Ecotoxicological Studies on the Effect of Roundup® (Glyphosate Formulation) on Marine Benthic Microalgae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Work
2.2. Preparation of Microalgal Suspensions and Experiment Design
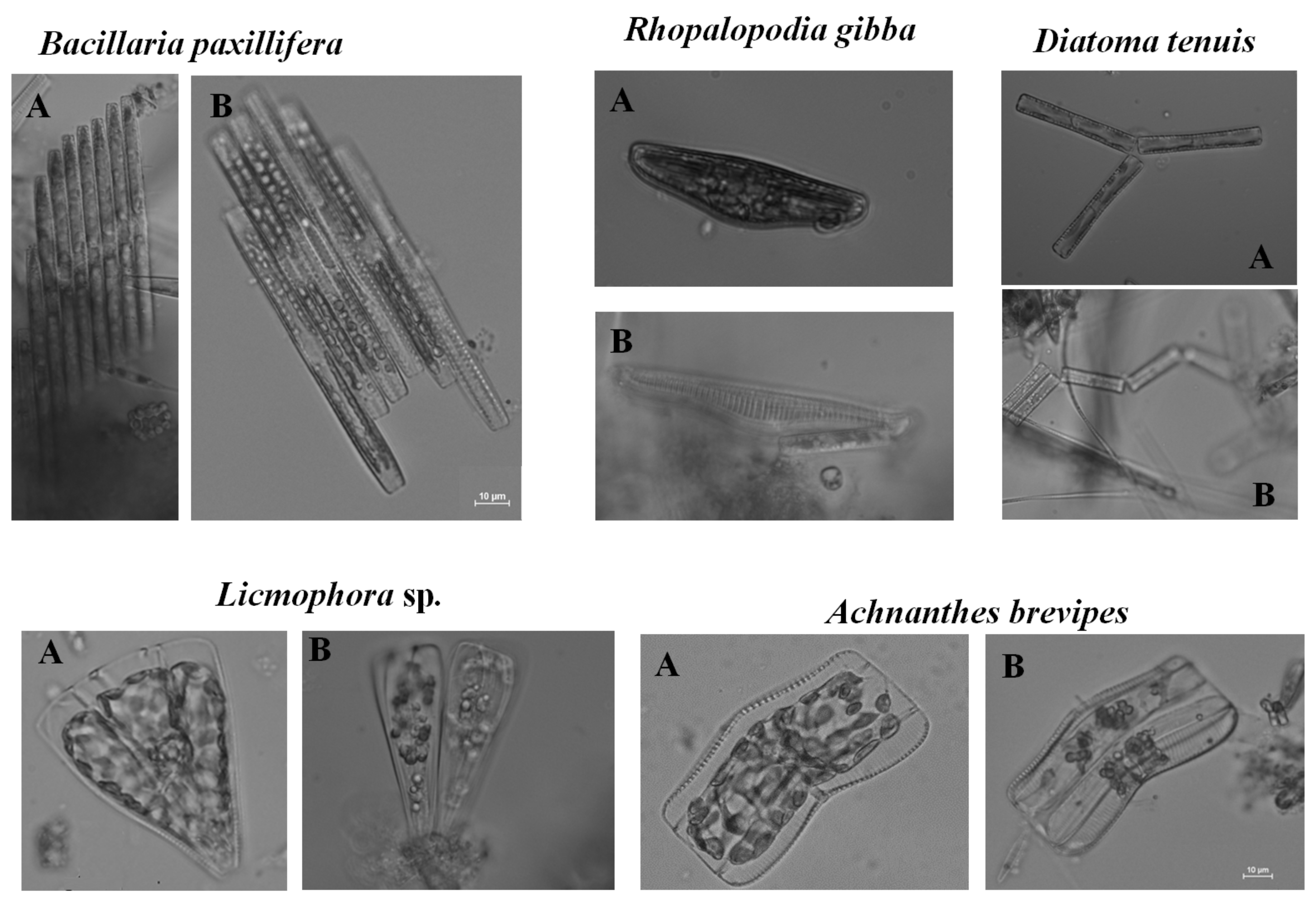
2.3. Microscopic Analysis
2.4. Statistical Analysis
3. Results
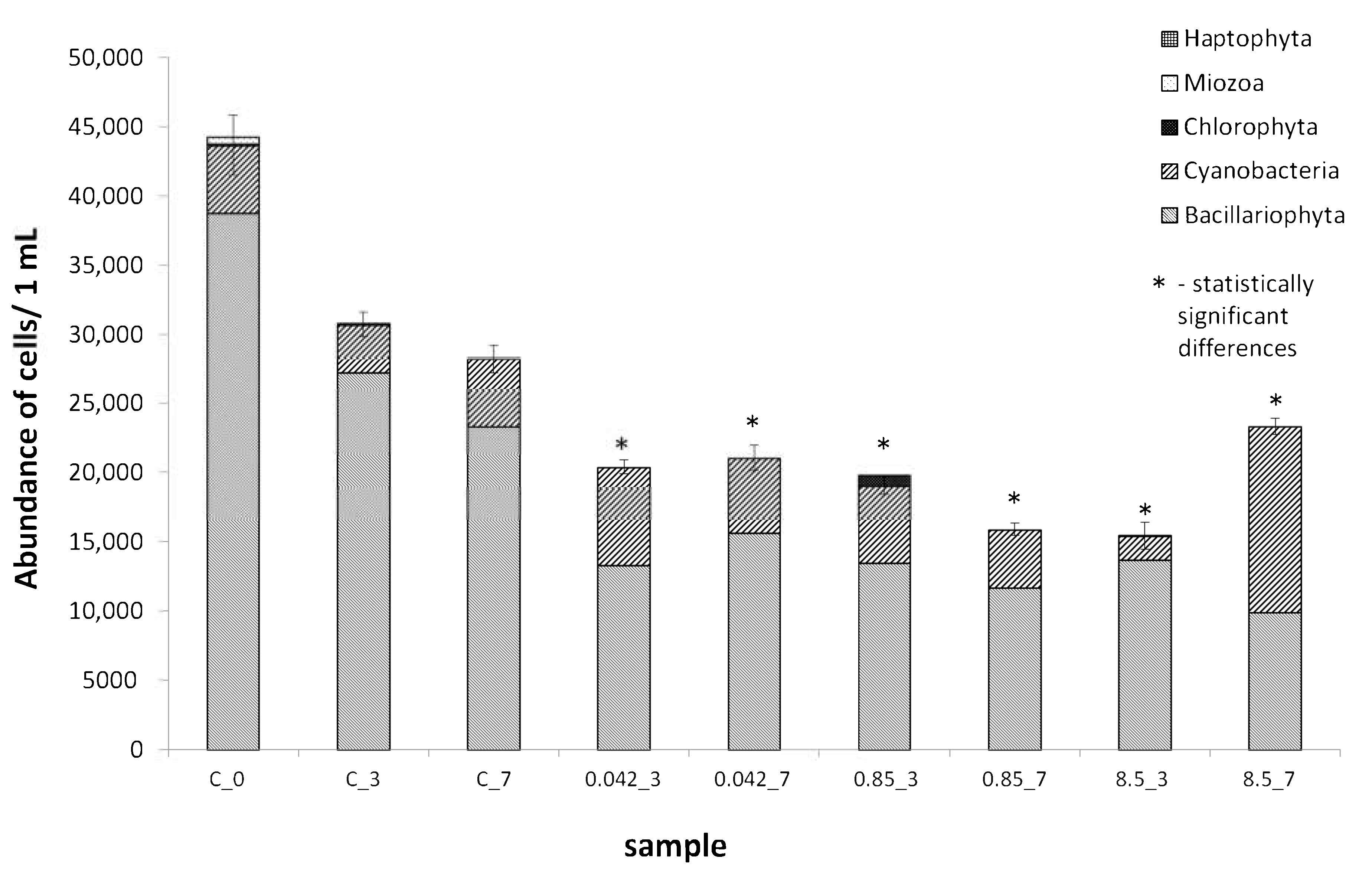
3.1. Quantitative and Qualitative Analysis of Assemblages
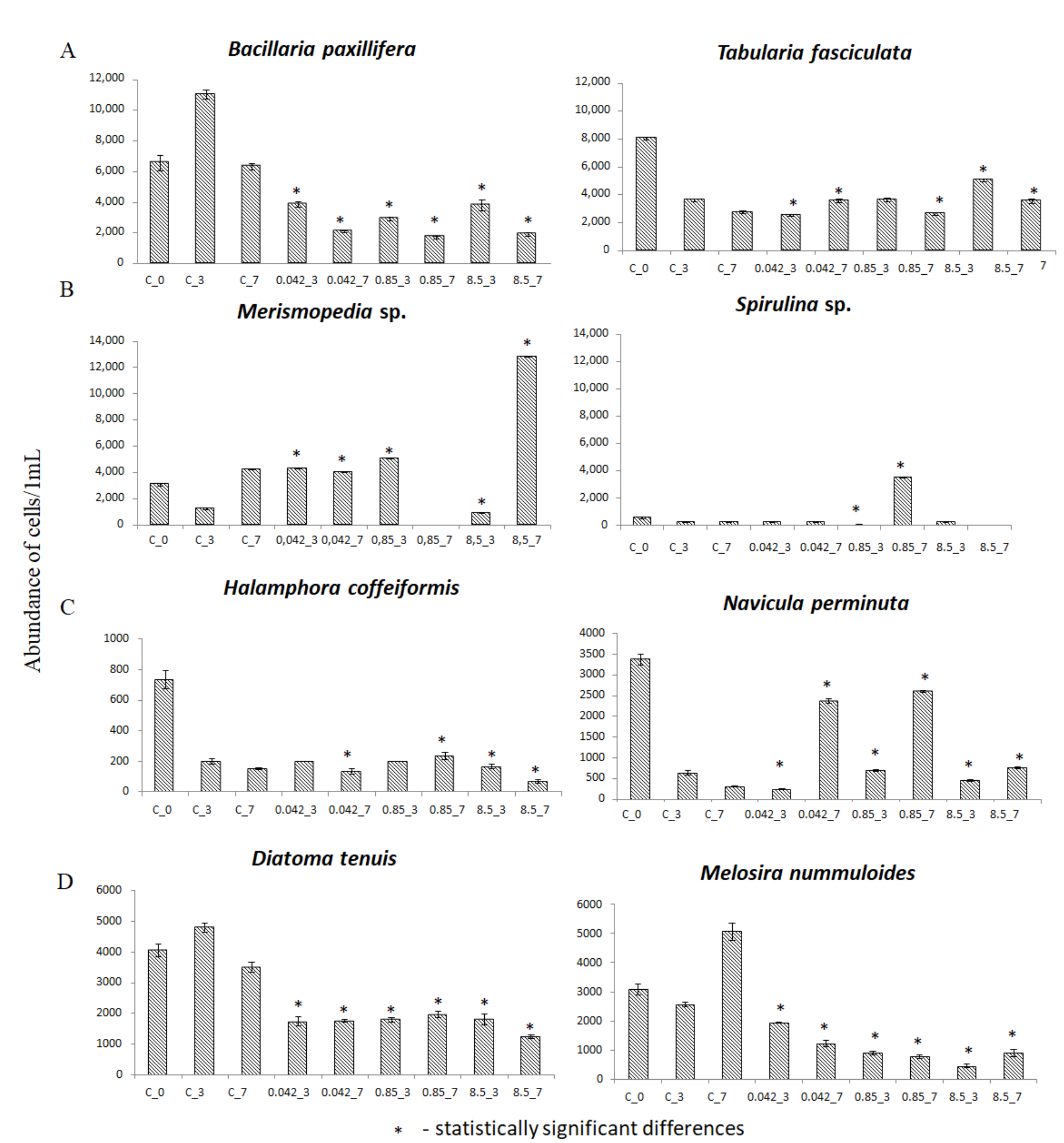
3.2. Abundance of Selected Taxa
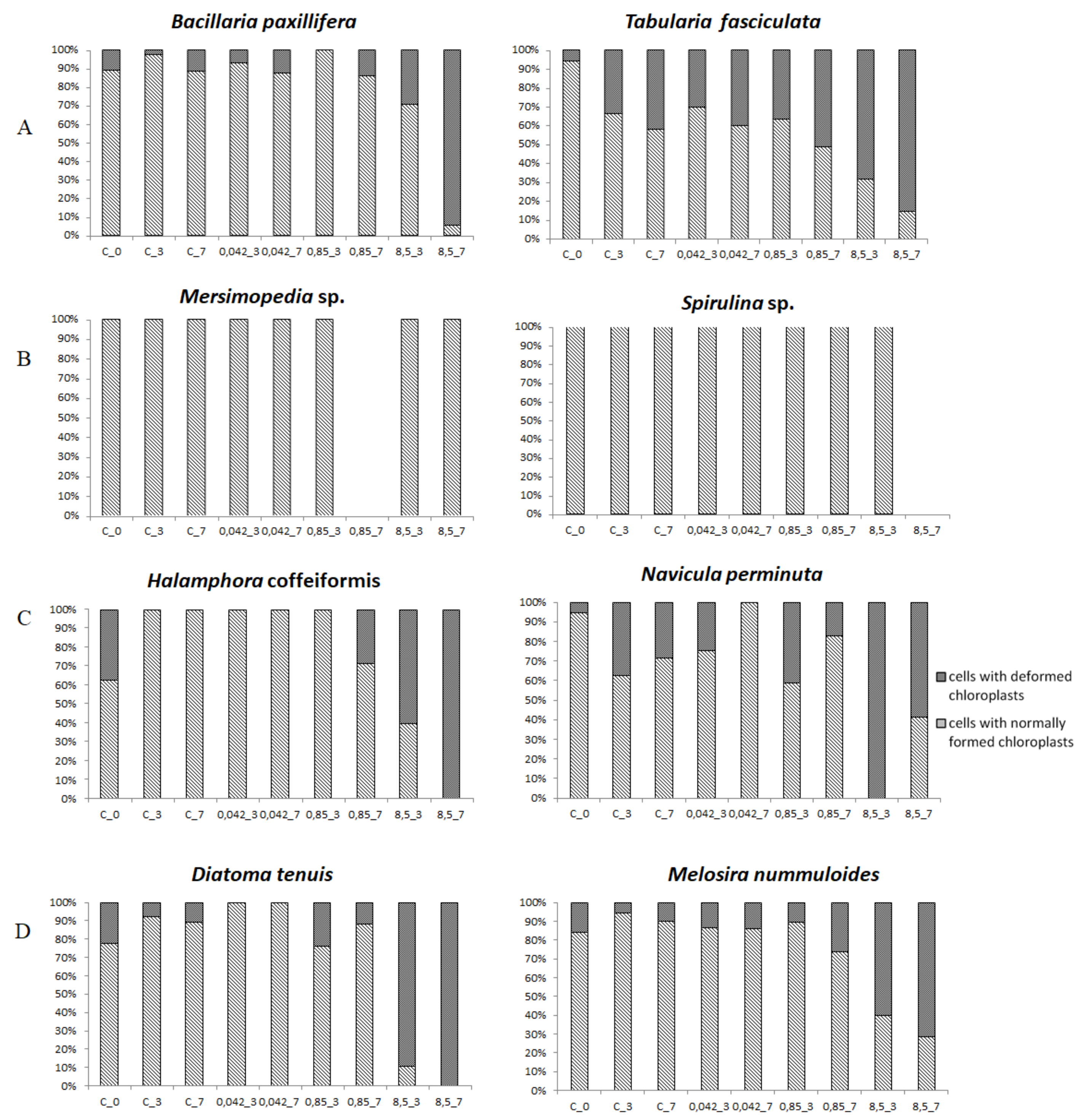
3.3. Cell Condition in Selected Taxa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Group of Algae | Taxon | Author |
|---|---|---|
| Bacillariophyta | Achnanthes brevipes | Bory |
| Achnanthes lemmermannii | Hustedt | |
| Amphora ovalis | (Kützing) Kützing | |
| Amphora pediculus | (Kützing) Grunow | |
| Amphora sp. | Kützing | |
| Bacillaria paxillifera | (O.F.Müller) T.Marsson | |
| Berkeleya rutilans | (Trentepohl ex Roth) Grunow | |
| Brebissonia lanceolata | (C.Agardh) R.K.Mahoney & Reimer | |
| Chaetoceros wighamii | Brightwell | |
| Cocconeis pediculus | Ehrenberg | |
| Cocconeis placentula | Ehrenberg | |
| Cocconeis sp. | Ehrenberg | |
| Cyclotella sp. | (Kützing) Brėbisson | |
| Cylindrotheca closterium | (Ehrenberg) Reimann & J.C.Lewin | |
| Diatoma moniliformis | Kützing | |
| Diatoma tenuis | C.Agardh | |
| Diatoma vulgaris | Bory | |
| Diploneis didyma | (Ehrenberg) Ehrenberg | |
| Diploneis interrupta | (Kützing) Cleve | |
| Encyonema prostratum | (Berkeley) Kützing | |
| Entomoneis paludosa | (W.Smith) Reimer | |
| Epithemia gibba | (Ehrenberg) Kützing 1844 | |
| Epithemia sp. | Kützing | |
| Fallacia sp. | Kütz | |
| Gomphonella olivacea | (Hornemann) Rabenhorst | |
| Grammatophora marina | (Lyngbye) Kützing | |
