Anhedonia as a Potential Risk Factor of Alzheimer’s Disease in a Community-Dwelling Elderly Sample: Results from the ZARADEMP Project
Abstract
:1. Introduction
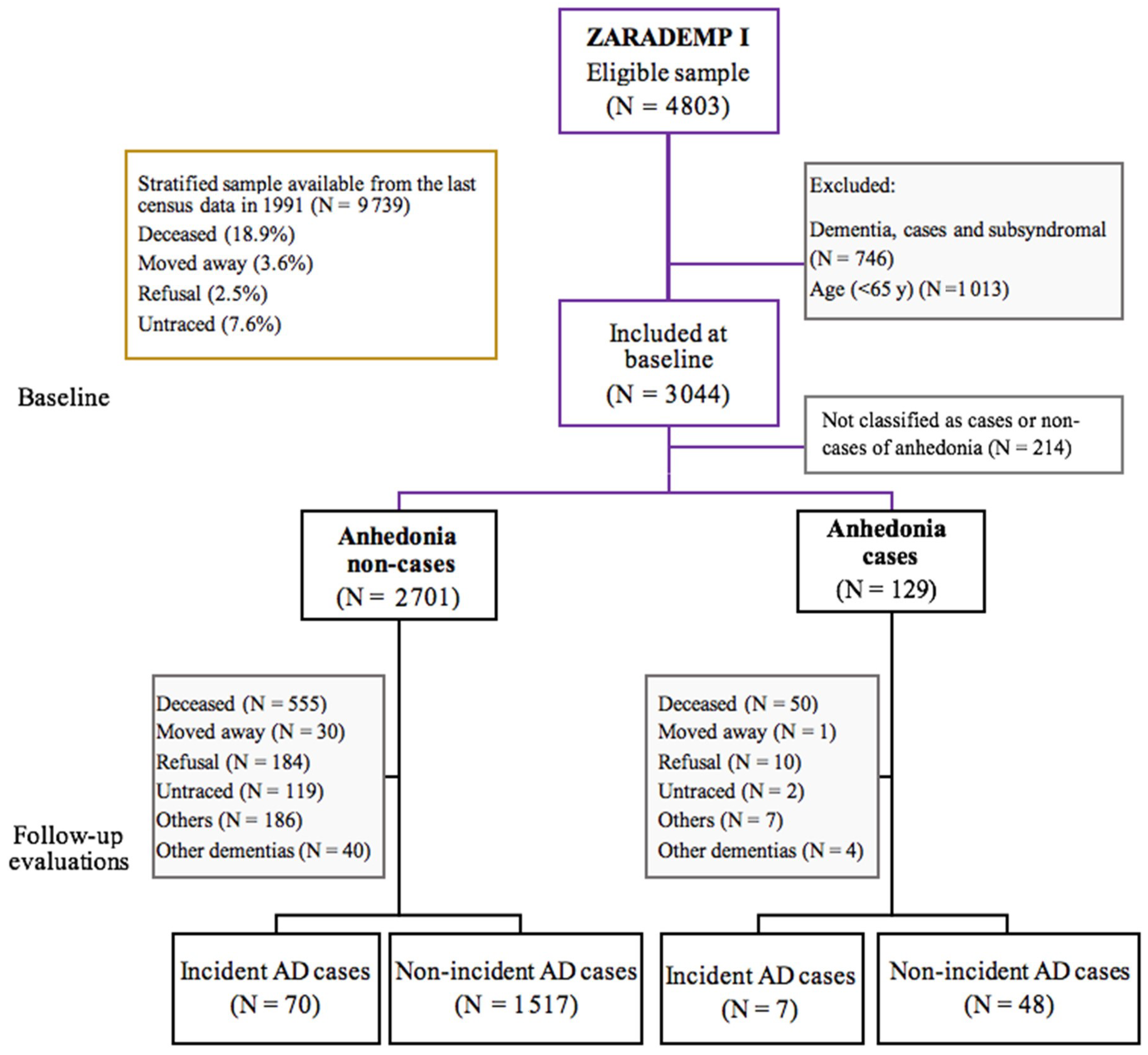
2. Materials and Methods
2.1. Sample
2.2. Procedure
2.3. Ethics
2.4. Clinical Measurements
2.4.1. Alzheimer’s Disease Assessment and Diagnosis
2.4.2. Anhedonia Assessment and Diagnosis
2.5. Covariates
2.6. Statistical Analysis
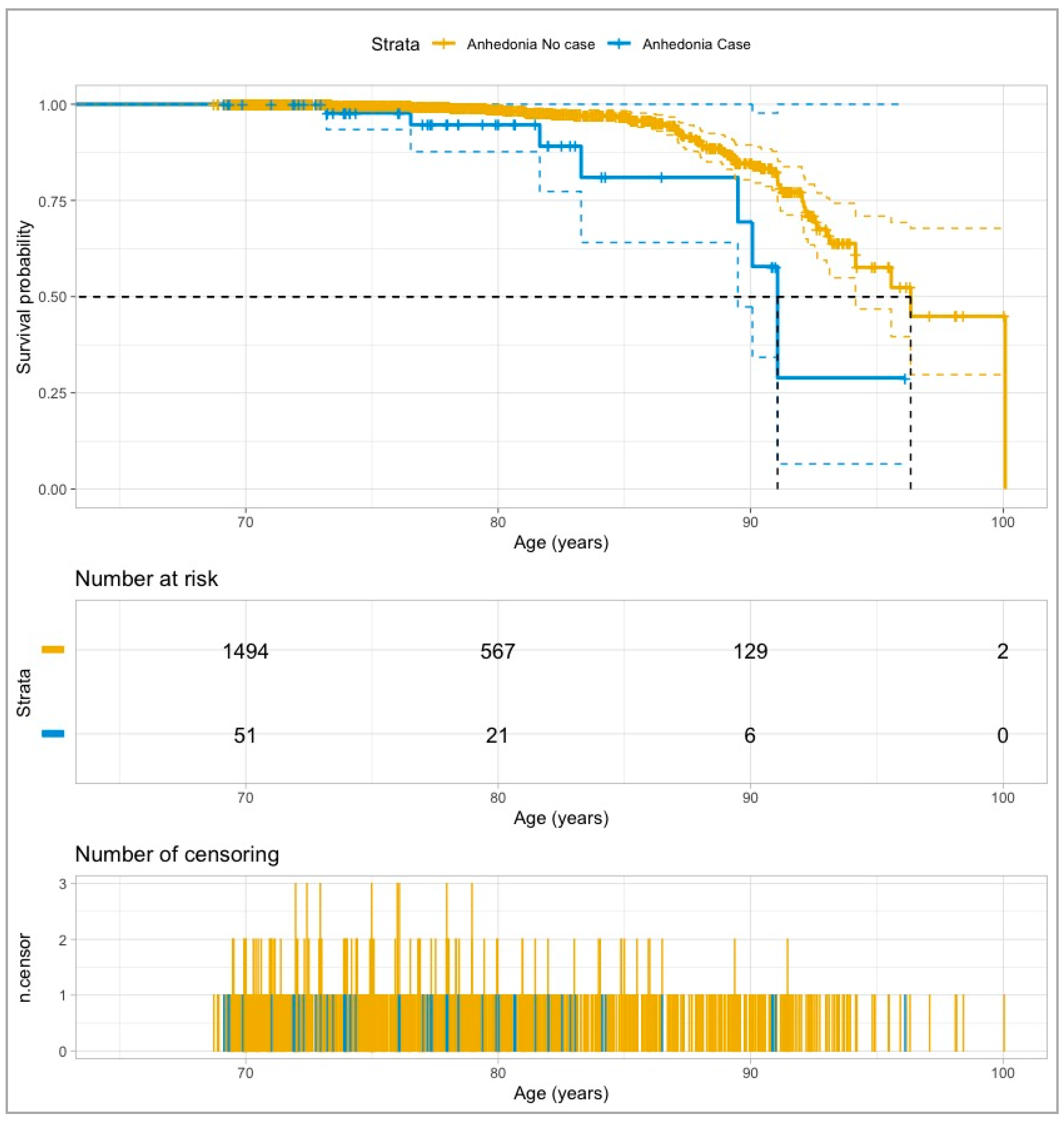
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Dua, T.; Seeher, K.M.; Sivananthan, S.; Chowdhary, N.; Pot, A.M.; Saxena, S. Risk reduction of cognitive decline and dementia. Alzheimers Dement. 2017, 13, 1450–1451. [Google Scholar] [CrossRef]
- World Health Organization. Towards A Dementia Plan: A WHO Guide; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; DSM-IV-TR; American Psychiatric Press: Washington DC, USA, 2000. [Google Scholar] [CrossRef]
- Chan, D.C.; Kasper, J.D.; Black, B.S.; Rabins, P.V. Presence of behavioral and psychological symptoms predicts nursing home placement in community-dwelling elders with cognitive impairment in univariate but not multivariate analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 548–554. [Google Scholar] [CrossRef]
- Shin, I.S.; Carter, M.; Materman, D.; Fairbanks, L.; Cummings, J.L. Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am. J. Geriatr. Psychiatry 2005, 13, 469–474. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Heser, K.; Fink, A.; Reinke, C.; Wagner, M.; Doblhammer, G. The temporal association between incident late-life depression and incident dementia. Acta Psychiatr. Scand. 2020, 142, 402–412. [Google Scholar] [CrossRef]
- De-la-Cámara, C.; Saz, P.; López-Antón, R.; Ventura, T.; Día, J.L.; Lobo, A. Depression in the elderly community: I. Prevalence by different diagnostic criteria and clinical profile. Eur. J. Psychiatry 2008, 22, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Gracia-García, P.; De-La-Cámara, C.; Santabárbara, J.; Lopez-Anton, R.; Quintanilla, M.A.; Ventura, T. Depression and incident alzheimer disease: The impact of disease severity. Am. J. Geriatr. Psychiatry 2015, 23, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Saz, P.; López-Antón, R.; Dewey, M.E.; Ventura, T.; Martín, A.; Marcos, G. Prevalence and implications of psychopathological non-cognitive symptoms in dementia. Acta Psychiatr. Scand. 2009, 119, 107–116. [Google Scholar] [CrossRef]
