Treatment of Stage 2 Medication-Induced Osteonecrosis of the Jaw: A Case Series
Abstract
:1. Introduction
- -
- Current or prior treatment with anti-resorptive or antiangiogenic agents.
- -
- Maxillofacial intra- or extraoral bone fistula persisting for >8 weeks.
- -
- No history of maxillary radiation therapy or bone metastases [8].
2. Case Reports
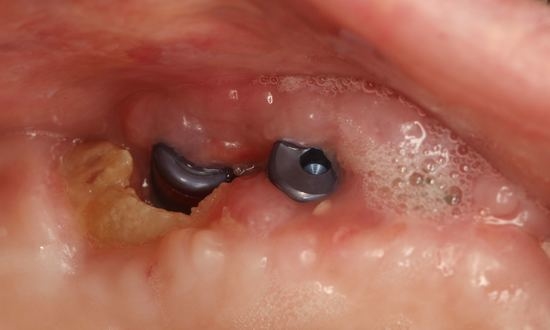
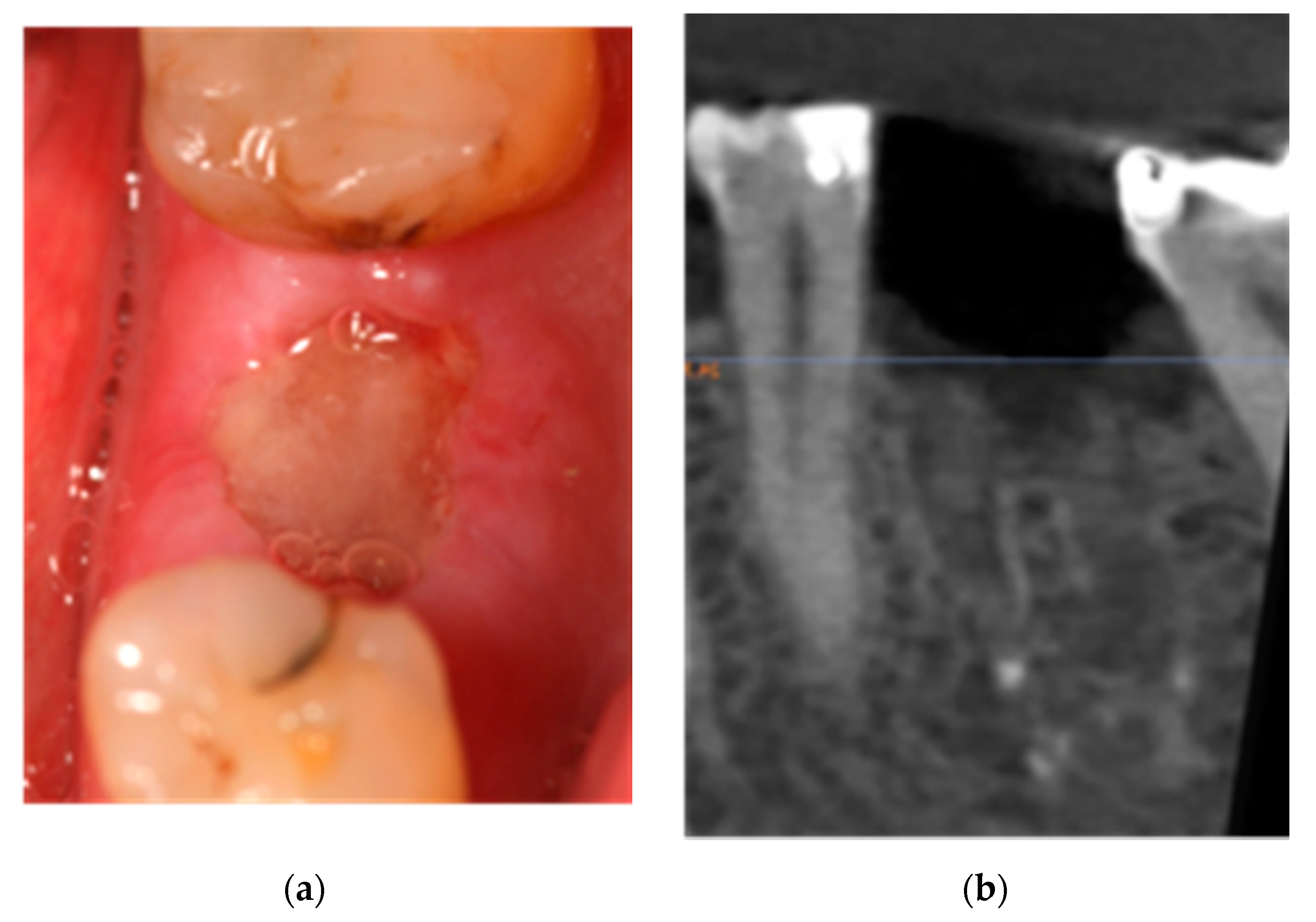
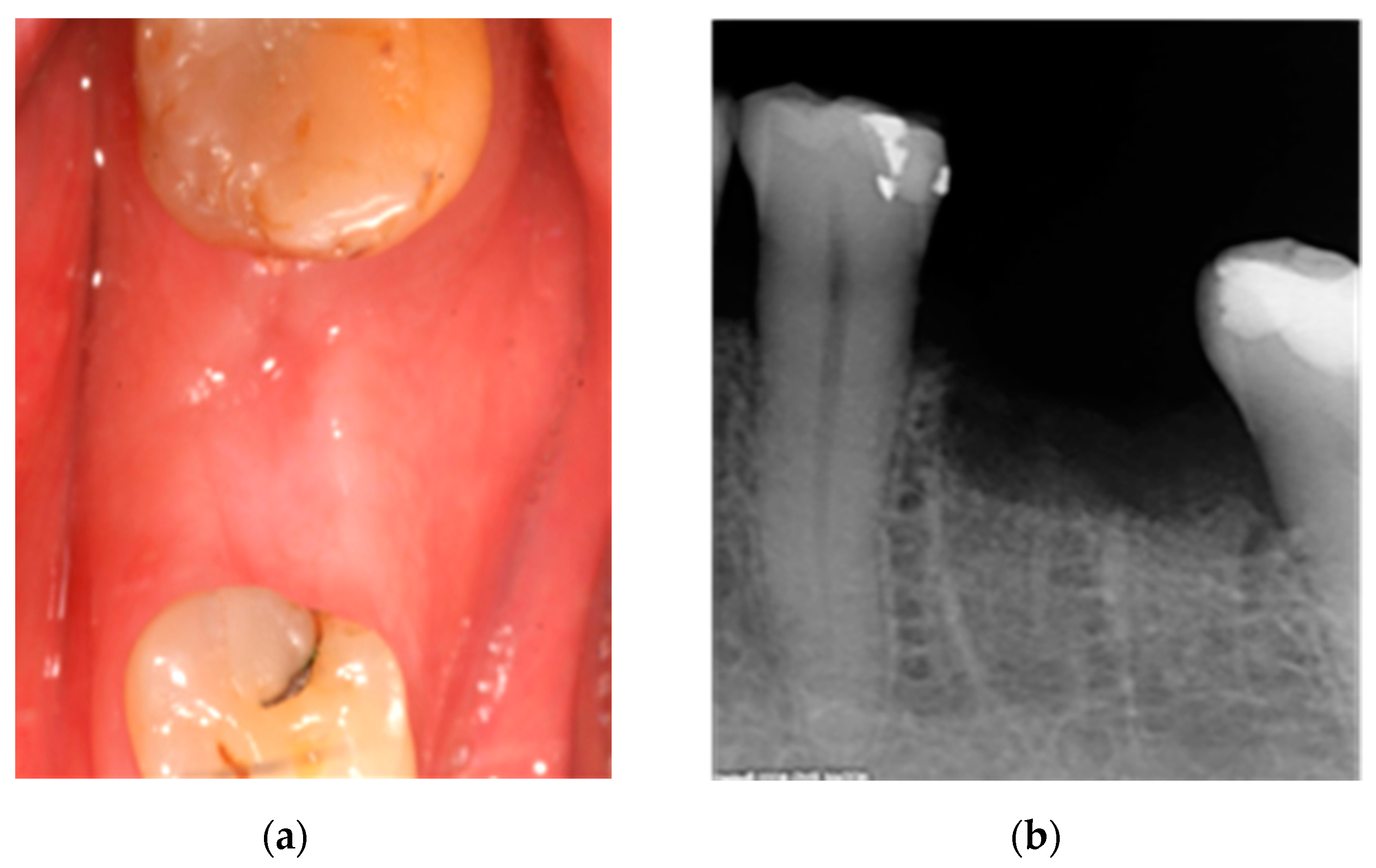
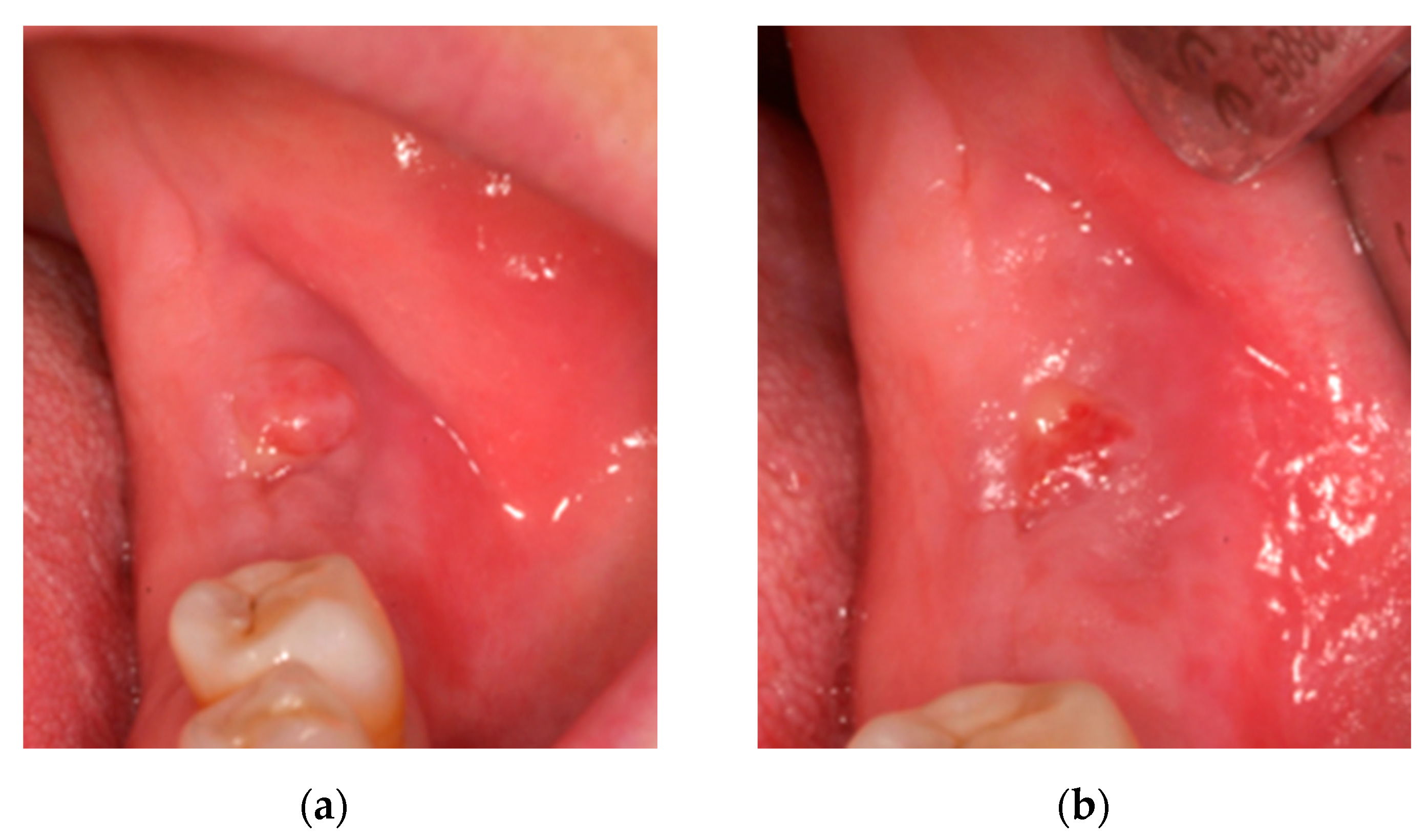
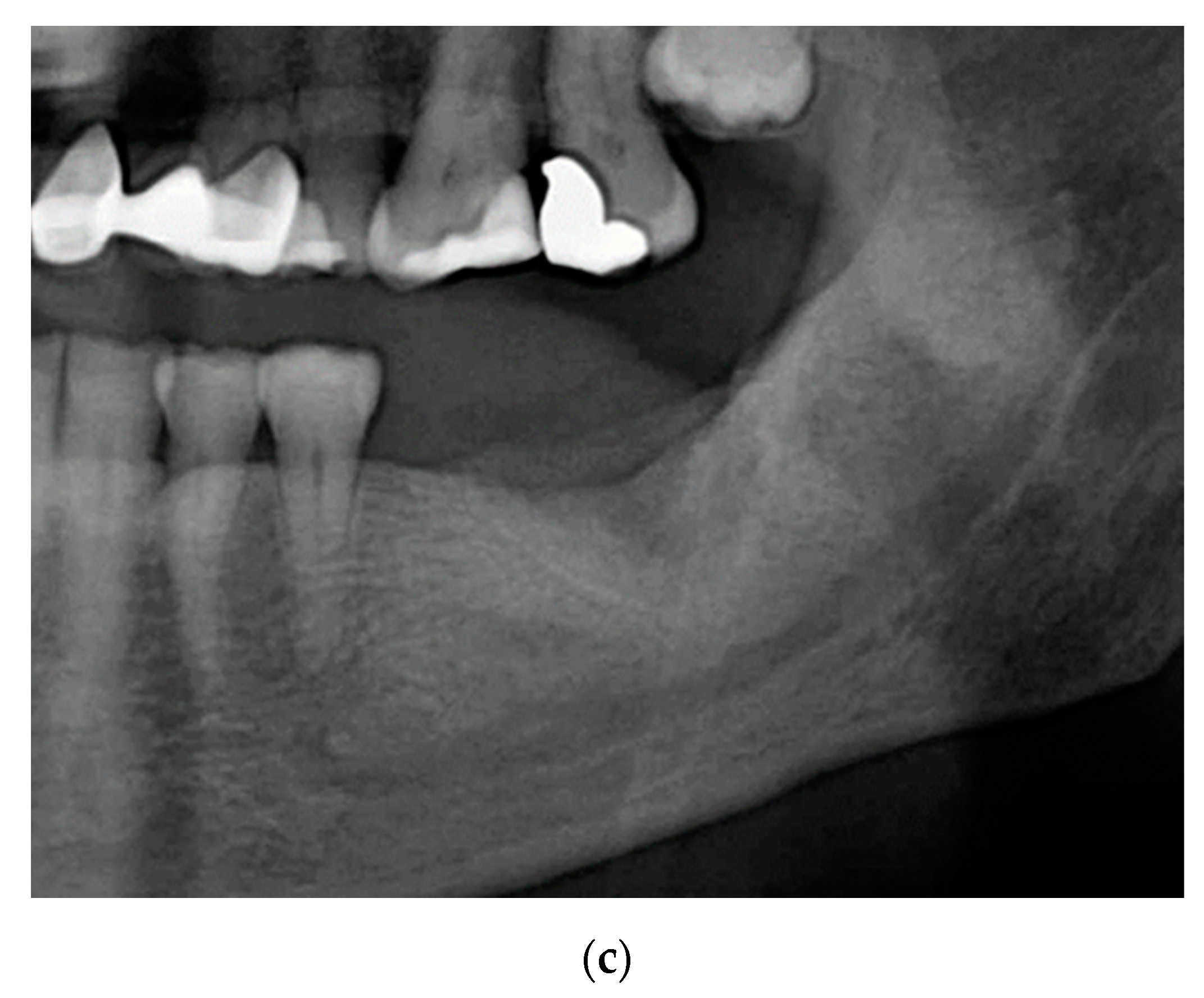
2.1. Case 1: Conservative Treatment
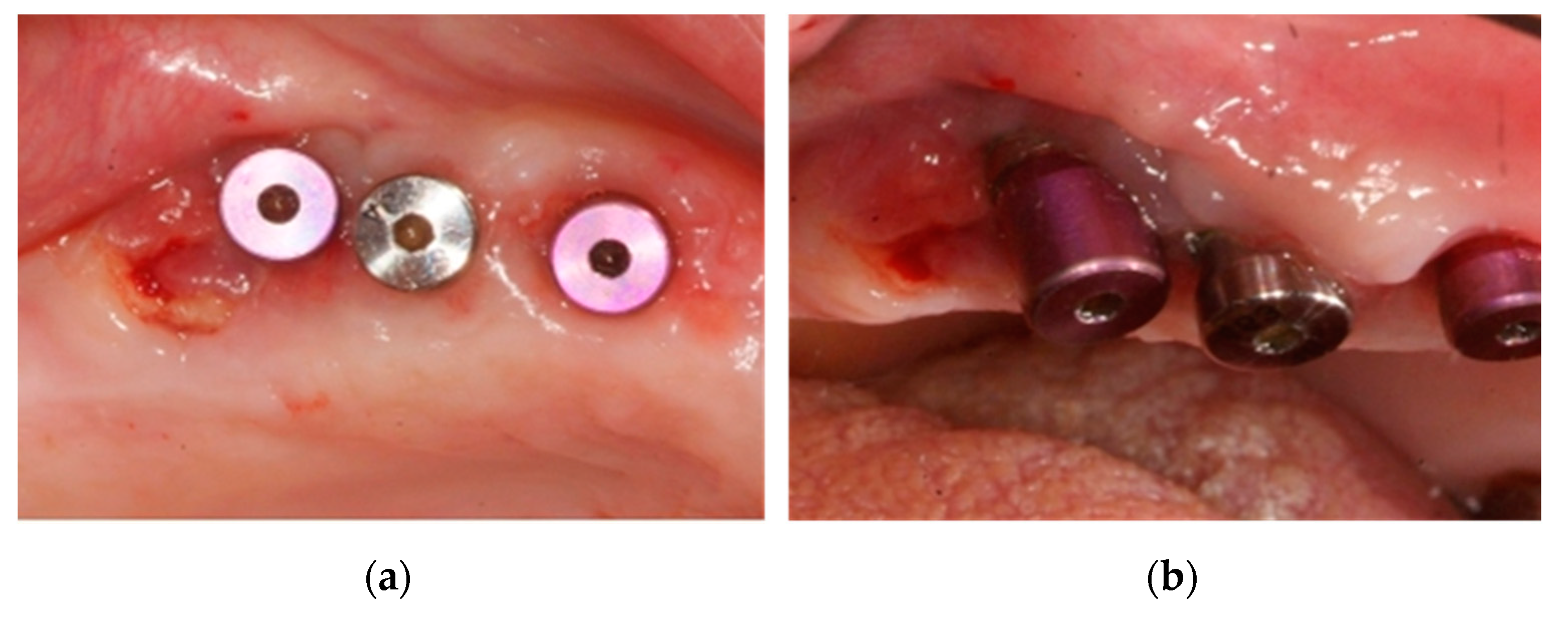
2.2. Case 2: Conservative Treatment Plus Surgery
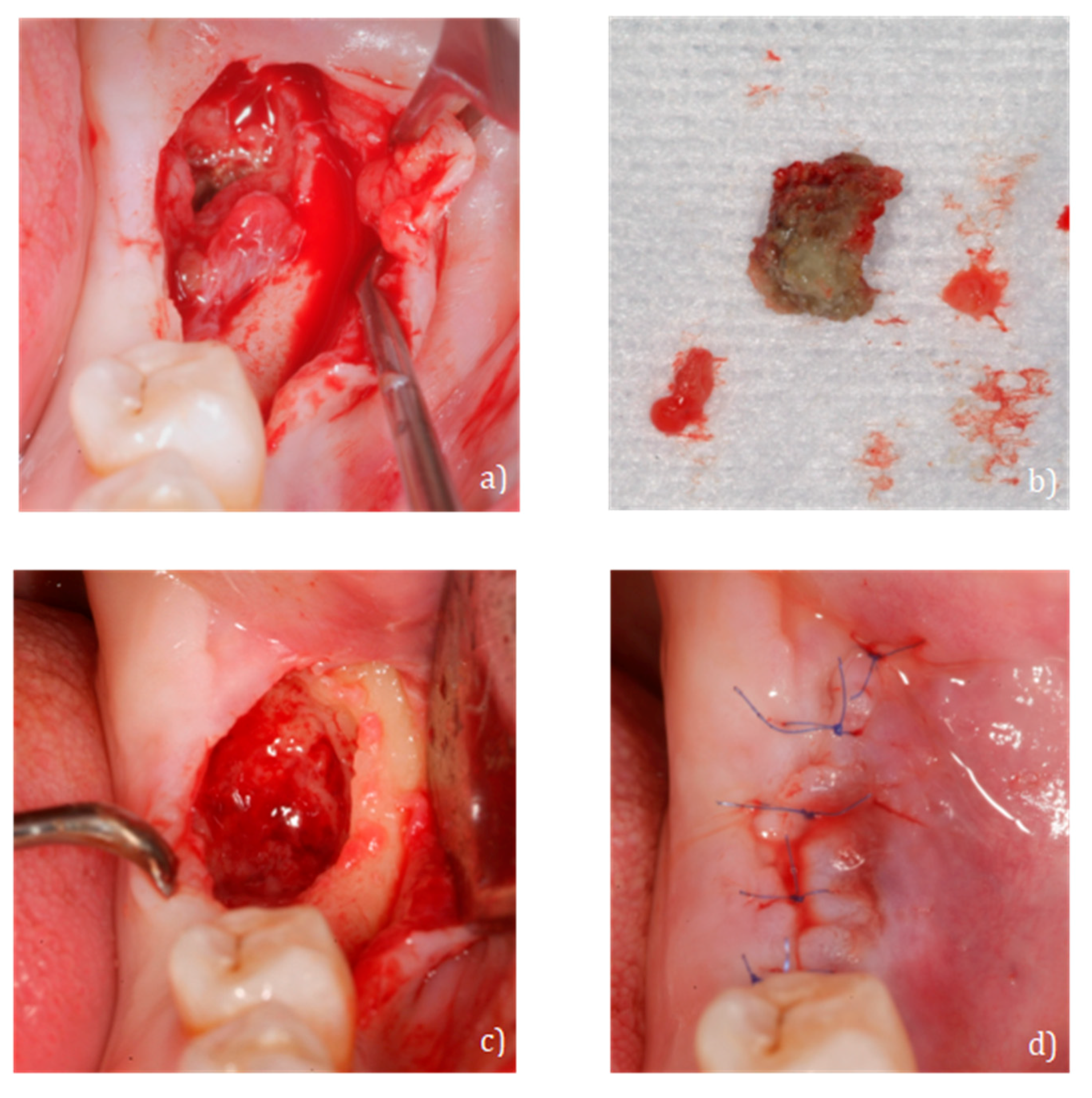
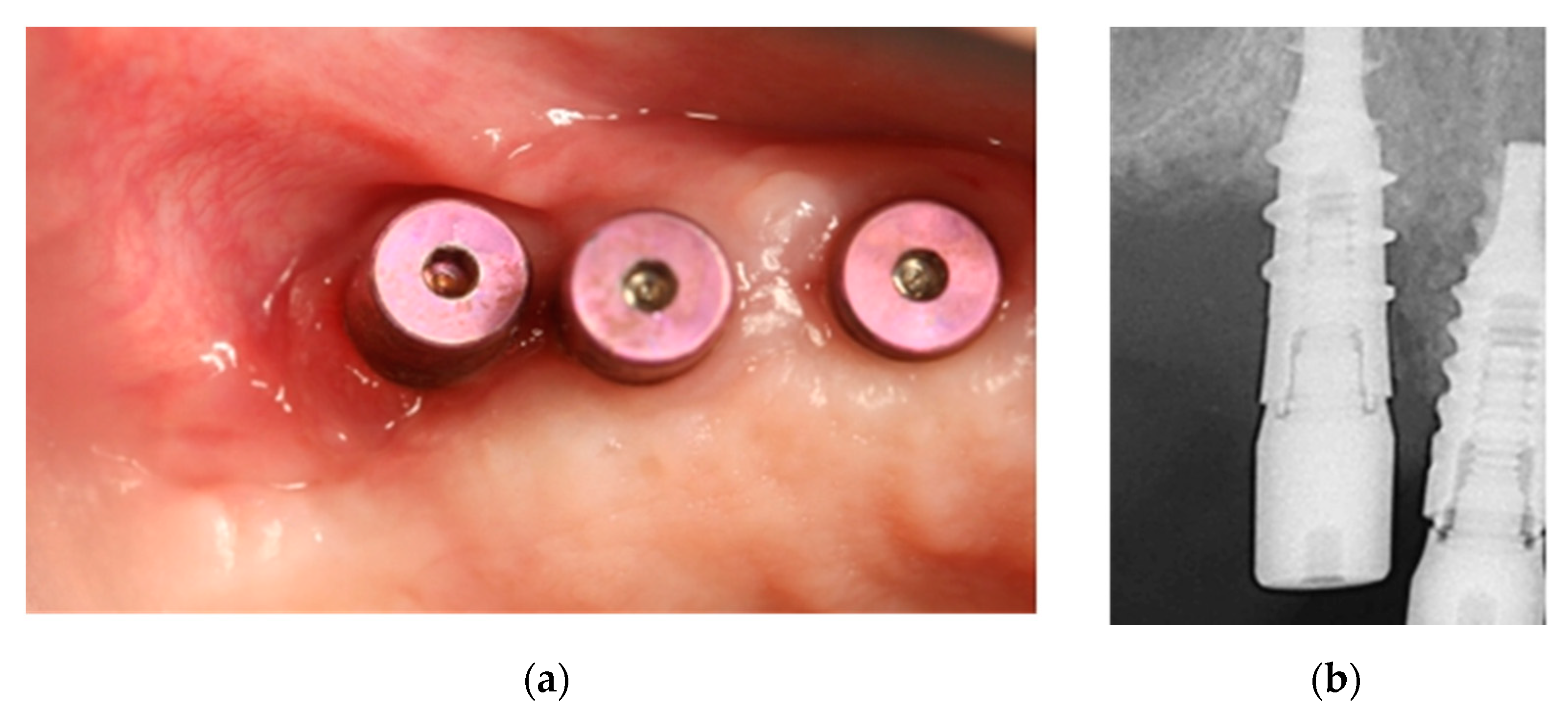
2.3. Case 3: Conservative Treatment + SURGERY + PRF-L
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Di Fede, O.; Panzarella, V.; Mauceri, R.; Fusco, V.; Bedogni, A.; Lo Muzio, L.; Board, S.O.; Campisi, G. The Dental Management of Patients at Risk of Medication-Related Osteonecrosis of the Jaw: New Paradigm of Primary Prevention. BioMed Res Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Akashi, M.; Kusumoto, J.; Takeda, D.; Shigeta, T.; Hasegawa, T.; Komori, T. A literature review of perioperative antibiotic administration in surgery for medication-related osteonecrosis of the jaw. Oral Maxillofac. Surg. 2018, 22, 369–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlDhalaan, N.A.; BaQais, A.; Al-Omar, A. Medication-related Osteonecrosis of the Jaw: A Review. Cureus 2020, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Gavaldá, C.; Bagan, J.V. Concept, diagnosis and classification of bisphosphonate-associated osteonecrosis of the jaws. A review of the literature. Med. Oral Patol. Oral Cir. Bucal. 2016, 21, e260–e270. [Google Scholar]
