Associations between Daily Movement Distribution, Bone Structure, Falls, and Fractures in Older Adults: A Compositional Data Analysis Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample and Study Design
2.2. Ethics Statement
2.3. Peripheral Quantitative Computed Tomography (pQCT)
2.4. Fear and Risk of Falling, Falls, and Fractures
2.5. Physical Activity and Sedentary Behaviors
2.6. Body Composition Measurements and Anthropometrics
2.7. Dietary Intake
2.8. Blood Samples and Serum 25(OH)D
2.9. Statistical Analyses
3. Results
3.1. Descriptive Characteristics of the Sample
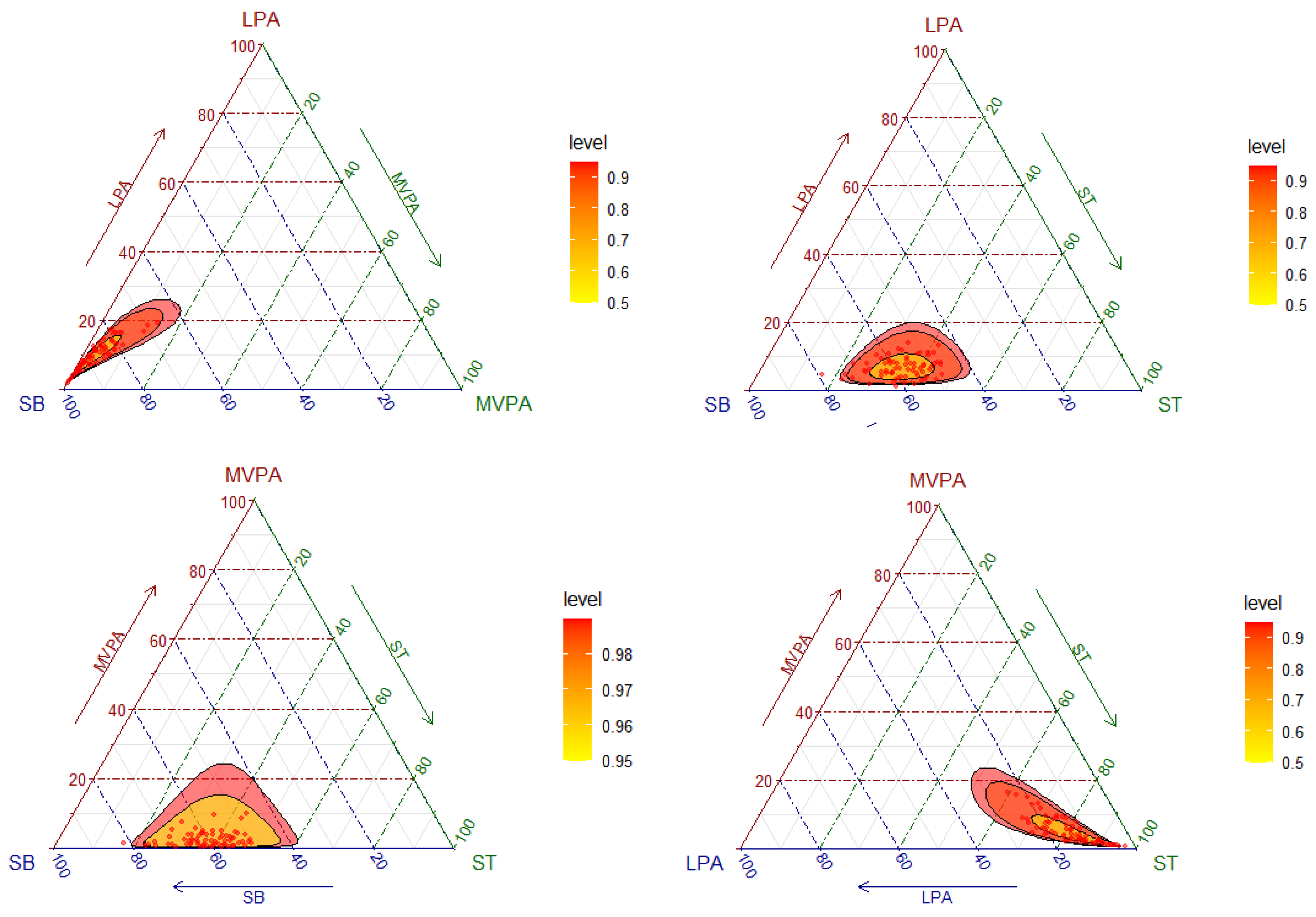
3.2. Composition of the Day and Movement Behavior Characteristics
3.3. Bone Mass and Structure and Movement Behaviors Analysed by Compositional Data
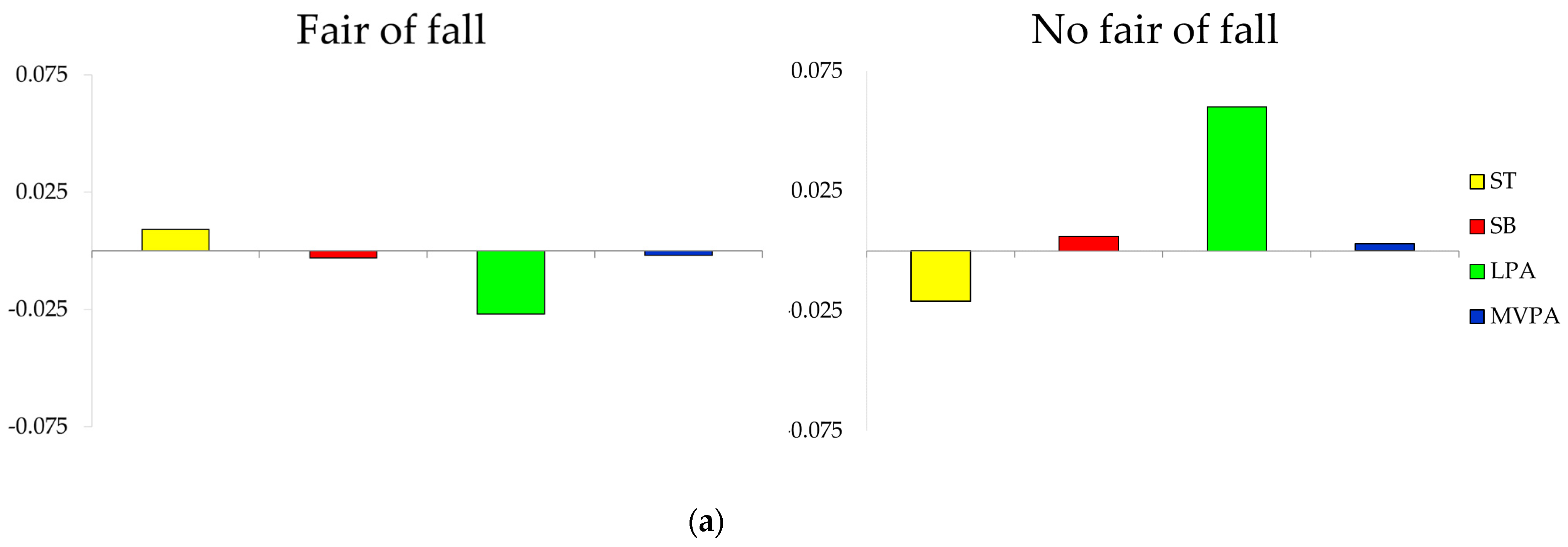
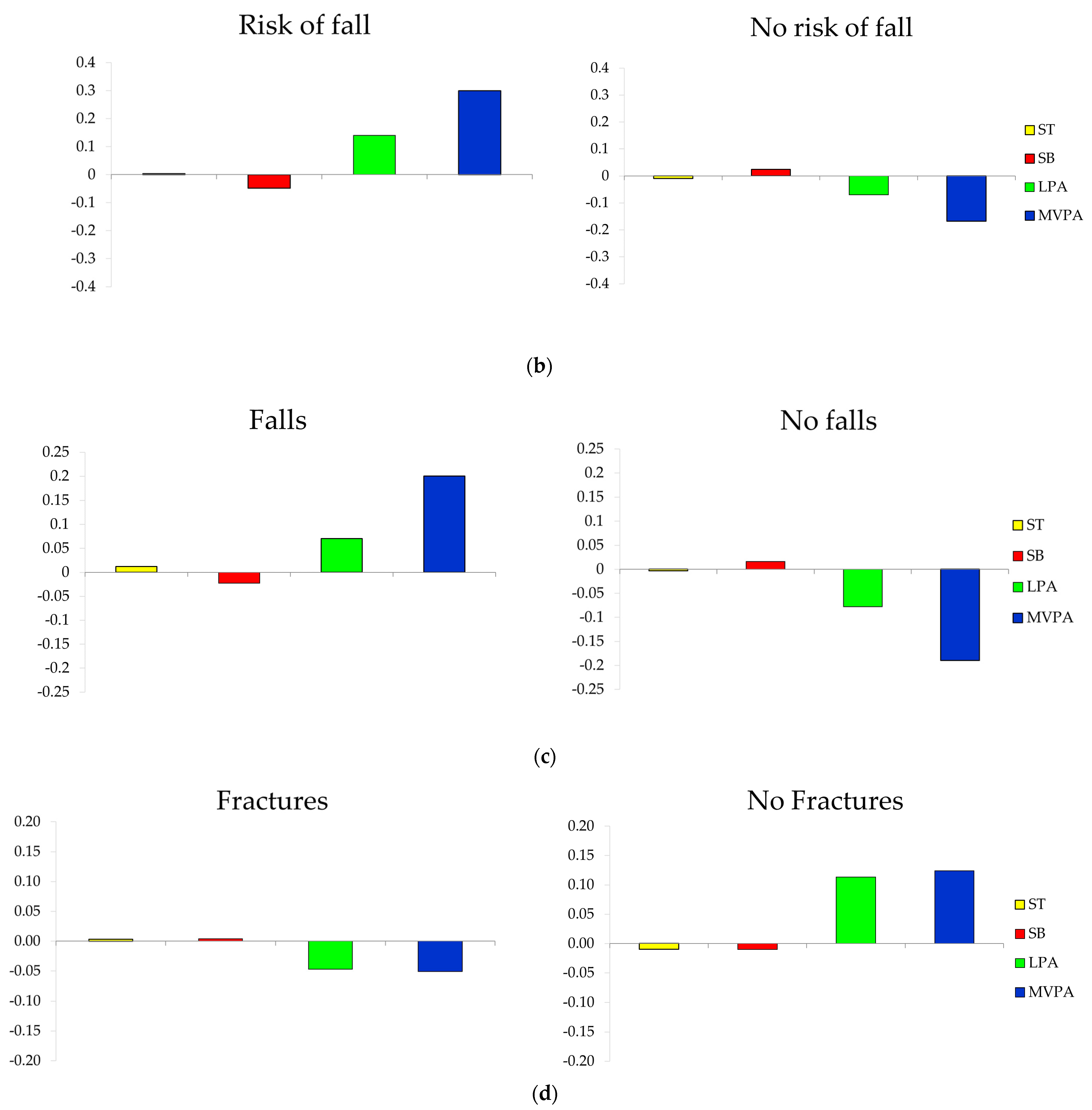
3.4. Composition of the Day by Groups of Fall and Fracture-Related Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; World Health Organization: Geneva, Switzerland, 2019; ISBN 9241514183. [Google Scholar]
- Lear, S.A.; Hu, W.; Rangarajan, S.; Gasevic, D.; Leong, D.; Iqbal, R.; Casanova, A.; Swaminathan, S.; Anjana, R.M.; Kumar, R.; et al. The effect of physical activity on mortality and cardiovascular disease in 130 000 people from 17 high-income, middle-income, and low-income countries: The PURE study. Lancet 2017, 390, 2643–2654. [Google Scholar] [CrossRef]
- Janz, K. Physical activity and bone development during childhood and adolescence. Implications for the prevention of osteoporosis. Minerva Pediatr. 2002, 54, 93–104. [Google Scholar] [PubMed]
- Castrogiovanni, P.; Trovato, F.M.; Szychlinska, M.A.; Nsir, H.; Imbesi, R.; Musumeci, G. The importance of physical activity in osteoporosis. From the molecular pathways to the clinical evidence. Histol. Histopathol. 2016, 31, 1183–1194. [Google Scholar] [PubMed]
- Siris, E.S.; Adler, R.; Bilezikian, J.; Bolognese, M.; Dawson-Hughes, B.; Favus, M.J.; Harris, S.T.; De Beur, S.M.J.; Khosla, S.; Lane, N.E.; et al. The clinical diagnosis of osteoporosis: A position statement from the National Bone Health Alliance Working Group. Osteoporos. Int. 2014, 25, 1439–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, S.C.; Wolk, A. Sedentary leisure-time in relation to mortality and survival time. J. Sci. Med. Sport 2019, 22, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; McNamara, E.; Tainio, M.; De Sá, T.H.; Smith, A.D.; Sharp, S.J.; Edwards, P.; Woodcock, J.; Brage, S.; Wijndaele, K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018, 33, 811–829. [Google Scholar] [CrossRef] [Green Version]