| Gyrosigma sp. | Kützing | |
| Halamphora cofeiformis | (C.Agardh) Mereschkowsky | |
| Licmophora sp. | C.Agardh | |
| Melosira moniliformis | C.Agardh | |
| Melosira nummuloides | C.Agardh | |
| Navicula gregaria | Donkin | |
| Navicula palpebralis | Brébisson ex W.Smith | |
| Navicula perminuta | Grunow | |
| Navicula ramosissima | (C.Agardh) Cleve | |
| Navicula sp. | Bory de Saint-Vincent | |
| Nitzschia sigma | (Kützing) W.Smith | |
| Opephora sp. | Petit | |
| Planothidium delicatulum | (Kützing) Round & Bukhtiyarova | |
| Pleurosigma aestuarii | (Brébisson ex Kützing) W.Smith | |
| Pleurosigma sp. | W. Smith | |
| Proschkinia poretzkajae | (Koretkevich) D.G.Mann | |
| Rhoicosphenia abbreviata | (C.Agardh) Lange-Bertalot | |
| Tabularia fasciculata | (C.Agardh) D.M.Williams & Round | |
| Tryblionella sp. | (Grunow) | |
| Cyanobacteria | Anabaena sp. | Bory ex Bornet & Flahault |
| Merismopedia sp. | Meyen | |
| Microcystis sp. | Lemmermann | |
| Nodularia sp. | Mertens ex Bornet & Flahault | |
| Oscillatoria sp. | Vaucher ex Gomont | |
| Spirulina major | Kützing ex Gomont | |
| Spirulina subsalsa | Oersted ex Gomont | |
| Woronichinia sp. | A.A.Elenkin | |
| Myzozoa | Peridinium sp. | Ehrenberg |
| Chlorophyta | Pseudopediastrum boryanum | (Turpin) E.Hegewald |
| Scenedesmus sp. | Meyen | |
| Haptophyta | Prymnesium sp. | N.Carter |
| Average Abundance | C_0 | C_3 | C_7 | 0.042_3 | 0.042_7 | 0.85_3 | 0.85_7 | 8.5_3 | 8.5_7 |
|---|---|---|---|---|---|---|---|---|---|
| Bacillaria paxillifera | 6550 | 11,033 | 6333 | 3867 | 2100 | 2900 | 1700 | 3800 | 1900 |
| Diatoma tenuis | 4050 | 4800 | 3500 | 1733 | 1750 | 1800 | 1967 | 1800 | 1250 |
| Melosira nummuloides | 3080 | 2567 | 5067 | 1950 | 1233 | 900 | 767 | 450 | 900 |
| Navicula perminuta | 3367 | 633 | 300 | 250 | 2367 | 700 | 2600 | 450 | 767 |