- Qaisi, M.; Hargett, J.; Loeb, M.; Brown, J.; Caloss, R. Denosumab Related Osteonecrosis of the Jaw with Spontaneous Necrosis of the Soft Palate: Report of a Life Threatening Case. Case Rep. Dent. 2016. [Google Scholar] [CrossRef]
- Diaz-Reverand, S.A.; Naval-Gíaz, L.; Muñoz-Guerra, M.F.; Sastre-Pérez, J.; Rodríguez-Campo, F.J.; Gil-Diez, J.L. Manejo de la osteonecrosis maxilar asociada al uso de medicamentos en virtud de su estadio clínico: Análisis de 19 casos. Rev. Esp. Cir. Oral Maxilofac. 2018, 40, 104–111. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Mergoni, G.; Vescovi, P.; Passerini, P.; Maestri, R.; Corradi, D.; Sala, R.; Govoni, P. Effects of zoledronic acid and dexamethasone on early phases of socket healing after tooth extraction in rats: A preliminary macroscopic and microscopic quantitative study. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e339–e345. [Google Scholar] [CrossRef]
- Pabst, A.M.; Krüger, M.; Blatt, S.; Ziebart, T.; Rahimi-Nedjat, R.; Goetze, E.; Walter, C. Angiogenesis in the Development of Medication-Related Osteonecrosis of the Jaws: An Overview. Dent. J. 2017, 5, 2. [Google Scholar] [CrossRef]
- Atanes-Bonome, P.; Atanes-Bonome, A.; Ríos-Lage, P.; Atanes-Sandoval, A.D. Bisphosphonate-realated osteonecrosis of the Jaw. SEMERGEN 2014, 40, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Pichardo, S.E.C.; van Merkesteyn, J.P.R. Bisphosphonate related osteonecrosis of the jaws: Spontaneous or dental origin? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, 287–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.M.; Rhee, Y.; Kwon, Y.