5.12 (1.83) | 5.21 (2.95) | 5.59 (2.71) |
| Total Cholesterol (mg/dL) | 188.27 (34.13) | 177.53 (24.44) | 184.64 (29.83) | 171.45 (28.07) | 178.86 (29.34) | 174.17 (26.86) |
| Triglycerides (mg/dL) | 111.77 (55.82) | 103.17 (58.87) | 151.43 (172.63) | 144.35 (139.16) | 98.62 (54.49) | 106.83 (60.85) |
| HDL (mg/dL) | 50.10 (13.09) | 48.47 (12.66) | 45.67 (9.33) | 44.28 (10.29) | 48.78 (10.62) | 47.08 (10.27) |
| LDL (mg/dL) | 115.73 (31.55) | 108.33 (26.80) | 111.71 (23.55) | 102.46 (23.14) | 112.31 (23.49) | 106.24 (23.19) |
| Fasting Glycemia (mg/dL) | 83.0 (7.0) | 81.0 (9.0) | 84.0 (12.0) | 84.0 (11.0) | 82.0 (11.0) | 82 (11.0) |
| Fasting Insulin (μIU/mL) | 12.83 (8.5) | 12.45 (9.79) | 11.31 (8.09) | 11.2 (8.3) | 9.52 (7.18) | 10.40 (7.03) |
| HOMA-IR | 2.64 (1.77) | 2.59 (2.17) | 2.45 (1.90) | 2.42 (1.97) | 2.02 (1.88) | 2.20 (1.79) |
| Homocysteine (µmol/L) | 7.62 (1.74) | 7.89 (1.64) | 8.05 (2.40) | 7.66 (1.70) | 7.17 (1.80) | 7.54 (1.94) |
| C-reative protein (mg/dL) | 0.51 (0.41) | 0.53 (0.54) | 0.36 (0.41) | 0.43 (0.54) | 0.36 (0.32) | 0.35 (0.32) |
| Body composition (DXA) | ||||||
| Body Fat (%) | 40.59 (6.26) | 41.83 (4.36) | 40.25 (4.67) | 39.20 (5.63) | 41.97 (3.79) | 41.80 (5.53) |
| Android (%) | 42.33 (8.09) | 43.90 (3.08) | 42.25 (6.30) | 40.69 (7.13) | 43.37 (4.77) | 43.17 (5.60) |
| Gynoid (%) | 43.79 (6.10) | 44.e93 (4.26) | 43.58 (4.97) | 42.41 (5.60) | 45.23 (4.59) | 44.22 (5.54) |
| Fat Mass_height2 (kg/m2) | 11.80 (3.45) | 12.13 (3.08) | 11.43 (3.38) | 11.09 (3.49) | 11.80 (2.60) | 11.67 (2.74) |
| Lean Mass_height2 (kg/m2) | 16.80 (2.76) | 16.53 (2.53) | 16.51 (2.49) | 16.64 (2.45) | 16.11 (2.39) | 16.32 (2.61) |
| Telomere Length | ||||||
| T/S ratio * | 1.40 (0.50) | 1.45 (0.46) | 1.43 (0.39) | 1.45 (0.46) | 1.53 (0.46) | 1.54 (0.51) |
| Variables | T/S Ratio | |
|---|---|---|
| r | p-Value | |
| Age (years) | −0.1633 | 0.0324 |
| BMI (kg/m2) | −0.1770 | 0.0194 |
| WC (cm) | −0.1613 | 0.0334 |
| Testosterone (ng/dL) | 0.1027 | 0.1773 |
| Androstenedione (ng/dL) | 0.0090 | 0.9057 |
| CRP (mg/dL) | −0.2161 | 0.0041 |
| Homocysteine (µmol/L) | −0.1635 | 0.0311 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, V.B.; Pedroso, D.C.C.; Kogure, G.S.; Lopes, I.P.; Santana, B.A.; Dutra de Souza, H.C.; Ferriani, R.A.; Calado, R.T.; Furtado, C.L.M.; Reis, R.M.d. Short-Term Aerobic Exercise Did Not Change Telomere Length While It Reduced Testosterone Levels and Obesity Indexes in PCOS: A Randomized Controlled Clinical Trial Study. Int. J. Environ. Res. Public Health 2021, 18, 11274. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph182111274
Ribeiro VB, Pedroso DCC, Kogure GS, Lopes IP, Santana BA, Dutra de Souza HC, Ferriani RA, Calado RT, Furtado CLM, Reis RMd. Short-Term Aerobic Exercise Did Not Change Telomere Length While It Reduced Testosterone Levels and Obesity Indexes in PCOS: A Randomized Controlled Clinical Trial Study. International Journal of Environmental Research and Public Health. 2021; 18(21):11274. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph182111274
Chicago/Turabian StyleRibeiro, Victor Barbosa, Daiana Cristina Chielli Pedroso, Gislaine Satyko Kogure, Iris Palma Lopes, Barbara Aparecida Santana, Hugo Celso Dutra de Souza, Rui Alberto Ferriani, Rodrigo Tocantins Calado, Cristiana Libardi Miranda Furtado, and Rosana Maria dos Reis. 2021. "Short-Term Aerobic Exercise Did Not Change Telomere Length While It Reduced Testosterone Levels and Obesity Indexes in PCOS: A Randomized Controlled Clinical Trial Study" International Journal of Environmental Research and Public Health 18, no. 21: 11274. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph182111274