- Higgins, S.; Belcher, S.L.; Lewis, R.D. Sedentary Behaviors in Children and Adolescents: What Is the Influence on Bone Strength? In Nutritional Influences on Bone Health; Metzler, J.B., Ed.; Springer: Cham, Switzerland, 2019; pp. 95–108. [Google Scholar]
- Koedijk, J.B.; Van Rijswijk, J.; Oranje, W.A.; van den Bergh, J.P.; Bours, S.P.; Savelberg, H.H.; Schaper, N.C. Sedentary behaviour and bone health in children, adolescents and young adults: A systematic review. Osteoporos. Int. 2017, 28, 2507–2519. [Google Scholar] [CrossRef]
- Vogel, T.; Brechat, P.-H.; Leprêtre, P.-M.; Kaltenbach, G.; Berthel, M.; Lonsdorfer, J. Health benefits of physical activity in older patients: A review. Int. J. Clin. Pract. 2009, 63, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Cadore, E.L.; Rodríguez-Mañas, L.; Sinclair, A.; Izquierdo, M. Effects of Different Exercise Interventions on Risk of Falls, Gait Ability, and Balance in Physically Frail Older Adults: A Systematic Review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Bea, J.W.; Thomson, C.A.; Wallace, R.B.; Wu, C.; Seguin, R.A.; Going, S.B.; Lacroix, A.; Eaton, C.; Ockene, J.K.; LaMonte, M.J.; et al. Changes in physical activity, sedentary time, and risk of falling: The Women’s Health Initiative Observational Study. Prev. Med. 2017, 95, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Moreira, N.B.; Rodacki, A.L.F.; Pereira, G.; Bento, P.C.B. Does functional capacity, fall risk awareness and physical activity level predict falls in older adults in different age groups? Arch. Gerontol. Geriatr. 2018, 77, 57–63. [Google Scholar] [CrossRef]
- Scott, D.; Johansson, J.; McMillan, L.B.; Ebeling, P.R.; Nordstrom, A.; Nordstrom, P. Mid-calf skeletal muscle density and its associations with physical activity, bone health and incident 12-month falls in older adults: The Healthy Ageing Initiative. Bone 2019, 120, 446–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferretti, J.L.; Cointry, G.R.; Capozza, R.F.; Frost, H.M. Bone mass, bone strength, muscle–bone interactions, osteopenias and osteoporoses. Mech. Ageing Dev. 2003, 124, 269–279. [Google Scholar] [CrossRef]