-D.; Kwon, T.-G.; Lee, J.K.; Kim, D.-Y. Medication Related Osteonecrosis of the Jaw: 2015 Position Statement of the Korean Society for Bone and Mineral Research and the Korean Association of Oral and Maxillofacial Surgeons. J. Bone Metab. 2015, 22, 151–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papapoulos, S.E.; Chapurlat, R.; Libanati, C.; Brandi, M.L.; Brown, J.P.; Czerwiński, E.; Krieg, M.; Man, Z.; Mellström, D.; Radominski, S.C.; et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: Results from the first two years of the FREEDOM extension. J. Bone Miner. Res. 2011, 27, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Carducci, M.; Smith, M.; Damião, R.; Brown, J.; Karsh, L.; Milecki, P.; Shore, N.; Rader, M.; Wang, H.; et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: A randomised, double-blind study. Lancet 2011, 377, 813–822. [Google Scholar] [CrossRef] [Green Version]
- Junquera, L.M.; Martín-Granizo, R. Diagnóstico, prevención y tratamiento de la osteonecrosis de los maxilares por bisfosfonatos: Recomendaciones de la Sociedad Española de Cirugía Oral y Maxilofacial (SECOM). Rev. Esp. Cir. Oral Maxilofac. 2008, 30, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Sarasquete, M.E.; González, M.; Miguel, J.S.; García-Sanz, R. Bisphosphonate-related osteonecrosis: Genetic and acquired risk factors. Oral Dis. 2009, 15, 382–387. [Google Scholar] [CrossRef]
- Tsao, C.; Darby, I.; Ebeling, P.R.; Walsh, K.; O’Brien-Simpson, N.; Reynolds, E.; Borromeo, G. Oral health risk factors for bisphosphonate-associated jaw osteonecrosis. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2013, 71, 1360–1366. [Google Scholar] [CrossRef]