- Lobo, A.; López-Antón, R.; De-La-Cámara, C.; Quintanilla, M.A.; Campayo, A.; Saz, P. Non-cognitive psychopathological symptoms associated with incident mild cognitive impairment and dementia, alzheimer’s type. Neurotox Res. 2008, 14, 263–272. [Google Scholar] [CrossRef]
- Marshall, G.A.; Donovan, N.J.; Lorius, N.; Gidicsin, C.M.; Maye, J.; Pepin, L.C. Apathy is associated with increased amyloid burden in mild cognitive impairment. J. Neuropsychiatry Clin. Neurosci. 2013, 25, 302–307. [Google Scholar] [CrossRef] [Green Version]
- Donovan, N.J.; Hsu, D.C.; Dagley, A.S.; Schultz, A.P.; Amariglio, R.E.; Mormino, E.C. Depressive symptoms and biomarkers of Alzheimer’s disease in cognitively normal older adults. J. Alzheimers Dis. 2015, 46, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Selles, M.C.; Oliveira, M.M.; Ferreira, S.T. Brain inflammation connects cognitive and non-cognitive symptoms in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 64, 313–327. [Google Scholar] [CrossRef]
- Lee, J.R.; Suh, S.W.; Han, J.W.; Byun, S.; Kwon, S.J.; Lee, K.H. Anhedonia and dysphoria are differentially associated with the risk of dementia in the cognitively normal elderly individuals: A prospective cohort study. Psychiatry Investig. 2019, 16, 575–580. [Google Scholar] [CrossRef] [Green Version]
- So, Y.; Kim, K.W.; Park, J.H.; Lee, J.J.; Lee, S.B.; TaeHui, K. P3–396: Anhedonia is associated with the risk of Alzheimer’s disease in elders with mild cognitive impairment: Results from the Korean Longitudinal Study on Health and Aging (KLOSHA). Alzheimers Dement. 2012, 8, 594. [Google Scholar] [CrossRef]
- Lobo, A.; Lopez-Anton, R.; Santabárbara, J.; De-la-Cámara, C.; Ventura, T.; Quintanilla, M.A. Incidence and lifetime risk of dementia and Alzheimer’s disease in a Southern European population. Acta Psychiatr. Scand. 2011, 124, 372–383. [Google Scholar] [CrossRef]
- Lobo, A.; Saz, P.; Marcos, G.; Día, J.L.; De-La-Cámara, C.; Ventura, T. The ZARADEMP Project on the incidence, prevalence and risk factors of dementia (and depression) in the elderly community: II. Methods and first results. Eur. J. Psychiatry 2005, 19, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Copeland, J.R.; Dewey, M.E.; Griffiths-Jones, H.N. A computerized psychiatric diagnostic system and case nomenclature for elderly subjects: GMS and AGECAT. Psychol. Med. 1986, 16, 89–99. [Google Scholar] [CrossRef]
