Marine Fish Primary Hepatocyte Isolation and Culture: New Insights to Enzymatic Dissociation Pancreatin Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Acclimatization
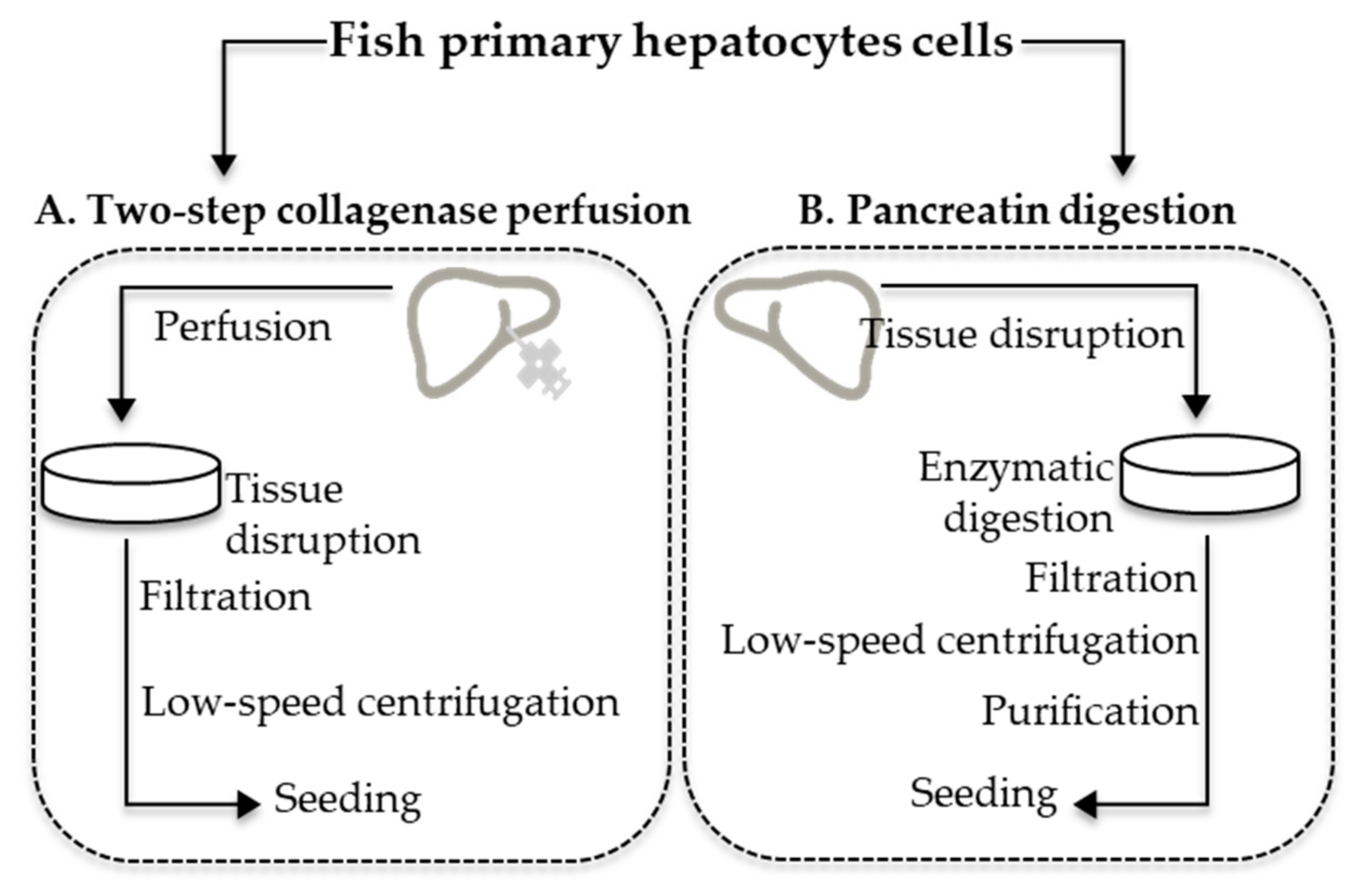
2.2. Primary Hepatocyte Cell Isolation
2.2.1. Two-Step Collagenase Perfusion
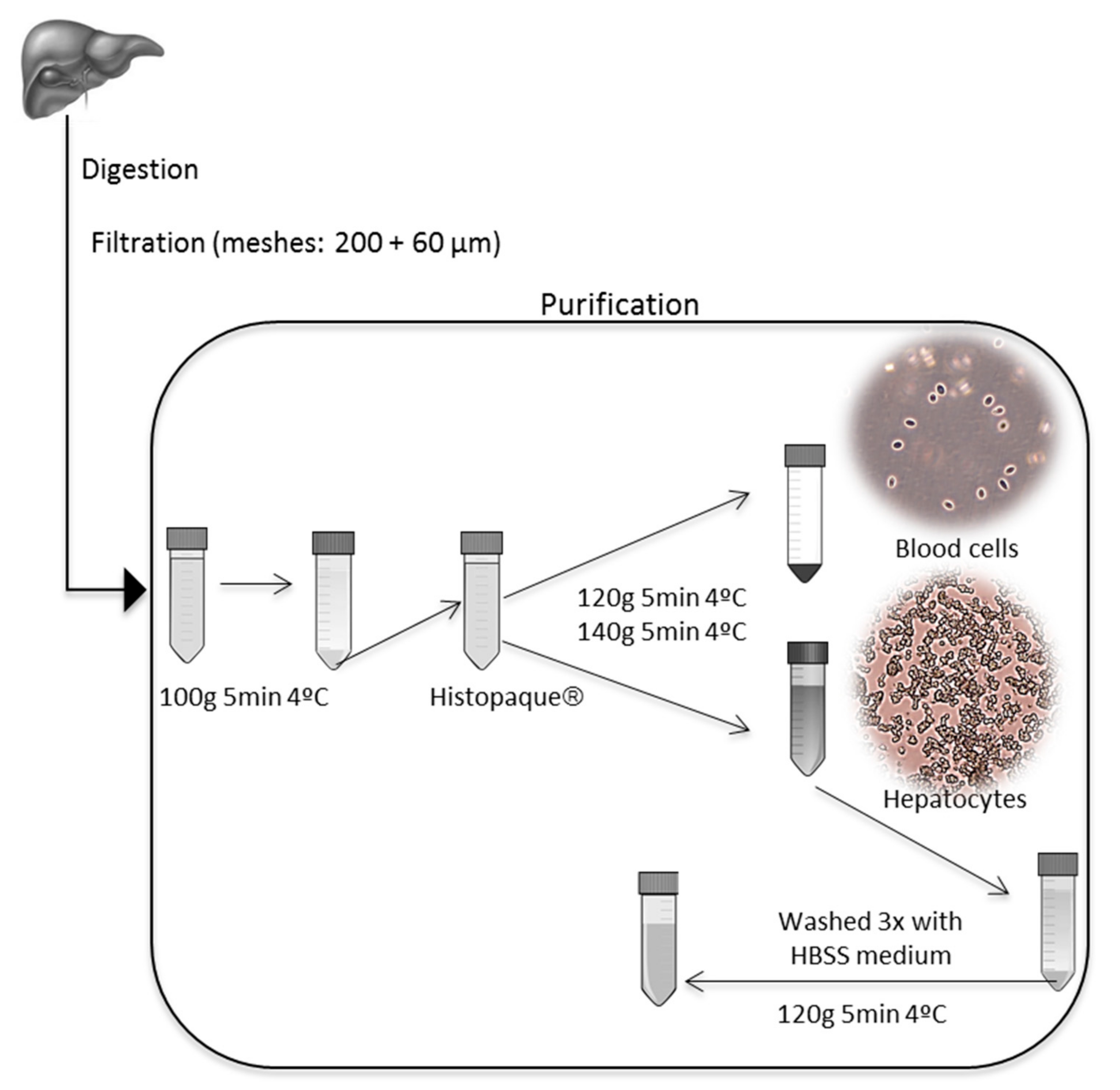
2.2.2. Pancreatin Digestion
2.3. Seeding Fish Primary Hepatocytes