- Nicoletti, P.; Cartsos, V.M.; Palaska, P.K.; Shen, Y.; Floratos, A.; Zavras, A.I. Genomewide pharmacogenetics of bisphosphonate-induced osteonecrosis of the jaw: The role of RBMS3. Oncologist 2012, 17, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Marini, F.; Tonelli, P.; Cavalli, L.; Cavalli, T.; Masi, L.; Falchetti, A.; Brandi, M.L. Pharmacogenetics of bisphosphonate-associated osteonecrosis of the jaw. Front. Biosci. Elite 2011, 3, 364–370. [Google Scholar]
- Wessel, J.H.; Dodson, T.B.; Zavras, A.I. Zoledronate, smoking, and obesity are strong risk factors for osteonecrosis of the jaw: A case-control study. Abstract Eur. PMC 2008, 66, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyrgidis, A.; Vahtsevanos, K.; Koloutsos, G.; Andreadis, C.; Boukovinas, I.; Teleioudis, Z.; Patrikidou, A.; Triaridis, S. Bisphosphonate-Related Osteonecrosis of the Jaws: A Case-Control Study of Risk Factors in Breast Cancer Patients. J. Clin. Oncol. 2008, 26, 4634–4638. [Google Scholar] [CrossRef] [PubMed]
- Vahtsevanos, K.; Kyrgidis, A.; Verrou, E.; Katodritou, E.; Triaridis, S.; Andreadis, C.G.; Boukovinas, I.; Koloutsos, G.E.; Teleioudis, Z.; Kitikidou, K.; et al. Longitudinal Cohort Study of Risk Factors in Cancer Patients of Bisphosphonate-Related Osteonecrosis of the Jaw. J. Clin. Oncol. 2009, 27, 5356–5362. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, M.; Boyd, N.M.; Smith, A. Denosumab and osteonecrosis of the jaws—The pharmacology, pathogenesis and a report of two cases. Aust. Dent. J. 2014, 59, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Ferrari, S.L.; Russell, R.G.G. Denosumab and bisphosphonates: Different mechanisms of action and effects. Bone 2011, 48, 677–692. [Google Scholar] [CrossRef]