- Acosta, I.; Borges, G.; Aguirre-Hernandez, R.; Sosa, A.L.; Prince, M. Neuropsychiatric symptoms as risk factors of dementia in a Mexican population: A 10/66 Dementia Research Group study. Alzheimers Dement. 2018, 14, 271–279. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. «Mini-mental state». A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Dewey, M.E.; Copeland, J.R.M. Diagnosis of dementia from the history and aetiology schedule. Int. J. Geriatr. Psychiatry 2001, 16, 912–917. [Google Scholar] [CrossRef]
- Katz, S.; Ford, A.B.; Moskowitz, R.W.; Jackson, B.A.; Jaffe, M.W. Studies of Illness in the Aged: The Index of ADL: A standardized measure of biological and psychosocial function. JAMA J. Am. Med. Assoc. 1963, 185, 914–919. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Lobo-Escolar, A.; Saz, P.; Marcos, G.; Quintanilla, M.A.; Campayo, A.; Lobo, A. Somatic and psychiatric comorbidity in the general elderly population: Results from the ZARADEMP Project. J. Psychosom. Res. 2008, 65, 347–355. [Google Scholar] [CrossRef]
- Lobo, A.; Saz, P.; Marcos, G.; Día, J.L.; De-la Cámara, C. The prevalence of dementia and depression in the elderly community in a southern European population: The Zaragoza Study. Arch. Gen. Psychiatry 1995, 52, 497–506. [Google Scholar] [CrossRef]
- Mazzanti-Di-Ruggiero, M.D.Á.M.D. Declaración de Helsinki, principios y valores bioéticos en juego en la investigación médica con seres humanos. Rev. Colomb. De Bioética 2015, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Saz, P.; Día, J.L.; De-la-Cámara, C.; Carreras, S.; Marcos, G.; Lobo, A. Reliability and validity of the Spanish version of the GMS-AGECATE package for the assessment of dementia and cognitive disturbances. Int. J. Geriatr. Psychiatry 1996, 11, 721–728. [Google Scholar] [CrossRef]
- Lobo, E.; Marcos, G.; Santabárbara, J.; Lobo-Escolar, L.; Salvador-Rosés, H.; De-la-Cámara, C. Gender differences in the association of cognitive impairment with the risk of hip fracture in the older population. Maturitas 2018, 109, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Sommerlad, A.; Ruegger, J.; Singh-Manoux, A.; Lewis, G.; Livingston, G. Marriage and risk of dementia: Systematic review and meta-analysis of observational studies. J. Neurol. Neurosurg. Psychiatry 2018, 89, 231–238. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, G.; Park, S. Widowhood status as a risk factor for cognitive decline among older adults. Am. J. Geriatr. Psychiatry 2018, 26, 778–787. [Google Scholar] [CrossRef]