2.4. Monitoring of Fish Primary Hepatocyte Cultures: Morphology, Viability, and Functionality
2.4.1. Cell Morphology
2.4.2. Cell Attachment and Viability
2.4.3. Cell Viability by MTT
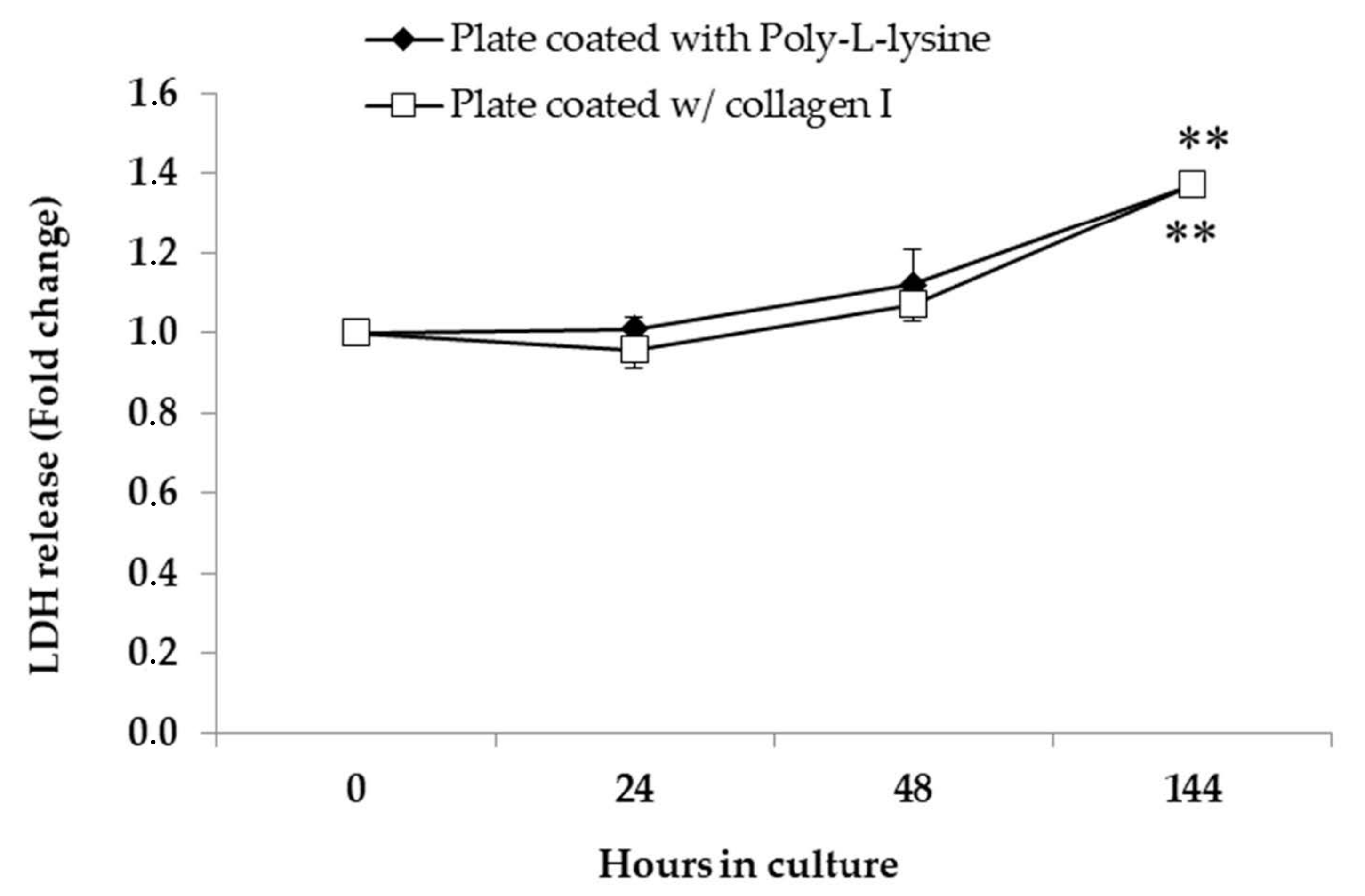
2.4.4. Detection of LDH in the Supernatant
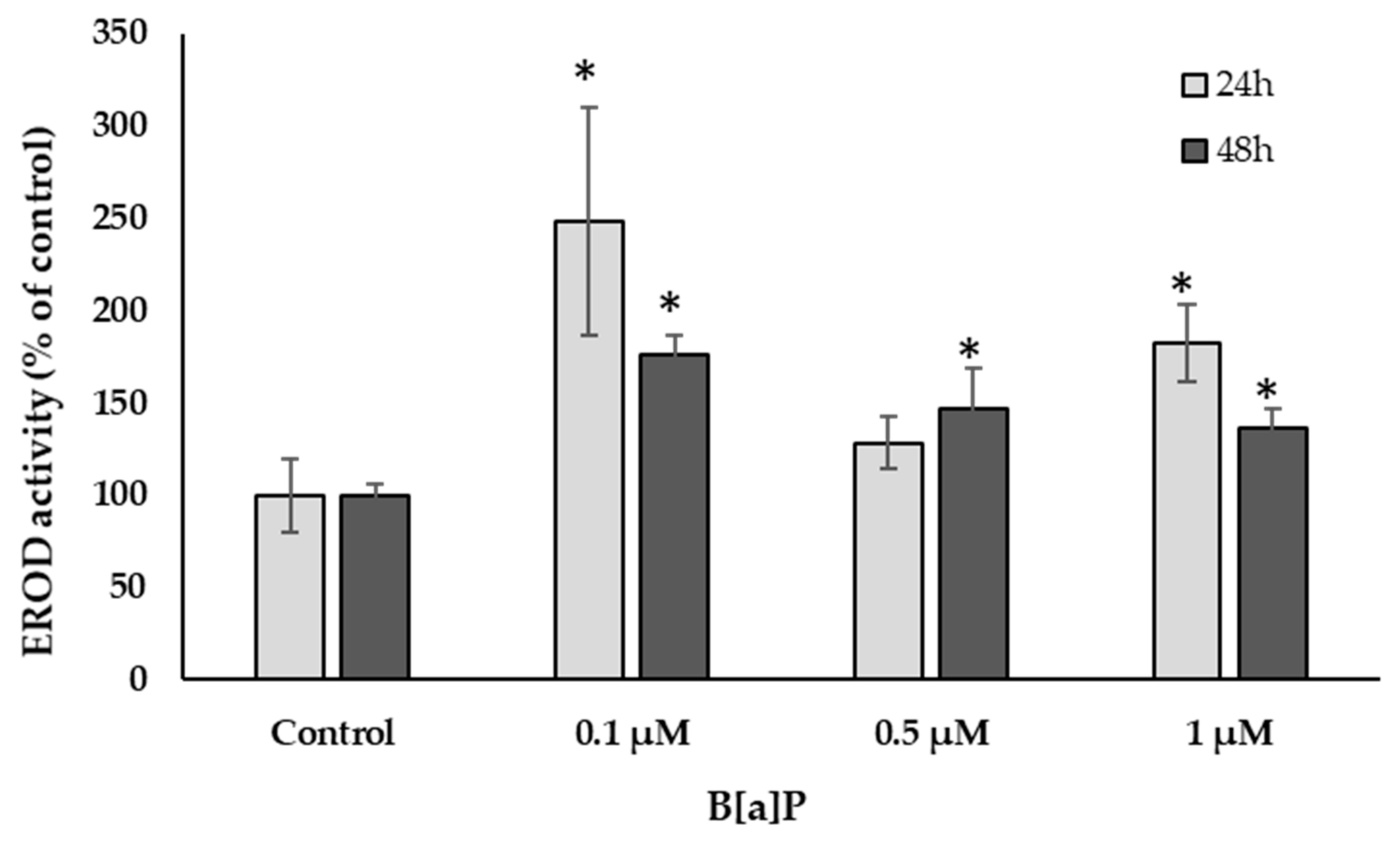
2.4.5. Ethoxyresorufin O-Deethylase (EROD) Activity
2.5. Data Analysis
3. Results