- Vicente-Rodríguez, G.; Ezquerra, J.; Mesana, M.I.; Fernández-Alvira, J.M.; Rey-López, J.P.; Casajús, J.A.; Moreno, L.A. Independent and combined effect of nutrition and exercise on bone mass development. J. Bone Miner. Metab. 2008, 26, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Clarke, A.E.; Carson, V.; Chaput, J.-P.; Giangregorio, L.M.; Kho, M.E.; Poitras, V.J.; Ross, R.; Saunders, T.J.; Ross-White, A.; et al. A systematic review of compositional data analysis studies examining associations between sleep, sedentary behaviour, and physical activity with health outcomes in adults. Appl. Physiol. Nutr. Metab. 2020, 45, S248–S257. [Google Scholar] [CrossRef] [PubMed]
- Chastin, S.F.M.; Palarea-Albaladejo, J.; Dontje, M.L.; Skelton, D.A. Combined Effects of Time Spent in Physical Activity, Sedentary Behaviors and Sleep on Obesity and Cardio-Metabolic Health Markers: A Novel Compositional Data Analysis Approach. PLoS ONE 2015, 10, e0139984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carson, V.; Tremblay, M.S.; Chastin, S.F.M. Cross-sectional associations between sleep duration, sedentary time, physical activity, and adiposity indicators among Canadian preschool-aged children using compositional analyses. BMC Public Health 2017, 17, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Gómez, I.; Mañas, A.; Losa-Reyna, J.; Rodríguez-Mañas, L.; Chastin, S.F.M.; Alegre, L.M.; García-García, F.J.; Ara, I. Associations between sedentary time, physical activity and bone health among older people using compositional data analysis. PLoS ONE 2018, 13, e0206013. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, I.; Mañas, A.; Losa-Reyna, J.; Rodríguez-Mañas, L.; Chastin, S.F.M.; Alegre, L.M.; García-García, F.J.; Ara, I. Compositional Influence of Movement Behaviors on Bone Health during Aging. Med. Sci. Sports Exerc. 2019, 51, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, I.; Rodríguez-Mañas, L.; Losa-Reyna, J.; Rodríguez-Mañas, L.; Chastin, S.F.M.; Alegre, L.M.; García-García, F.J.; Ara, I. Prospective Changes in the Distribution of Movement Behaviors Are Associated With Bone Health in the Elderly According to Variations in their Frailty Levels. J. Bone Miner. Res. 2020, 35, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, I.; Mañas, A.; Losa-Reyna, J.; Rodríguez-Mañas, L.; Chastin, S.F.M.; Alegre, L.M.; García-García, F.J.; Ara, I. The Impact of Movement Behaviors on Bone Health in Elderly with Adequate Nutritional Status: Compositional Data Analysis Depending on the Frailty Status. Nutrients 2019, 11, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treacy, D.; Hassett, L. The Short Physical Performance Battery. J. Physiother. 2018, 64, 61. [Google Scholar] [CrossRef]