- Rodan, G. Bone mass homeostasis and bisphosphonate action. Bone 1997, 20, 1–4. [Google Scholar] [CrossRef]
- Anesi, A.; Generali, L.; Sandoni, L.; Pozzi, S.; Grande, A. From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights. Int. J. Mol. Sci. 2019, 20, 4925. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Altman, R.B.; Klein, T.E. Bisphosphonates pathway. Pharmacogenet. Genom. 2011, 21, 50–53. [Google Scholar] [CrossRef] [Green Version]
- Kimmel, D.B. Mechanism of action, pharmacokinetic and pharmacodynamic profile, and clinical applications of nitrogen-containing bisphosphonates. J. Dent. Res. 2007, 86, 1022–1033. [Google Scholar] [CrossRef]
- Khan, A.A.; Morrison, A.A.; Hanley, D.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; Taguchi, A.; Tetradis, S.; et al. Diagnosis and Management of Osteonecrosis of the Jaw: A Systematic Review and International Consensus. J. Bone Miner. Res. 2015, 30, 3–23. [Google Scholar] [CrossRef]
- Boquete-Castro, A.; Gómez-Moreno, G.; Calvo-Guirado, J.-L.; Aguilar-Salvatierra, A.; Delgado-Ruiz, R.A. Denosumab and osteonecrosis of the jaw. A systematic analysis of events reported in clinical trials. Clin. Oral Implant. Res. 2015, 27, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Limones, A.; Alcaide, L.M.S.; Díaz-Parreño, S.A.; Helm, A.; Bornstein, M.M.; Mourelle, P.M. Osteonecrosis de los maxilares inducida por medicamentos (MRONJ) en pacientes oncológicos tratados con denosumab vs. ácido zoledrónico: Una revisión sistemática y meta-análisis. Med. Oral. Patol. Oral Cir. Bucal. Ed. Esp. 2020, 25, 307–316. [Google Scholar]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-Related Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020. Int. J. Environ. Res. Public Health 2020, 17, 5998. [Google Scholar] [CrossRef] [PubMed]