| Tabularia fasciculata | 8033 | 3600 | 2733 | 2533 | 3567 | 3633 | 2633 | 5033 | 3533 |
| Cylindrotheca closterium | 5233 | 200 | 100 | 100 | 400 | 150 | 100 | 667 | 700 |
| Entomoneis paludosa | 300 | 267 | 100 | 150 | 100 | 133 | 0 | 300 | 0 |
| Merismopedia sp. | 3060 | 1200 | 4200 | 4300 | 4000 | 5000 | 0 | 900 | 12,800 |
| Halamphora cofeiformis | 733 | 200 | 150 | 200 | 133 | 200 | 233 | 167 | 67 |
| Scenedesmus sp. | 1100 | 450 | 400 | 0 | 600 | 0 | 600 | 600 | 600 |
| Spirulina major | 83 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 0 |
| Spirulina subsalsa | 483 | 100 | 100 | 100 | 100 | 0 | 3400 | 100 | 0 |
| Standard Deviations | C_0 | C_3 | C_7 | 0.042_3 | 0.042_7 | 0.85_3 | 0.85_7 | 8.5_3 | 8.5_7 |
| Bacillaria paxillifera | 476 | 311 | 200 | 178 | 66 | 132 | 70 | 332 | 98 |
| Diatoma tenuis | 208 | 147 | 156 | 146 | 49 | 72 | 116 | 170 | 64 |
| Melosira nummuloides | 201 | 80 | 297 | 21 | 110 | 70 | 64 | 64 | 121 |
| Navicula perminuta | 126 | 49 | 14 | 7 | 57 | 20 | 14 | 21 | 21 |
| Tabularia fasciculata | 93 | 111 | 85 | 76 | 133 | 140 | 85 | 87 | 188 |
| Cylindrotheca closterium | 283 | 0 | 0 | 0 | 14 | 21 | 0 | 46 | 0 |
| Entomoneis paludosa | 10 | 12 | 0 | 7 | 0 | 6 | 0 | 20 | 0 |
| Merismopedia sp. | 111 | 57 | 0 | 0 | 26 | 0 | 0 | 14 | 0 |
| Halamphora cofeiformis | 59 | 17 | 7 | 0 | 15 | 0 | 25 | 15 | 12 |
| Scenedesmus sp. | 99 | 7 | 0 | 0 | 28 | 28 | 0 | 0 | 0 |
| Spirulina major | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spirulina subsalsa | 99 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
References