- Fernández-García, Á.I.; Gómez-Cabello, A.; Moradell, A.; Navarrete-Villanueva, D.; Pérez-Gómez, J.; Ara, I.; Pedrero-Chamizo, R.; Subías-Perié, J.; Muniz-Pardos, B.; Casajús, J.A.; et al. How to Improve the Functional Capacity of Frail and Pre-Frail Elderly People? Health, Nutritional Status and Exercise Intervention. The EXERNET-Elder 3.0 Project. Sustainability 2020, 12, 6246. [Google Scholar] [CrossRef]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stagi, S.; Cavalli, L.; Cavalli, T.; De Martino, M.; Brandi, M.L. Peripheral quantitative computed tomography (pQCT) for the assessment of bone strength in most of bone affecting conditions in developmental age: A review. Ital. J. Pediatr. 2016, 42, 1–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Aguero, A.; Vicente-Rodriguez, G.; Gómez-Cabello, A.; Casajús, J.A. Cortical and trabecular bone at the radius and tibia in male and female adolescents with Down syndrome: A peripheral quantitative computed tomography (pQCT) study. Osteoporos. Int. 2012, 24, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Blew, R.M.; Lee, V.R.; Farr, J.N.; Schiferl, D.J.; Going, S.B. Standardizing Evaluation of pQCT Image Quality in the Presence of Subject Movement: Qualitative Versus Quantitative Assessment. Calcif. Tissue Int. 2014, 94, 202–211. [Google Scholar] [CrossRef]
- Izquierdo, M.; Casas Herrero, A.; Zambom Ferraresi, F.; Velilla, N.; Bouzón, C.; Rodríguez-Mañas, L. Guía Práctica para la Prescripción de un Programa de Entrenamiento Físico Multicomponente para la Prevención de la Fragilidad y Caídas en Mayores de 70 Años; VIVIFRAIL Erasmus+: Pamplona, Spain, 2016. [Google Scholar]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Kim, H.-J.; Park, I.; Lee, H.J.; Lee, O. The reliability and validity of gait speed with different walking pace and distances against general health, physical function, and chronic disease in aged adults. J. Exerc. Nutr. Biochem. 2016, 20, 46–50. [Google Scholar] [CrossRef]
- Zhang, S.; Murray, P.; Zillmer, R.; Eston, R.G.; Catt, M.; Rowlands, A.V. Activity Classification Using the GENEA: Optimum sampling frequency and number of axes. Med. Sci. Sports Exerc. 