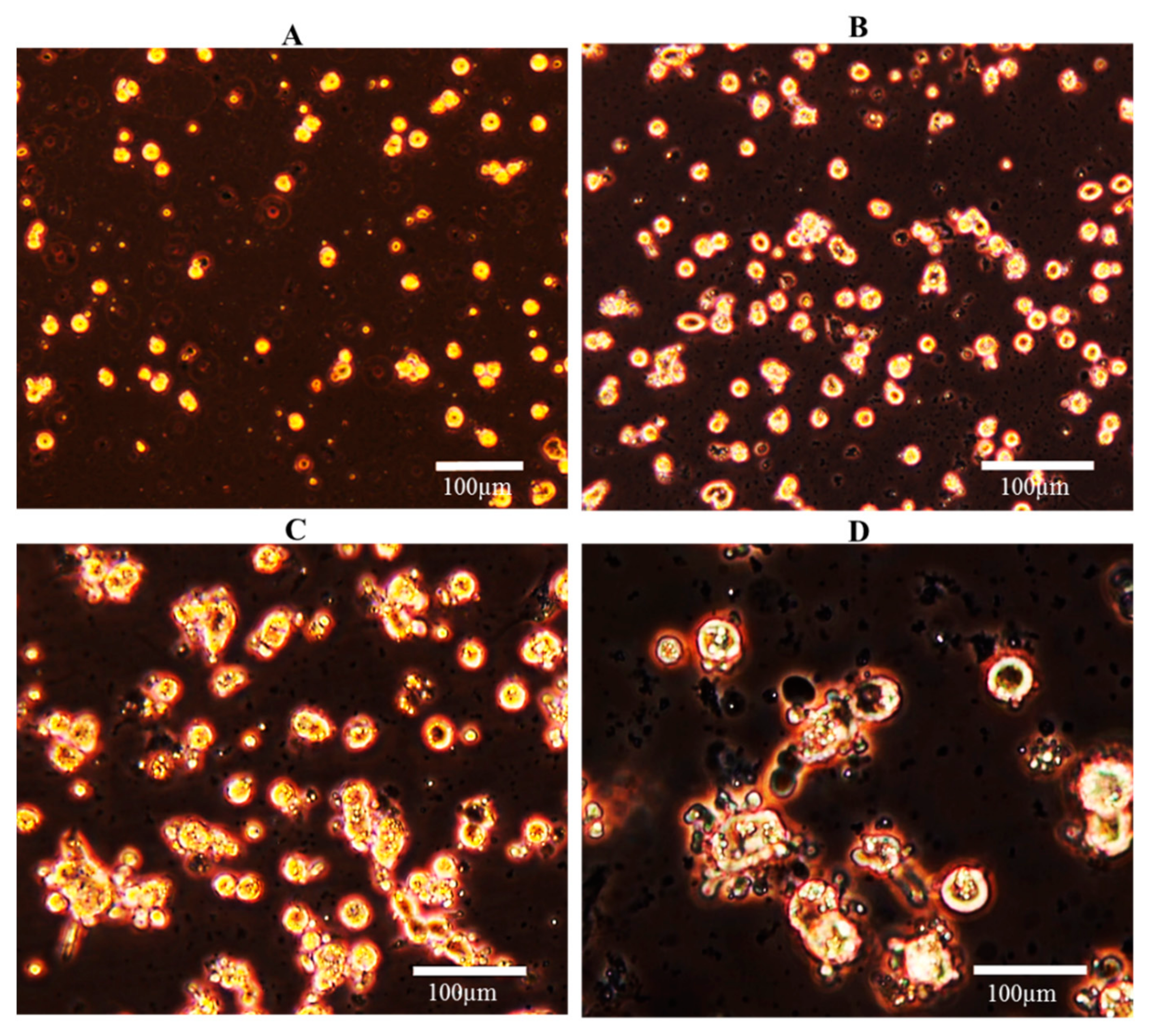
3.1. Cell Yield and Viability
Cell Culture Purity
3.2. Cell Culture Condition: Morphology, Viability, and Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antunes, J.; Frias, J.; Sobral, P. Microplastics on the Portuguese coast. Mar. Pollut. Bull. 2018, 131, 294–302. [Google Scholar] [CrossRef]
- Karri, V.; Kumar, V.; Ramos, D.; Oliveira, E.; Schuhmacher, M. Comparative in vitro toxicity evaluation of heavy metals (lead, cadmium, arsenic, and methylmercury) on HT-22 hippocampal cell line. Biol. Trace Elem. Res. 2018, 184, 226–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IPEN and the National Toxics Network (NTN). Ocean Pollutants Guide: Toxic Threats to Human and Marine Life. 2018. Available online: www.ipen.org (accessed on 25 June 2019).