- Gonzalez, D.; Juárez, A.B.; Krug, C.P.; Santos, M.; Vera, M.S. Freshwater periphyton response to technical-grade and two commercial formulations of glyphosate. Ecol. Austral 2019, 29, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Steinmann, H.H.; Dickeduisberg, M.; Theuvsen, L. Uses and benefits of glyphosate in German arable farming. Crop Prot. 2012, 42, 164–169. [Google Scholar] [CrossRef]
- Peixoto, F. Comparative Effects of the Roundup and Glyphosate on Mitochondrial Oxidative Phosphorylation. Chemosphere 2005, 61, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska, M.; Jarosiewicz, P.; Bukowska, B. Glifosat i jego preparaty—Toksyczność, narażenie zawodowe i środowiskowe. Med. Pracy 2013, 64, 717–729. [Google Scholar]
- Qiu, H.; Geng, J.; Ren, H.; Xia, X.; Wang, X.; Yu, Y. Physiological and biochemical responses of Microcystis aeruginosa to glyphosate and its Roundup® formulation. J. Hazard. Mater. 2013, 248, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Modesto, A.K.; Martinez, B.C.R. Roundup causes oxidative stress in liver and inhibits acetylocholinesterase in muscle and brain of the fish Prochilodus lineatus. Chemosphere 2010, 78, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Franz, J.E.; Mao, M.K.; Sikorski, J.A. Glyphosate A Unique Global Herbicyde (ACS Monograph 189); American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Sylwestrzak, Z.; Zgrundo, A.; Jurowska, J.; Śliwińska, S.; Pniewski, F.; Latała, A. Ocena kondycji zbiorowisk mikrofitobentosu jako metoda monitoringu zanieczyszczeń w Morzu Bałtyckim. In Stan, Trendy Zmian Oraz Współczesne Metody Monitorowania Środowiska Morza Bałtyckiego “Bałtyk 2015”; Dera, J., Ostrowska, M., Eds.; Wydawnictwo Instytutu Oceanologii PAN: Sopot, Poland, 2015; ISBN 978-83-941037-1-2. [Google Scholar]
- Pieniążek, D.; Bukowska, B.; Duda, W. Glifosat—Nietoksyczny pestycyd? Med. Pracy 2003, 54, 579–583. [Google Scholar]