2012, 44, 2228–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Hees, V.T.; Renström, F.; Wright, A.; Gradmark, A.; Catt, M.; Chen, K.Y.; Löf, M.; Bluck, L.; Pomeroy, J.; Wareham, N.J.; et al. Estimation of Daily Energy Expenditure in Pregnant and Non-Pregnant Women Using a Wrist-Worn Tri-Axial Accelerometer. PLoS ONE 2011, 6, e22922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, G.J.; Boddy, L.M.; Sparks, S.A.; Curry, W.B.; Roe, B.; Kaehne, A.; Fairclough, S.J. Evaluation of wrist and hip sedentary behaviour and moderate-to-vigorous physical activity raw acceleration cutpoints in older adults. J. Sports Sci. 2019, 37, 1270–1279. [Google Scholar] [CrossRef] [PubMed]
- Tasheva, P.; Kraege, V.; Vollenweider, P.; Roulet, G.; Méan, M.; Marques-Vidal, P. Accelerometry assessed physical activity of older adults hospitalized with acute medical illness—An observational study. BMC Geriatr. 2020, 20, 382. [Google Scholar] [CrossRef] [PubMed]
- Van Hees, V.T.; Sabia, S.; Jones, S.E.; Wood, A.R.; Anderson, K.N.; Kivimäki, M.; Frayling, T.M.; Pack, A.I.; Bucan, M.; Trenell, M.I.; et al. Estimating sleep parameters using an accelerometer without sleep diary. Sci. Rep. 2018, 8, 12975. [Google Scholar] [CrossRef] [PubMed]
- Martin-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-Rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and Validation of a Food Frequency Questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ballart, J.D.; Pinol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Martin-Moreno, J.M. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef] [Green Version]
- Moreiras, O.; Carbajal, A.; Cabrera, L.C.C. Tablas de Composición de los Alimentos; GUÍA de Prácticas; Ed.Pirámide: Madrid, Spain, 2015. [Google Scholar]
- Mataix, J. Tabla de Composición de Alimentos, 5th ed.; Universidad de Granada: Granada, Spain, 2009. [Google Scholar]
- Dumuid, D.; Pedišić, Ž.; Palarea-Albaladejo, J.; Martín-Fernández, J.A.; Hron, K.; Olds, T. Compositional Data Analysis in Time-Use Epidemiology: What, Why, How. Int. J. Environ. Res. Public Health 2020, 17, 2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chastin, S.F.M.; Mandrichenko, O.; Helbostadt, J.L.; Skelton, D.A. Associations between objectively-measured sedentary behaviour and physical activity with bone mineral density in adults and older adults, the NHANES study. Bone 2014, 64, 254–262. [Google Scholar] [CrossRef]
- Jain, R.K.; Vokes, T. Physical activity as measured by accelerometer in NHANES 2005–2006 is associated with better bone density and trabecular bone score in older adults. Arch. Osteoporos. 2019, 14, 29. [Google Scholar] [CrossRef]