- Biddle, K.D.; Jacobs, H.I.L.; D’Oleire-Uquillas, F.; Zide, B.S.; Kirn, D.R.; Properzi, M.R. Associations of widowhood and β-Amyloid with cognitive decline in cognitively unimpaired older adults. JAMA Netw. Open 2020, 3, e200121. [Google Scholar] [CrossRef] [Green Version]
- Launer, L.J.; Brayne, C.; Breteler, M.M.B. Epidemiologic approach to the study of dementing diseases: A nested case-control study in European incidence studies of dementia. Neuroepidemiology 1992, 11, 114–118. [Google Scholar] [CrossRef]
- Lamar, M.; Boots, E.A.; Arfanakis, K.; Barnes, L.L.; Schneider, J.A. Common brain structural alterations associated with cardiovascular disease risk factors and Alzheimer’s dementia: Future directions and implications. Neuropsychol. Rev. 2020, 30, 546–557. [Google Scholar] [CrossRef]
- Gracia-García, P.; López-Antón, R.; Santabárbara, J.; Quintanilla, M.A.; De-la-Cámara, C.; Marcos, G. Cognition and daily activities in a general population sample aged +55. Aging Neuropsychol. Cogn. 2021, 28, 270–283. [Google Scholar] [CrossRef]
- Santabárbara, J.; Villagrasa, B.; Gracia-García, P. Clinically relevant late-life depression as a risk factor of dementia: A systematic review and meta-anaysis of prospective cohort studies. Rev. Neurol. 2019, 68, 493–502. [Google Scholar] [CrossRef]
- Firth, J.; Solmi, M.; Wootton, R.E.; Vancampfort, D.; Schuch, F.B.; Hoare, E. A meta-review of “lifestyle psychiatry”: The role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry 2020, 19, 360–380. [Google Scholar] [CrossRef]
- Thiébaut, A.C.M.; Bénichou, J. Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: A simulation study. Stat. Med. 2004, 23, 3803–3820. [Google Scholar] [CrossRef]
- Grambsch, P.M.; Therneau, T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994, 81, 515–526. [Google Scholar] [CrossRef]
- Davidson, K.W.; Burg, M.M.; Kronish, I.M.; Shimbo, D.; Dettenborn, L.; Mehran, R. Association of anhedonia with recurrent major adverse cardiac events and mortality 1 year after acute coronary syndrome. Arch. Gen. Psychiatry 2010, 67, 480–488. [Google Scholar] [CrossRef] [Green Version]
- Doyle, F. Anhedonia predicts adverse cardiac events in people with acute coronary syndromes. Evid. Based Ment. Health 2010, 13, 109. [Google Scholar] [CrossRef]
- Leroy, M.; Loas, G.; Perez-Diaz, F. Anhedonia as predictor of clinical events after acute coronary syndromes: A 3-year prospective study. Compr. Psychiatry. 2010, 51, 8–14. [Google Scholar] [CrossRef]