- Peterson, H.G.; Boutin, C.; Martin, P.A.; Freemark, K.E.; Ruecker, N.J.; Moody, M.J. Aquatic phyto-toxicity of 23 pesticides applied at expected environmental concentrations. Aquat. Toxicol. 1994, 28, 275–292. [Google Scholar] [CrossRef]
- Wong, P.K. Effects of 2,4-D, glyphosate and paraquat on growth, photosynthesis and chlorophyll a synthesis of Scenedesmus quadricauda Berb 614. Chemosphere 2000, 41, 177–182. [Google Scholar] [CrossRef]
- Latała, A.; Nędzi, M.; Stepnowski, P. Toxity of imidazolium and pyridinium based ionic liquids towards algae Bacillaria paxillifera (a microphytobenthic diatom) and Geitlerinema amphibium (a microphytobenthic blue green alga). Green Chem. 2009, 11, 1371–1376. [Google Scholar] [CrossRef]
- Śliwińska, S.; Bubak, I.; Sylwestrzak, Z.; Pniewski, F.; Latała, A. Allelopathic effects and anthropogenic substances on cyanobacteria and microalgae in aquatic ecosystems. Eur. J. Phycol. 2015, 50 (Suppl. 1), 187. [Google Scholar]
- Iummato, M.M.; Fassiano, A.; Graziano, M.; dos Santos Afonso, M.; de Molina, M.D.C.R.; Juárez, Á.B. Effect of glyphosate on the growth, morphology, ultrastructure and metabolism of Scenedesmus vacuolatus. Ecotoxicol. Environ. Saf. 2019, 172, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Lakeman, M.B.; Von Dassow, P.; Cattolico, R.A. The strain concept in phytoplankton ecology. Harmful Algae 2009, 8, 746–758. [Google Scholar] [CrossRef]
- Rengefors, K.; Kremp, A.; Reusch, T.B.H.; Wood, A.M. Genetic diversity and evolution ineukaryotic phytoplankton: Revelations from population genetic studies. J. Plankton Res. 2017, 39, 165–179. [Google Scholar]
- Rosenberg, R.; Hellman, B.; Johansson, B. Hypoxic tolerance of marine benthic fauna. Mar. Ecol. Progress Ser. Oldendorf 1991, 79, 127–131. [Google Scholar] [CrossRef]