- Johansson, J.; Nordström, A.; Nordström, P. Objectively measured physical activity is associated with parameters of bone in 70-year-old men and women. Bone 2015, 81, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Hannam, K.; Deere, K.C.; Hartley, A.; Al-Sari, U.A.; Clark, E.M.; Fraser, W.D.; Tobias, J.H. Habitual levels of higher, but not medium or low, impact physical activity are positively related to lower limb bone strength in older women: Findings from a population-based study using accelerometers to classify impact magnitude. Osteoporos. Int. 2017, 28, 2813–2822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farr, J.N.; Khosla, S. Skeletal changes through the lifespan—from growth to senescence. Nat. Rev. Endocrinol. 2015, 11, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Füzéki, E.; Engeroff, T.; Banzer, W. Health Benefits of Light-Intensity Physical Activity: A Systematic Review of Accelerometer Data of the National Health and Nutrition Examination Survey (NHANES). Sports Med. 2017, 47, 1769–1793. [Google Scholar] [CrossRef] [PubMed]
- Cowan, P.T.; Kahai, P. Anatomy, Bones; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabello, A.; Pedrero-Chamizo, R.; Olivares, P.R.; Hernández-Perera, R.; Rodriguez-Marroyo, J.A.; Mata, E.; Aznar, S.; Villa, J.G.; Espino-Torón, L.; Gusi, N.; et al. Sitting time increases the overweight and obesity risk independently of walking time in elderly people from Spain. Maturitas 2012, 73, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Mañas, A.; Del Pozo-Cruz, B.; Rodríguez-Gómez, I.; Losa-Reyna, J.; Rodríguez-Mañas, L.; García-García, F.J.; Ara, I. Can Physical Activity Offset the Detrimental Consequences of Sedentary Time on Frailty? A Moderation Analysis in 749 Older Adults Measured with Accelerometers. J. Am. Med. Dir. Assoc. 2019, 20, 634–638.e1. [Google Scholar] [CrossRef]
- Min, C.; Yoo, D.M.; Wee, J.H.; Lee, H.-J.; Byun, S.H.; Choi, H.G. Mortality and cause of death in physical activity and insufficient physical activity participants: A longitudinal follow-up study using a national health screening cohort. BMC Public Health 2020, 20, 1469. [Google Scholar] [CrossRef] [PubMed]
- Mañas, A.; Del Pozo-Cruz, B.; Rodríguez-Gómez, I.; Losa-Reyna, J.; Júdice, P.B.; Sardinha, L.B.; Rodríguez-Mañas, L.; García-García, F.J.; Ara, I. Breaking Sedentary Time Predicts Future Frailty in Inactive Older Adults: A Cross-Lagged Panel Model. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2020. [Google Scholar] [CrossRef]