- Martins, M.; Silva, A.; Costa, M.H.; Miguel, C.; Costa, P. Co-exposure to environmental carcinogens in vivo induces neoplasia-related hallmarks in low genotoxicity events, even after removal of insult. Sci. Rep. 2018, 8, 3649. [Google Scholar] [CrossRef]
- Martins, M.; Ferreira, A.M.; Costa, M.H.; Costa, P.M. Comparing the genotoxicity of a potentially carcinogenic and a noncarcinogenic PAH, singly, and in binary combination, on peripheral blood cells of the European sea bass. Environ. Toxicol. 2016, 31, 1307–1318. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Santos, J.M.; Diniz, M.S.; Ferreira, A.M.; Costa, M.H.; Costa, P.M. Effects of carcinogenic versus non-carcinogenic AHR-active PAHs and their mixtures: Lessons from ecological relevance. Environ. Res. 2015, 138, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Costa, P.M.; Ferreira, A.M.; Costa, M.H. Comparative DNA damage and oxidative effects of carcinogenic and non-carcinogenic sediment-bound PAHs in the gills of a bivalve. Aquat. Toxicol. 2013, 142–143, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Costa, P.M.; Raimundo, J.; Vale, C.; Ferreira, A.M.; Costa, M.H. Impact of remobilized contaminants in Mytilus edulis during dredging operations in a harbour area: Bioaccumulation and biomarker responses. Ecotoxicol. Environ. Saf. 2012, 85, 96–103. [Google Scholar] [CrossRef]
- Martins, M.; Ferreira, A.M.; Vale, C. The influence of Sarcocornia fruticosa on retention of PAHs in salt marsh sediments (Sado estuary, Portugal). Chemosphere 2008, 71, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Tasca, A.L.; Fletcher, A. State of the art of the environmental behaviour and removal techniques of the endocrine disruptor 3,4-dichloroaniline. J. Environ. Sci. Health A Tox. Hazard Subst. Environ. Eng. 2018, 53, 260–270. [Google Scholar] [CrossRef]
- Celandar, M.C. Cocktail effects on biomarker responses in fish. Aquat. Toxicol. 2011, 105, 72–77. [Google Scholar] [CrossRef]
- Tan, L.; Schirmer, K. Cell culture-based biosensing techniques for detecting toxicity in water. Curr. Opin. Biotechnol. 2017, 45, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Kramer, N.I.; Hermens, J.L.M.; Schirmer, K. The influence of modes of action and physicochemical properties of chemicals on the correlation between in vitro and acute fish toxicity data. Toxicol. Vitro. 2009, 23, 1372–1379. [Google Scholar] [CrossRef]
- Bickley, L.K.; Langea, A.; Winterb, M.J.; Tylera, C.R. Evaluation of a carp primary hepatocyte culture system for screening chemicals for oestrogenic activity. Aquat. Toxicol. 2009, 94, 195–203. [Google Scholar] [CrossRef]
- Baksi, S.M.; Frazier, J.M. Isolated fish hepatocytes- model systems for toxicology research. Aquat. Toxicol. 1990, 16, 229–256. [Google Scholar] [CrossRef]
- Poteser, M. Cell-based in vitro models in environmental toxicology: A review. Biomonitoring 2017, 4, 11–26. [Google Scholar] [CrossRef]
- Segner, H.; Cravedi, J.P. Metabolic activity in primary cultures of fish hepatocytes. Altern. Lab. Anim. 2001, 29, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Rodd, A.L.; Messier, N.J.; Vaslet, C.A.; Kane, A.B. A 3D fish liver model for aquatic toxicology: Morphological changes and Cyp1a induction in PLHC-1 microtissues after repeated benzo(a)pyrene exposures. Aquat. Toxicol. 2017, 186, 134–144. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.; Santos, P.; Rey-Salgueiro, L.; Zaja, R.; Reis-Henriques, M.A.; Smital, T. The first demonstration of CYP1A and the ABC protein(s) gene expression and activity in European seabass (Dicentrarchus labrax) primary hepatocytes. Chemosphere 2014, 100, 152–159. [Google Scholar] [CrossRef]