- Bagán, J.; Blade, J.; Cozar, J.M.; Constela, M.; Sanz, R.G.; Gómez-Veiga, F.; Lahuerta, J.J.; Lluch, A.; Massuti, B.; Morote, J.; et al. Recommendations for the prevention, diagnosis, and treatment of osteonecrosis of the jaw (ONJ) in cancer patients treated with bisphosphonates. Med. Oral Patol. Oral Cir. Bucal 2007, 12, 336–340. [Google Scholar]
- Jaimes, M.; Oliveira, G.R.; Olate, S.; de Albergaria Barbosa, J.R. Bifosfonatos asociado a osteonecrosis de los maxilares: Revisión de la literatura. Av. Odontoestomatol. 2008, 24, 219–226. [Google Scholar] [CrossRef]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-Induced Exposed Bone (Osteonecrosis/Osteopetrosis) of the Jaws: Risk Factors, Recognition, Prevention, and Treatment. J. Oral Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef]
- Barrientos Lezcano, F.J.; Peral Cagigal, B.; la Peña Varela G de Sánchez Cuéllar, L.A.; García Cantera, J.M.; Serrat Soto, A.; Verrier Hernández, A. Osteonecrosis de los maxilares inducida por bifosfonatos: Prevención y actitud terapéutica. Rev. Esp. Cir. Oral Maxilofac. 2007, 29, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Damato, K.; Gralow, J.; Hoff, A.; Huryn, J.; Marx, R.; Ruggiero, S.; Schubert, M.; Toth, B.; Valero, V. Expert panel recommendations for the prevention, diagnosis and treatment of osteonecrosis of the Jaws: June 2004. Report of the Council on Scientific Affairs. 2004. Available online: www.ada.org/~/media/ADA/Member%20Center/FIles/topics_osteonecrosis_whitepaper.pdf (accessed on 23 January 2021).