- Steeves, J.A.; Shiroma, E.J.; Conger, S.A.; Van Domelen, D.; Harris, T.B. Physical activity patterns and multimorbidity burden of older adults with different levels of functional status: NHANES 2003. Disabil. Health J. 2019, 12, 495–502. [Google Scholar] [CrossRef]
- Cauley, J.A.; Hovey, K.M.; Stone, K.L.; Andrews, C.A.; Barbour, K.E.; Hale, L.; Jackson, R.D.; Johnson, K.C.; Leblanc, E.S.; Li, W.; et al. Characteristics of Self-Reported Sleep and the Risk of Falls and Fractures: The Women’s Health Initiative (WHI). J. Bone Miner. Res. 2019, 34, 464–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Zhao, X.; Lu, H.; Jiang, F.; Ma, X.; Zhu, S. Association between sleep duration and bone mineral density in Chinese women. Bone 2011, 49, 1062–1066. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; O’Sullivan, R.; Caserotti, P.; Tully, M.A. Consequences of physical inactivity in older adults: A systematic review of reviews and meta-analyses. Scand. J. Med. Sci. Sports 2020, 30, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Rivasi, G.; Kenny, R.A.; Ungar, A.; Romero-Ortuno, R. Predictors of Incident Fear of Falling in Community-Dwelling Older Adults. J. Am. Med. Dir. Assoc. 2020, 21, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.W. Fear of falling in robust community-dwelling older people: Results of a cross-sectional study. J. Clin. Nurs. 2015, 24, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Keegan, T.H.M.; Sternfeld, B.; Sidney, S.; Quesenberry, C.P.J.; Kelsey, J.L. Outdoor Falls Among Middle-Aged and Older Adults: A Neglected Public Health Problem. Am. J. Public Health 2006, 96, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, X.; Shen, Y.; Yao, P.; Chen, J.; Zhou, Y.; Gu, Y.; Qian, Z.; Cao, X. Effectiveness of exercise intervention on fall-related fractures in older adults: A systematic review and meta-analysis of randomized controlled trials. BMC Geriatr. 2020, 20, 322. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.R.; Close, J.C.T. New horizons in falls prevention. Age Ageing 2018, 47, 492–498. [Google Scholar] [CrossRef]
- Marusic, U.; Verghese, J.; Mahoney, J.R. Cognitive-Based Interventions to Improve Mobility: A Systematic Review and Meta-analysis. J. Am. Med. Dir. Assoc. 2018, 19, 484–491.e3. [Google Scholar] [CrossRef]
- Papalia, G.F.; Papalia, R.; Balzani, L.A.D.; Torre, G.; Zampogna, B.; Vasta, S.; Fossati, C.; Alifano, A.M.; Denaro, V. The Effects of Physical Exercise on Balance and Prevention of Falls in Older People: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2595. [Google Scholar] [CrossRef]