- Lakra, W.S.; Raja Swaminathan, T.; Joy, K.P. Development, characterization, conservation and storage of fish cell lines: A review. Fish Physiol. Biochem. 2011, 37, 1–20. [Google Scholar] [CrossRef]
- Pesonen, M.; Andersson, T.B. Fish primary hepatocyte culture; an important model for xenobiotic metabolism and toxicity studies. Aquat. Toxicol. 1997, 37, 253–267. [Google Scholar] [CrossRef]
- Berry, M.N.; Friend, D.S. High-yield preparation of isolated rat liver parenchymal cells. J. Cell Biol. 1969, 43, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, M.J.; Schulta, J.; Fain, J.N. Hormone-stimulated glycogenolysis in isolated goldfish hepatocytes. Am. J. Physiol. 1976, 231, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanhong, F.; Chenghua, H.; Guofang, L.; Haibin, Z. Optimization of the isolation and cultivation of Cyprinus carpio primary hepatocytes. Cytotechnology 2008, 58, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Bermejo-Nogales, A.; Connolly, M.; Rosenkranz, P.; Fernández-Cruz, M.L.; Navas, J.M. Negligible cytotoxicity induced by different titanium dioxide nanoparticles in fish cell lines. Ecotoxicol. Environ. Saf. 2017, 138, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.; Hultman, M.T.; Tollefsen, K.E. Primary hepatocytes from Arctic char (Salvelinus alpinus) as a relevant Arctic in vitro model for screening contaminants and environmental extracts. Aquat. Toxicol. 2017, 187, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Stott, L.C.; Schnell, S.; Hogstrand, C.; Owen, S.F.; Bury, N.R. A primary fish gill cell culture model to assess pharmaceutical uptake and efflux: Evidence for passive and facilitated transport. Aquat. Toxicol. 2015, 159, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Bury, N.R.; Schnell, S.; Hogstrand, C. Gill cell culture systems as models for aquatic environmental monitoring. J. Exp. Biol. 2014, 217, 639–650. [Google Scholar] [CrossRef] [Green Version]
- Thomas, K.V.; Farkas, J.; Farmen, E.; Christian, P.; Langford, K.; Wu, Q.; Tollefsen, K.E. Effects of dispersed aggregates of carbon and titanium dioxide engineered nanoparticles on rainbow trout hepatocytes. J. Toxicol. Environ. Health A 2011, 74, 466–477. [Google Scholar] [CrossRef]
- Banni, M.; Bouraoui, Z.; Ghedira, J.; Clerandeau, C.; Guerbej, H.; Narbonne, J.F.; Boussetta, H. Acute effects of benzo[a]pyrene on liver phase I and II enzymes, and DNA damage on sea bream Sparus aurata. Fish Physiol. Biochem. 2009, 35, 293–299. [Google Scholar] [CrossRef]
- Santacroce, M.P.; Pastore, A.S.; Tinelli, A.; Colamonaco, M.; Crescenzo, G. Implications for Chronic Toxicity of Benzo[a]Pyrene in Sea Bream Cultured Hepatocytes: Cytotoxicity, Inflammation, and Cancerogenesis. Environ Toxicol. 2015, 30, 1045–1062. [Google Scholar] [CrossRef]
- Pastore, A.S.; Santacroce, M.P.; Narracci, M.; Cavallo, R.A.; Acquaviva, M.I.; Casalino, E.; Colamonaco, M.; Crescenzo, G. Genotoxic damage of benzo[a]pyrene in cultured sea bream (Sparus aurata L.) hepatocytes: Harmful effects of chronic exposure. Mar. Environ. Res. 2014, 100, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Zacchino, V.; Centoducati, G.; Narracci, M.; Selvaggi, M.; Santacroce, M.P. Effects of benzo[a]pyrene on gilthead sea bream (Sparus aurata L.) hepatocytes exposed in vitro to short and long term trials. Ital. J. Anim. Sci. 2013, 12, e17. [Google Scholar] [CrossRef] [Green Version]
- Neiffer, D.L.; Stamper, M.A. Fish sedation, analgesia, anesthesia, and euthanasia: Considerations, methods, and types of drugs. ILAR J. 2009, 50, 343–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigma-Aldrich. Available online: https://www.sigmaaldrich.com (accessed on 25 June 2019).