- Byrne, C.J.; Toukhsati, S.R.; Toia, D.; O’Halloran, P.D.; Hare, D.L. Hopelessness and cognitive impairment are risk markers for mortality in systolic heart failure patients. J. Psychosom. Res. 2018, 109, 12–18. [Google Scholar] [CrossRef]
- Denollet, J.; Pedersen, S.S.; Daemen, J.; De-Jaegere, P.; Serruys, P.W.; Van-Domburg, R.T. Reduced positive affect (anhedonia) predicts major clinical events following implantation of coronary-artery stents. J. Intern. Med. 2008, 263, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Nefs, G.; Pop, V.J.M.; Denollet, J.; Pouwer, F. Depressive symptoms and all-cause mortality in people with type 2 diabetes: A focus on potential mechanisms. Br. J. Psychiatry 2016, 209, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Campayo, A.; De-Jonge, P.; Roy, J.F.; Saz, P.; De-la-Cámara, C.; Quintanilla, M.A. Depressive disorder and incident diabetes mellitus: The effect of characteristics of depression. Am. J. Psychiatry 2010, 167, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Justice, N.J. The relationship between stress and Alzheimer’s disease. Neurobiol. Stress 2018, 8, 127–133. [Google Scholar] [CrossRef]
- Bisht, K.; Sharma, K.; Tremblay, M.È. Chronic stress as a risk factor for Alzheimer’s disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol. Stress 2018, 9, 9–21. [Google Scholar] [CrossRef]
- Briones, A.; Gagno, S.; Martisova, E.; Dobarro, M.; Aisa, B.; Solas, M. Stress-induced anhedonia is associated with an increase in Alzheimer’s disease-related markers. Br. J. Pharmacol. 2012, 165, 897–907. [Google Scholar] [CrossRef] [Green Version]
- Han, J.W.; Kim, T.H.; Kwak, K.P.; Kim, K.; Kim, B.J.; Kim, S.G. Overview of the Korean longitudinal study on cognitive aging and dementia. Psychiatry Investig. 2018, 15, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Der-Avakian, A.; Markou, A. The Neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012, 35, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Jonker, F.A.; Jonker, C.; Scheltens, P.; Scherder, E.J.A. The role of the orbitofrontal cortex in cognition and behavior. Rev. Neurosci. 2015, 26, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Delgado, M.R. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci. 2007, 1104, 70–88. [Google Scholar] [CrossRef] [PubMed]