- Sedghizadeh, P.P.; Yooseph, S.; Fadrosh, D.W.; Zeigler-Allen, L.; Thiagarajan, M.; Salek, H.; Farahnik, F.; Williamson, S.J. Metagenomic investigation of microbes and viruses in patients with jaw osteonecrosis associated with bisphosphonate therapy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 764–770. [Google Scholar] [CrossRef] [Green Version]
- Hinson, A.M.; Smith, C.W.; Siegel, E.R.; Stack, J.B.C. Is Bisphosphonate-Related Osteonecrosis of the Jaw an Infection? A Histological and Microbiological Ten-Year Summary. Int. J. Dent. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Magopoulos, C.; Karakinaris, G.; Telioudis, Z.; Vahtsevanos, K.; Dimitrakopoulos, I.; Antoniadis, K.; Delaroudis, S. Osteonecrosis of the jaws due to bisphosphonate use. A review of 60 cases and treatment proposals. Am. J. Otolaryngol. 2007, 28, 158–163. [Google Scholar] [CrossRef]
- Joshi Otero, J.; Rollón Mayordomo, A.; Coello Suanzes, J.; Lledó Villar, E.; Lozano Rosado, R.; Sánchez-Moliní, M.; Berart, P. Osteonecrosis de los maxilares asociada al uso de bifosfonatos: Revisión de ocho casos. Rev. Esp. Cir. Oral Maxilofac. 2011, 33, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Heifetz-Li, J.J.; Abdelsamie, S.; Campbell, C.B.; Roth, S.; Fielding, A.F.; Mulligan, J.P. Systematic review of the use of pentoxifylline and tocopherol for the treatment of medication-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 491–497.e2. [Google Scholar] [CrossRef] [PubMed]
- Alons, K.; Kuijpers, S.C.C.; de Jong, E.; van Merkesteyn, J.P.R. Treating low- and medium-potency bisphosphonate–related osteonecrosis of the jaws with a protocol for the treatment of chronic suppurative osteomyelitis: Report of 7 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Stanton, D.C.; Balasanian, E. Outcome of Surgical Management of Bisphosphonate-Related Osteonecrosis of the Jaws: Review of 33 Surgical Cases. J. Oral Maxillofac. Surg. 2009, 67, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Ferlito, S.; Puzzo, S.; Palermo, F.; Verzì, P. Treatment of bisphosphonate-related osteonecrosis of the jaws: Presentation of a protocol and an observational longitudinal study of an Italian series of cases. Br. J. Oral Maxillofac. Surg. 2012, 50, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Bejarano, E.-B.; Serrera-Figallo, M.Á.; Gutiérrez-Corrales, A.; Romero-Ruiz, M.-M.; Castillo-De-Oyagüe, R.; Gutiérrez-Pérez, J.-L.; Torres-Lagares, D. Prophylaxis and antibiotic therapy in management protocols of patients treated with oral and intravenous bisphosphonates. J. Clin. Exp. Dent. 2017, 9, e141–e149. [Google Scholar]
- Eckert, A.W.; Maurer, P.; Meyer, L.; Kriwalsky, M.; Rohrberg, R.; Schneider, D.; Bilkenroth, U.; Schubert, J. Bisphosphonate-related jaw necrosis—Severe complication in Maxillofacial surgery. Cancer Treat. Rev. 2007, 33, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, P.; Merigo, E.; Meleti, M.; Manfredi, M.; Guidotti, R.; Namour, M. Bisphosphonates-related osteonecrosis of the jaws: A concise review of the literature and a report of a single-centre experience with 151 patients. J. Oral Pathol. Med. 2011, 41, 214–221. [Google Scholar] [CrossRef]
- Junquera, L.; Gallego, L.; Cuesta, P.; Pelaz, A.; De Vicente, J.-C. Clinical experiences with bisphosphonate-associated osteonecrosis of the jaws: Analysis of 21 cases. Am. J. Otolaryngol. 2009, 30, 390–395. [Google Scholar] [CrossRef]
- Lenz, J.-H.; Steiner-Krammer, B.; Schmidt, W.; Fietkau, R.; Mueller, P.C.; Gundlach, K.K.H. Does avascular necrosis of the jaws in cancer patients only occur following treatment with bisphosphonates? J. Cranio-Maxillo-Fac. Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Fac. Surg. 2005, 33, 395–403. [Google Scholar] [CrossRef]
- Bamias, A.; Kastritis, E.; Bamia, C.; Moulopoulos, L.A.; Melakopoulos, I.; Bozas, G.; Koutsoukou, V.; Gika, D.; Anagnostopoulos, A.; Papadimitriou, C.; et al. Osteonecrosis of the Jaw in Cancer After Treatment with Bisphosphonates: Incidence and Risk Factors. J. Clin. Oncol. 2005, 23, 8580–8587. [Google Scholar] [CrossRef] [PubMed]
- Fleiter, N.; Walter, G.; Bösebeck, H.; Vogt, S.; Büchner, H.; Hirschberger, W.; Hoffmann, R. Clinical use and safety of a novel gentamicin-releasing resorbable bone graft substitute in the treatment of osteomyelitis/osteitis. Bone Jt Res. 2014, 3, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Chia, C.L.K.; Shelat, V.G.; Low, W.; George, S.; Rao, J. The Use of Collatamp G, Local Gentamicin-Collagen Sponge, in Reducing Wound Infection. Int. Surg. 2014, 99, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Bianchi, B.; Savi, A.; Poli, T.; Multinu, A.; Balestreri, A.; Ferri, A. Fibula Free Flap With Endosseous Implants for Reconstructing a Resected Mandible in Bisphosphonate Osteonecrosis. J. Oral Maxillofac. Surg. 2008, 66, 999–1003. [Google Scholar] [CrossRef]
- Pautke, C.; Bauer, F.; Otto, S.; Tischer, T.; Steiner, T.; Weitz, J.; Kreutzer, K.; Hohlweg-Majert, B.; Wolff, K.-D.; Hafner, S.; et al. Fluorescence-Guided Bone Resection in Bisphosphonate-Related Osteonecrosis of the Jaws: First Clinical Results of a Prospective Pilot Study. J. Oral Maxillofac. Surg. 2011, 69, 84–91. [Google Scholar] [CrossRef]