- Sylwestrzak, Z.; Zgrundo, A.; Pniewski, F.; Latała, A. Wpływ glifosatu na wodne mikroorganizmy roślinne—przegląd dotychczasowych badań. In Zagadnienia Aktualnie Poruszane Przez Młodych Naukowców 3; Kuczera, M., Piech, K., Eds.; Creativetime Publisher: Kraków, Poland, 2015; pp. 227–230. ISBN 978-83-63058-50-0. [Google Scholar]
- Sylwestrzak, Z.; Zgrundo, A.; Pniewski, F.; Lejk, K.; Latała, A. Wpływ glifosatu w postaci preparatu Roundup na zbiorowiska mikrofitobentosu Zatoki Gdańskiej—nowe doniesienia. In Zagadnienia Aktualnie Poruszane Przez Młodych Naukowców 8; Kuczera, M., Piech, K., Eds.; Creativetime Publisher: Kraków, Poland, 2016; pp. 163–167. ISBN 978-83-63058-62-3. [Google Scholar]
- Sylwestrzak, Z.; Zgrundo, A.; Pniewski, F.; Lejk, K.; Latała, A. Effect of glyphosate (Roundup® formulation) on microphytobenthic communities of Gulf of Gdansk—New report. In Wpływ glifosatu w postaci preparatu Roundup na zbiorowiska mikrofitobentosu Zatoki Gdańskiej—Nowe doniesienia; Tech. Issues: Advseo Szczecin, Poland, 2017. [Google Scholar]
- Śliwińska, S.; Sylwestrzak, Z.; Zgrundo, A.; Pniewski, F.; Latała, A. The effects of allelochemicals and selected anthropogenic substances on the diatom Bacillaria paxillifera. Eduk. Biologiczna Śr. 2016, 1, 23–30. [Google Scholar]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test; OECD Publishing: Paris, France, 2011; Available online: https://search.oecd.org/env/test-no-201-alga-growth-inhibition-test-9789264069923-en.htm (accessed on 3 November 2020). [CrossRef] [Green Version]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt Int. Ver Limnol. 1958, 9, 38. [Google Scholar] [CrossRef]
- HELCOM. Manual for Marine Monitoring in the COMBINE Programme of HELCOM. 2014. Available online: https://mcc.jrc.ec.europa.eu/documents/Manual_for_Marine_Monitoring_COMBINE_Programme_HELCOM.pdf (accessed on 29 October 2020).