- Ferraris, M.; Radice, S.; Catalani, P.; Francolini, M.; Marabini, L.; Chiesara, E. Early oxidative damage in primary cultured trout hepatocytes: A time course study. Aquat. Toxicol. 2002, 59, 283–296. [Google Scholar] [CrossRef]
- Vinken, M.; Hengstler, J.G. Characterization of hepatocyte-based in vitro systems for reliable toxicity testing. Arch. Toxicol. 2018, 92, 2981–2986. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimentric assay for cellular growth and survival: Application to proliferation and cytotoxicity assay. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Carvalho, C.M.L.; Chew, E.; Hashemy, S.I.; Lu, J.; Holmgren, A. Inhibition of human thioredoxin system: A molecular mechanism of mercury toxicity. J. Biol. Chem. 2008, 283, 11913–11923. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.D.; Li, H.P.; Liu, F.J.; Wang, X.J.; Guo, Y.J.; Wang, L.F.; Lu, W.F.; Li, H.J.; Li, X.P.; Yang, G.Y. Isolation and identification of bovine primary hepatocytes. Genet. Mol. Res. 2013, 12, 5186–5194. [Google Scholar] [CrossRef]
- Ling, J.; Lewis, J.; Douglas, D.; Kneteman, N.M.; Vance, D.E. Characterization of lipid and lipoprotein metabolism in primary human hepatocytes. Biochim. Biophys. Acta 2013, 1831, 387–397. [Google Scholar] [CrossRef]
- Toloudi, M.; Apostolou, P.; Chatziioannou, M.; Papasotiriou, I. Correlation between cancer stem cells and circulating tumor cells and their value. Case Rep. Oncol. 2011, 4, 44–54. [Google Scholar] [CrossRef]
- Lee, L.E.J.; Dayeh, V.R.; Schirmer, K.; Bols, N.C. Applications and potential uses of fish gill cell lines: Examples with RTgill-W1. Vitro Cell Dev. Biol. Anim. 2009, 45, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.M.; Kelly, S.P.; Zhou, B.; Fletcher, M.; O’Donnell, M.; Eletti, B.; Pärt, P. Cultured gill epithelia as models for the freshwater fish gill. Biochim. Biophys. Acta 2002, 1566, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Hultman, M.T.; Songa, Y.; Tollefsen, K.E. 17α-Ethinylestradiol (EE2) effect on global gene expression in primary rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat. Toxicol. 2015, 169, 90–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hultman, M.T.; Rundberget, J.T.; Tollefsen, K.E. Evaluation of the sensitivity, responsiveness and reproducibility of primary rainbow trout hepatocyte vitellogenin expression as a screening assay for estrogen mimics. Aquat. Toxicol. 2015, 159, 233–244. [Google Scholar] [CrossRef] [Green Version]
- Madureira, T.V.; Malhão, F.; Pinheiro, I.; Lopes, C.; Ferreira, N.; Urbatzka, R.; Castro, L.F.C.; Rocha, E. Estrogenic and anti-estrogenic influences in cultured brown trout hepatocytes: Focus on the expression of some estrogen and peroxisomal related genes and linked phenotypic anchors. Aquat. Toxicol. 2015, 169, 133–142. [Google Scholar] [CrossRef]
- Tollefsen, K.E.; Blikstada, C.; Eikvara, S.; Finnea, E.F.; Gregersena, I.K. Cytotoxicity of alkylphenols and alkylated non-phenolics in a primary culture of rainbow trout (Onchorhynchus mykiss) hepatocytes. Ecotoxicol. Environ. Saf. 2008, 69, 64–73. [Google Scholar] [CrossRef]
- Mortensen, A.S.; Arukwe, A. Dimethyl sulfoxide is a potent modulator of estrogen receptor isoforms and xenoestrogen biomarker responses in primary culture of salmon hepatocytes. Aquat. Toxicol. 2006, 79, 99–103. [Google Scholar] [CrossRef]
- Peyon, P.; Calvayrac, R.; Baloche, S.; Burzawa-Gérard, E. Metabolic studies on eel (Anguilla anguilla L.) hepatocytes in primary culture: Effect of 17i-estradiol and growth hormone. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998, 121, 35–44. [Google Scholar] [CrossRef]
- Rankouhi, T.R.; Sanderson, J.T.; Holsteijn, I.; Leeuwen, C.; Vethaak, A.D.; den Berg, M. Effects of natural and synthetic estrogens and various environmental contaminants on vitellogenesis in fish primary hepatocytes: Comparison of Bream (Abramis brama) and Carp (Cyprinus carpio). Toxicol. Sci. 2004, 81, 90–102. [Google Scholar] [CrossRef] [Green Version]