- Robbins, T.W. Illuminating anhedonia: Optogenetics and fMRI reveal the brain circuitry of anhedonia. Science 2016, 351, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Naguy, A.; Alwetayan, S.; AlKhadhari, S. Anhedonia as a transdiagnostic construct. Asian J. Psychiatry 2020, 48, 101604. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, D.A.; Holmes, A.J.; Dillon, D.G.; Goetz, E.L.; Birk, J.L.; Bogdan, R. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry 2009, 166, 702–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, E.A. The amygdala, reward and emotion. Trends Cogn. Sci. 2007, 11, 489–497. [Google Scholar] [CrossRef]
- Han, S.; Yang, S.H.; Kim, J.Y.; Mo, S.; Yang, E.; Song, K.M. Down-regulation of cholinergic signaling in the habenula induces anhedonia-like behavior. Sci. Rep. 2017, 7, 900. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Yoon, S.; Nakajima, R.; Lee, H.J.; Lim, H.J.; Lee, Y.K. Dopamine D2 receptor-mediated circuit from the central amygdala to the bed nucleus of the stria terminalis regulates impulsive behavior. Proc. Natl. Acad. Sci. USA 2018, 115, E10730–E10739. [Google Scholar] [CrossRef] [Green Version]
- Pessiglione, M.; Seymour, B.; Flandin, G.; Dolan, R.J.; Frith, C.D. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 2006, 442, 1042–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, R.S.; Begeny, C.T.; Boyle, P.A.; Schneider, J.A.; Bennett, D.A. Vulnerability to stress, anxiety, and development of dementia in old age. Am. J. Geriatr. Psychiatry 2011, 19, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Gracia-García, P.; Modrego, P.; Lobo, A. Apathy and neurocognitive correlates: Review from the perspetive of ‘precision psychiatry’. Curr. Opin. Psychiatry 2021, 34, 193–198. [Google Scholar] [CrossRef]
- Ma, Y.; Liang, L.; Zheng, F.; Shi, L.; Zhong, B.; Xie, W. Association between sleep duration and cognitive decline. JAMA Netw. Open. 2020, 3, e2013573. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.T.; Xu, W.; Tan, C.C.; Andrieu, S.; Suckling, J.; Evangelou, E. Evidence-based prevention of Alzheimer’s disease: Systematic review and meta-analysis of 243 observational prospective studies and 153 randomised controlled trials. J. Neurol. Neurosurg. Psychiatry 2020, 91, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
| Follow-Up AD Status | |||
|---|---|---|---|
| Variables | Non-Incident AD (N = 1565) | Incident AD (N = 77) | p-Value |
| Socio-demographic characteristics | |||
| Age (years) | 73.4 (6.5) | 84.1 (6.6) | <0.001 |
| Female sex | 874 (55.8%) | 55 (71.4%) | 0.010 |
| Education (years) | 7.6 (3.9) | 5.7 (3.5) | <0.001 |