- Snoeijs, P.; Potapova, M. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Uppsala 1; Opulus Press: Uppsala, Sweden, 1993; p. 129. [Google Scholar]
- Snoeijs, P.; Vilbaste, S. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Uppsala 2; Opulus Press: Uppsala, Sweden, 1994; p. 126. [Google Scholar]
- Snoeijs, P.; Potapova, M. Intercalibration and distribution of diatom species in the Baltic Sea; Uppsala 3; Opulus Press: Uppsala, Sweden, 1995; p. 126. [Google Scholar]
- Snoeijs, P.; Kasperovičiene, J. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Uppsala 4; Opulus Press: Uppsala, Sweden, 1996; p. 126. [Google Scholar]
- Snoeijs, P.; Balashova, N. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Uppsala 5; Opulus Press: Uppsala, Sweden, 1998; p. 127. [Google Scholar]
- Witkowski, A.; Lange-Bertalot, H.; Metzeltin, D. Diatom Flora of Marine Coasts I. Iconographia Diatomologica 7; ARG Gantner Verlag KG: Ruggell, Liechtenstein, 2000; p. 925. [Google Scholar]
- Pliński, M.; Hindák, F. Green Algae with the English key for the identification to the genus. In Flora of the Gulf of Gdańsk and Adjacent Waters (The Southern Baltic Sea); Wydawnictwo Uniwersytetu Gdańskiego: Gdańsk, Poland, 2010; p. 172. ISBN 978-83-7326-437-3. (In Polish) [Google Scholar]
- Pliński, M.; Komárek, J. Cyanoprokaryota with the English key for the identification to the genus. In Flora of the Gulf of Gdańsk and Adjacent Waters (The Southern Baltic Sea); Wydawnictwo Uniwersytetu Gdańskiego: Gdańsk, Poland, 2007; p. 172. ISBN 978-83-7326-437-3. (In Polish) [Google Scholar]
- Halling-Sørensen, B. Algal toxicity of antibacterial agents used in intensive farming. Chemosphere 2000, 40, 731–739. [Google Scholar] [CrossRef]
- Dutka, B.J.; Bitton, G. Toxicity Testing Using Microorganisms; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Rozkrut, D. (Ed.) Statistical Yearbook of the Republic of Poland; Statistical Publishing Establishment: Warsaw, Poland, 2019; p. 784. [Google Scholar]
- Skeff, W.; Neumann, C.; Schulz-Bull, D.E. Glyphosate and AMPA in the estuaries of the Baltic Sea method optimization and field study. Mar. Pollut. Bull. 2015, 100, 577–585. [Google Scholar] [CrossRef]
- The Roundup® Safety Data Sheet. Available online: https://www.monsanto-ag.co.uk/media/1947/msds-roundup-proactive-04052017.pdf (accessed on 23 October 2020).