- Blus, C.; Szmukler-Moncler, S.; Giannelli, G.; Denotti, G.; Orrù, G. Use of Ultrasonic Bone Surgery (Piezosurgery) to Surgically Treat Bisphosphonate-Related Osteonecrosis of the Jaws (BRONJ). A Case Series Report with at Least 1 Year of Follow-Up. Open Dent. J. 2013, 7, 94–101. [Google Scholar] [CrossRef]
- Franco, S.; Miccoli, S.; Limongelli, L.; Tempesta, A.; Favia, G.; Favia, G.; Favia, G. New Dimensional Staging of Bisphosphonate-Related Osteonecrosis of the Jaw Allowing a Guided Surgical Treatment Protocol: Long-Term Follow-Up of 266 Lesions in Neoplastic and Osteoporotic Patients from the University of Bari. Int. J. Dent. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Temmerman, A.; Vandessel, J.; Castro, A.; Jacobs, R.; Teughels, W.; Pinto, N.; Quirynen, M. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: A split-mouth, randomized, controlled clinical trial. J. Clin. Periodontol. 2016, 43, 990–999. [Google Scholar] [CrossRef]
- Curi, M.M.; Cossolin, G.S.I.; Koga, D.H.; Araújo, S.R.; Feher, O.; Dos Santos, M.O.; Zardetto, C. Treatment of Avascular Osteonecrosis of the Mandible in Cancer Patients with a History of Bisphosphonate Therapy by Combining Bone Resection and Autologous Platelet-Rich Plasma: Report of 3 Cases. J. Oral Maxillofac. Surg. 2007, 65, 349–355. [Google Scholar] [CrossRef]
- Dragonas, P.; Katsaros, T.; Avila-Ortiz, G.; Chambrone, L.; Schiavo, J.; Palaiologou, A. Effects of leukocyte–platelet-rich fibrin (L-PRF) in different intraoral bone grafting procedures: A systematic review. Int. J. Oral Maxillofac. Surg. 2019, 48, 250–262. [Google Scholar] [CrossRef]
- Dincă, O.; Zurac, S.; Stăniceanu, F.; Bucur, M.B.; Bodnar, D.C.; Vlădan, C.; Bucur, A. Clinical and histopathological studies using fibrin-rich plasma in the treatment of bisphosphonate-related osteonecrosis of the jaw. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2014, 55, 961–964. [Google Scholar]
- Kim, J.-W.; Kim, S.-J.; Kim, M.-R. Leucocyte-rich and platelet-rich fibrin for the treatment of bisphosphonate-related osteonecrosis of the jaw: A prospective feasibility study. Br. J. Oral Maxillofac. Surg. 2014, 52, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, L.; Zhao, L.; He, Y.; Xiao, E.; Zhang, Y. Adipose-derived stem cells prevent the onset of bisphosphonate-related osteonecrosis of the jaw through transforming growth factor β-1-mediated gingival wound healing. Stem Cell Res. Ther. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Gonzálvez-García, M.; Rodríguez-Lozano, F.J.; Villanueva, V.; Segarra-Fenoll, D.; Rodriguez-Gonzalez, M.A.; Oñate-Sánchez, R.E.; Blanquer, M.B.; Moraleda, J.M. Cell Therapy in Bisphosphonate-Related Osteonecrosis of the Jaw. J. Cranio. Surg. 2013, 24, e226–e228. [Google Scholar] [CrossRef]







| MRONJ Grades | Description |
|---|---|
| At risk: | No apparent necrotic bone in patients treated with oral or intravenous anti-resorptive or anti-angiogenic agents. |
| Grade 0 | Without necrotic bone exposure, but with clinical findings, radiographic changes, and nonspecific symptoms. |
| Grade 1 | Exposure of necrotic bone and fistulas, asymptomatic and without signs of infection. |
| Grade 2 | Exposure of necrotic bone and fistulas associated with signs of infection; pain and erythema in the affected area with or without purulent drainage. |
| Grade 3. | Exposure of necrotic bone and fistulas associated with pain, infection and ≥1 of the following: exposed necrotic bone extending beyond the alveolar bone (i.e., lower border and branch of the jaw, maxillary sinus, and maxillary zygoma) resulting in pathological fracture, extraoral fistula, oroantral or oronasal communication, or osteolysis extending to the lower border of the jaw or the floor of the sinus [8] |
| MRONJ Grades | Treatment |
|---|---|
| At risk: |
|
| Grade 0 |
|
| Grade 1 |
|
| Grade 2 |
|
| Grade 3. |
|
| MRONJ Stages | Treatment |
|---|---|
| Stage 1 |
|
| Stage 2 |
|
| Stage 3 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo-Zamora, G.; Martínez, Y.; Moreno, J.A.; Ortiz-Ruíz, A.J. Treatment of Stage 2 Medication-Induced Osteonecrosis of the Jaw: A Case Series. Int. J. Environ. Res. Public Health 2021, 18, 1018. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18031018
Pardo-Zamora G, Martínez Y, Moreno JA, Ortiz-Ruíz AJ. Treatment of Stage 2 Medication-Induced Osteonecrosis of the Jaw: A Case Series. International Journal of Environmental Research and Public Health. 2021; 18(3):1018. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18031018
Chicago/Turabian StylePardo-Zamora, Guillermo, Yanet Martínez, Jose Antonio Moreno, and Antonio J. Ortiz-Ruíz. 2021. "Treatment of Stage 2 Medication-Induced Osteonecrosis of the Jaw: A Case Series" International Journal of Environmental Research and Public Health 18, no. 3: 1018. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18031018