| Marital status (ref. single) | <0.001 | ||
| Married/in couple | 1001 (63.9%) | 23 (29.9%) | |
| Formerly married | 409 (26.1%) | 53 (68.8%) | |
| Psychopathological risk factors | |||
| Depression | 110 (7%) | 10 (13%) | 0.082 |
| Anhedonia | 48 (3%) | 7 (9%) | 0.011 |
| Behavioural risk factors | |||
| BMI | 27.1 (6.3) | 26.1 (5.1) | <0.001 |
| Vascular risk factors | |||
| Diabetes | 192 (12.2%) | 8 (10.4%) | 0.770 |
| Hypertension | 1116 (71.3%) | 49 (63.6%) | 0.181 |
| Previous vascular disease | 110 (7%) | 5 (6.5%) | 0.304 |
| Functional and cognitive status | |||
| Basic ADLs | 69 (4.4%) | 13 (16.9%) | <0.001 |
| Instrumental ADLs | 131 (8.4%) | 32 (41.6%) | <0.001 |
| MMSE score | 30.8 (2.8) | 27.4 (2.9) | <0.001 |
| UNIVARIATE MODEL | MULTIVARIATE MODEL | |||||||
|---|---|---|---|---|---|---|---|---|
| ANHEDONIA STATUS AT BASELINE | No. (%) of AD Incident Cases | Person-Years | IR (per 1000 Person-Years) (95% CI) | IRR (95% CI) | HR (95% CI) | p-Value | HR (95% CI) | p-Value |
| No case (n = 1587) | 70 (4.4%) | 10,529 | 6.6 (5.2–8.4) | 1 | 1 | 1 | ||
| Case (n = 55) | 7 (12.7%) | 424 | 16.5 (6.6–34.0) | 2.48 (1.14–5.40) | 2.37 (1.04–5.40) | 0.039 | 2.95 (1.03–8.47) | 0.043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaquero-Puyuelo, D.; De-la-Cámara, C.; Olaya, B.; Gracia-García, P.; Lobo, A.; López-Antón, R.; Santabárbara, J. Anhedonia as a Potential Risk Factor of Alzheimer’s Disease in a Community-Dwelling Elderly Sample: Results from the ZARADEMP Project. Int. J. Environ. Res. Public Health 2021, 18, 1370. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18041370
Vaquero-Puyuelo D, De-la-Cámara C, Olaya B, Gracia-García P, Lobo A, López-Antón R, Santabárbara J. Anhedonia as a Potential Risk Factor of Alzheimer’s Disease in a Community-Dwelling Elderly Sample: Results from the ZARADEMP Project. International Journal of Environmental Research and Public Health. 2021; 18(4):1370. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18041370
Chicago/Turabian StyleVaquero-Puyuelo, David, Concepción De-la-Cámara, Beatriz Olaya, Patricia Gracia-García, Antonio Lobo, Raúl López-Antón, and Javier Santabárbara. 2021. "Anhedonia as a Potential Risk Factor of Alzheimer’s Disease in a Community-Dwelling Elderly Sample: Results from the ZARADEMP Project" International Journal of Environmental Research and Public Health 18, no. 4: 1370. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18041370