- Behrens, A.; Schirmer, K.; Bols, N.; Segner, H. Polycyclic aromatic hydrocarbons as inducers of cytochrome p4501a enzyme activity in the rainbow trout liver cell line, RTL-W1, and in primary cultures of rainbow trout hepatocytes. Environ. Toxicol. Chem. 2001, 20, 632–643. [Google Scholar] [CrossRef]
- Kim, B.H.; Takemura, A. Culture conditions affect induction of vitellogenin synthesis by estradiol-17b in primary cultures of tilapia hepatocytes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 231–239. [Google Scholar] [CrossRef]
- Eide, M.; Rustenb, M.; Maleb, R.; Jensena, K.H.M.; Goksøyra, A. A characterization of the ZFL cell line and primary hepatocytes as in vitro liver cell models for the zebrafish (Danio rerio). Aquat. Toxicol. 2014, 147, 7–17. [Google Scholar] [CrossRef]
- Fay, K.A.; Fitzsimmons, P.N.; Hoffman, A.D.; Nichols, J.W. Comparison of trout hepatocytes and liver s9 fractions as in vitro models for predicting hepatic clearance in fish. Environ. Toxicol. Chem. 2017, 36, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, A.; Naderi, M.; Niyogi, S. An in vitro examination of selenium–cadmium antagonism using primary cultures of rainbow trout (Oncorhynchus mykiss) hepatocytes. Metallomics 2016, 8, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, M.Q.; Luo, Z.; Wu, K.; Zhu, Q.L.; Zheng, J.L.; Zhang, L.H.; Chen, Q.L. Regulation of insulin on lipid metabolism in freshly isolated hepatocytes from yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 177–178, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Ma, T.; Wu, W.; Wang, Z. EROD activities in a primary cell culture of grass carp (Ctenopharyngodon idellus) hepatocytes exposed to polychlorinated aromatic hydrocarbonas. Ecotoxicol. Environ. Saf. 2004, 58, 84–89. [Google Scholar] [CrossRef]
- Strmac, M.; Braunbeck, T. Isolated hepatocytes of rainbow trout (Oncorhynchus mykiss) as a tool to discriminate between differently contaminated small river systems. Toxicol. Vitro 2000, 14, 361–377. [Google Scholar] [CrossRef]
- Gravato, C.; Santos, M.A. Genotoxicity biomarkers’ association with B(a)P biotransformation in Dicentrarchus labrax L. Ecotoxicol. Environ. Saf. 2003, 55, 352–358. [Google Scholar] [CrossRef]
- Wessel, N.; Ménard, D.; Pichavant-Rafini, K.; Ollivier, H.; Le Goff, J.; Burgeot, T.; Akcha, F. The Toxicity of Benzo[a]pyrene on sole (Solea Solea) hepatocytes: Assessment of genotoxic and enzymatic effects. Polycycl. Aromat. Compd. 2010, 30, 346–354. [Google Scholar] [CrossRef]
| Method | Fish Species | Weight (g) | 1 Number of Cells (×107) | % of Viability | |
|---|---|---|---|---|---|
| Fish | Liver | ||||
| Two-step perfusion | Sparus aurata | 234 ± 43.3 | 4.80 ± 1.66 | 1.04–2.47 = 1.91 ± 0.77 a | 70.7–72.8 |
| Pancreatin digestion | Sparus aurata | 236 ± 41.2 | 4.47± 1.37 | 3.3–7.31 = 4.5 ± 1.9 b | 95.1–100 |
| Psetta maxima | 62.5 ± 8.57 | 0.47 ± 0.19 | 1.08–3.44 = 1.97 ± 0.76 c | 100 | |
| Viability Tests | Plate Coating | |
|---|---|---|
| Plate Coated w/Poly-L Lysine | Plate Coated w/Collagen I | |
| TBA (24 h) | 98.1 ± 11.5 | 94.6 ± 14.1 |
| LDH (24 h) | 97.5 ± 3.4 | 95.5 ± 4.9 |
| MTT | ||
| 24 h | 84.1 ± 8.13 * | 91.8 ± 2.73 |
| 48 h | 82.7 ± 4.157 * | 89.0 ± 2.93 * |
| 72 h | 82.7 ± 8.15 * | 78.7 ± 0.47 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueiredo, N.; Matos, B.; Diniz, M.; Branco, V.; Martins, M. Marine Fish Primary Hepatocyte Isolation and Culture: New Insights to Enzymatic Dissociation Pancreatin Digestion. Int. J. Environ. Res. Public Health 2021, 18, 1380. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18041380
Figueiredo N, Matos B, Diniz M, Branco V, Martins M. Marine Fish Primary Hepatocyte Isolation and Culture: New Insights to Enzymatic Dissociation Pancreatin Digestion. International Journal of Environmental Research and Public Health. 2021; 18(4):1380. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18041380
Chicago/Turabian StyleFigueiredo, Neusa, Beatriz Matos, Mário Diniz, Vasco Branco, and Marta Martins. 2021. "Marine Fish Primary Hepatocyte Isolation and Culture: New Insights to Enzymatic Dissociation Pancreatin Digestion" International Journal of Environmental Research and Public Health 18, no. 4: 1380. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18041380