- Coupe, R.H.; Kalkhoff, S.J.; Capel, P.D.; Gregoire, C. Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag. Sci. 2012, 68, 16–30. [Google Scholar] [CrossRef]
- Ye, G.N.; Hajdukiewicz, P.T.; Broyles, D.; Rodriguez, D.; Xu, C.W.; Nehra, N.; Staub, J.M. Plastid-expressed 5-enolpyruvylshikimate-3-phosphate synthase genes provide high level glyphosate tolerance in tobacco. Plant J. 2001, 25, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Della-Cioppa, G.; Kishore, G.M. Import of a precursor protein into chloroplasts is inhibited by the herbicide glyphosate. EMBO J. 1988, 7, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.T.; Chu, L.M. Aquatic toxicity of glyphosate-based formulations: Comparison between different organisms and the effects of environmental factors. Chemosphere 2003, 52, 1189–1197. [Google Scholar] [CrossRef]
- Forlani, A.; Fontana, F.; Congiu, L. Isolation of microsatellite loci from the endemic and endangered Adriatic sturgeo (Acipenser naccarii). Conserv. Genet. 2008, 9, 461–463. [Google Scholar] [CrossRef]
- Berman, M.C.; Llames, M.E.; Minotti, P.; Fermani, P.; Quiroga, M.V.; Ferraro, M.A.; Zagarese, H.E. Field evidence supports former experimental claims on the stimulatory effect of glyphosate on picocyanobacteria communities. Sci. Total Environ. 2020, 701, 134601. [Google Scholar] [CrossRef]
- Malik, J.; Barry, G.; Kishore, G. A mini-review of the herbicide glyphosate. BioFActors 1989, 2, 17–25. [Google Scholar]
- Marsalek, B.; Rojickova, R. Stress factors enhancing production of algal exudates: A potential self-protective mechanism? J. Biosci. 1996, 51, 646–650. [Google Scholar]
- Sáenz, M.E.; Di Marzio, W.D.; Alberdi, J.L.; del Carmen Tortorelli, M. Effects of technical grade and a commercial formulation of glyphosate on algal population growth. Bull. Environ. Contam. Toxicol. 1997, 59, 638–644. [Google Scholar] [CrossRef]
- Hernando, F.; Royuela, M.; Muñoz-Rueda, A.; Gonzalez-Murua, C. Effect of glyphosate on the greening process and photosynthetic metabolism in Chlorella pyrenoidosa. J. Plant Physiol. 1989, 134, 26–31. [Google Scholar] [CrossRef]
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological risk assessment for Roundup® herbicide. In Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 2000; pp. 35–120. [Google Scholar]
- Vera, M.S.; Lagomarsino, L.; Sylvester, M.; Perez, G.L.; Rodriguez, P.; Mugni, H.; Sinistro, R.; Ferraro, M.; Bonetto, C.; Zagarese, H.; et al. New evidences of Roundup® (glyphosate formulation) impact on the periphyton community and the water quality of freshwater ecosystems. Ecotoxicology 2010, 19, 710–721. [Google Scholar] [CrossRef]
- Lipok, J.; Studnik, H.; Gruyaert, S. The toxicity of Roundup® 360 SL formulation and its main constituents: Glyphosate and isopropylamine towards non-target water photoautotrophs. Ecotoxicol. Environ. Saf. 2010, 73, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Stal, L.J.; Albertano, P.; Bergman, B.; von Bröckel, K.; Gallon, J.R.; Hayes, P.K.; Sivonen, K.; Walsby, A.E. BASIC: Baltic Sea cyanobacteria. An investigation of the structure and dynamics of water blooms of cyanobacteria in the Baltic Sea—Responses to a changing environment. Cont. Shelf Res. 2003, 23, 1695–1714. [Google Scholar] [CrossRef]
- Kahru, M.; Elmgren, R.; Di Lorenzo, E.; Savchuk, O. Unexplained interannual oscillations of cyanobacterial blooms in the Baltic Sea. Sci. Rep. 2018, 8, 6365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange-Bertalot, H.; Witkowski, A.; Bogaczewicz-Adamczak, B.; Zgrundo, A. Rare and new small-celled taxa of Naviculas s. str. in the Gulf of Gdansk and in its freshwater affluents. Limnologica 2003, 33, 258–270. [Google Scholar] [CrossRef] [Green Version]
- Zgrundo, A.; Dziengo-Czaja, M.; Bubak, I.; Bogaczewicz-Adamczak, B. Studies on the biodiversity of contemporary diatom assemblages in the Gulf of Gdańsk. Oceanol. Hydrobiol. Stud. 2009, 37, 139–153. [Google Scholar]
- Majewska, R.; Zgrundo, A.; Lemke, P.; De Stefano, M. Benthic diatoms of the Vistula River estuary (Northern Poland)—Seasonality, substrata preferences and the influence of water chemistry. Phycol. Res. 2012, 60, 1–19. [Google Scholar] [CrossRef]
- Arrhenius, Å; Backhaus, T.; Hilvarsson, A.; Wendt, I.; Zgrundo, A.; Blanck, H. A novel bioassay for evaluating the efficacy of biocides to inhibit settling and early establishment of marine biofilms. Mar. Pollut. Bull. 2014, 87, 292–299. [Google Scholar] [CrossRef] [PubMed]
- AlgaeBase. Available online: http://www.algaebase.org (accessed on 27 November 2020).

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sylwestrzak, Z.; Zgrundo, A.; Pniewski, F. Ecotoxicological Studies on the Effect of Roundup® (Glyphosate Formulation) on Marine Benthic Microalgae. Int. J. Environ. Res. Public Health 2021, 18, 884. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18030884
Sylwestrzak Z, Zgrundo A, Pniewski F. Ecotoxicological Studies on the Effect of Roundup® (Glyphosate Formulation) on Marine Benthic Microalgae. International Journal of Environmental Research and Public Health. 2021; 18(3):884. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18030884
Chicago/Turabian StyleSylwestrzak, Zuzanna, Aleksandra Zgrundo, and Filip Pniewski. 2021. "Ecotoxicological Studies on the Effect of Roundup® (Glyphosate Formulation) on Marine Benthic Microalgae" International Journal of Environmental Research and Public Health 18, no. 3: 884. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18030884