| Variables | Whole Sample (N = 70) |
|---|---|
| Age (years) | 80.4 ± 6.4 |
| Body composition | |
| BMI (kg/m2) | 28.9 ± 4.8 |
| Body Fat % | 37.8 ± 6.7 |
| Tibial Muscle Area | 5491.9 ± 1090.6 |
| Bone health-related confounders | |
| Smoking | 3(3.8) |
| Alcohol (g) | 4.3 ± 7.5 |
| Serum Vitamin D (ng/dL) | 28.5 ± 16.9 |
| Calcium (mg/day) | 1123.7 ± 398.5 |
| Fall-related variables | |
| Fear of falling | 51(64.6) |
| Risk of fall | 28(35.4) |
| Falls | 32(40.5) |
| Fractures | 54(68.4) |
| Bone variables | |
| Tt.BMC 4% | 2.6 ± 0.7 |
| Tt.BMD 4% | 229.4 ± 48.0 |
| Tt.Area 4% | 1405.9 ± 199.6 |
| Tb.BMD 4% | 203.8 ± 41.6 |
| Tt.BMC 38% | 4.1 ± 0.3 |
| Tt.BMD 38% | 781.3 ± 109.1 |
| Tt.Area 38% | 387.8 ± 59.7 |
| Ct.Th 38% | 4.3 ± 0.8 |
| Crt.BMD 38% | 122.3 ± 50.5 |
| SSIp | 1406.2 ± 339.4 |
| Fracture Load | 5133.4 ± 1432.9 |
| Behaviors | Minutes/Day | % Wearing Hours |
|---|---|---|
| ST | 507.4 | 35.2% |
| SB | 827.6 | 57.5% |
| LPA | 82.3 | 5.7% |
| MVPA | 22.7 | 1.5% |
| Behaviors | ST | SB | LPA | MVPA |
|---|---|---|---|---|
| ST | 0.000 | 0.098 | 0.331 | 0.930 |
| SB | 0.098 | 0.000 | 0.355 | 0.977 |
| LPA | 0.331 | 0.355 | 0.000 | 0.286 |
| MVPA | 0.930 | 0.977 | 0.286 | 0.000 |
| Bone Variables. | Model p-Value | γST | p-Value | γSB | p-Value | γLPA | p-Value | γMVPA | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| Tt.BMC 4% | 0.099 | −0.417 | 0.111 | 0.399 | 0.134 | −0.257 | 0.332 | 0.272 | 0.069 |
| Tt.BMD 4% | 0.689 | −8.698 | 0.727 | 9.813 | 0.553 | −16.114 | 0.387 | 14.998 | 0.300 |
| Tt.Area 4% | 0.099 | −0.414 | 0.110 | 0.399 | 0.134 | 0.257 | 0.332 | 0.272 | 0.069 |
| Tb.BMD 4% | 0.689 | −8.698 | 0.727 | 9.813 | 0.703 | −16.114 | 0.553 | 14.998 | 0.300 |
| Tt.BMC 38% | 0.105 | −0.288 | 0.133 | 0.383 | 0.052 | −0.313 | 0.132 | 0.218 | 0.041 |
| Tt.BMD 38% | 0.883 | 0.012 | 0.965 | −0.146 | 0.585 | 0.195 | 0.488 | −0.061 | 0.675 |
| Tt.Area 38% | 0.264 | 21.487 | 0.297 | −12.774 | 0.530 | 6.254 | 0.770 | −14.967 | 0.180 |
| Crt.BMD 38% | 0.773 | 9.937 | 0.785 | 8.932 | 0.793 | −36.899 | 0.328 | 18.029 | 0.332 |
| Ct.Th | 0.012 | −0.533 | 0.163 | 0.446 | 0.245 | −0.502 | 0.190 | 0.590 | 0.005 |
| SSIp | 0.477 | −150.65 | 0.120 | 121.51 | 0.202 | 24.466 | 0.801 | 4.677 | 0.926 |
| Fracture Load X | 0.700 | −371.13 | 0.420 | 176.36 | 0.702 | 70.65 | 0.886 | 124.12 | 0.627 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradell, A.; Rodríguez-Gómez, I.; Fernández-García, Á.I.; Navarrete-Villanueva, D.; Marín-Puyalto, J.; Pérez-Gómez, J.; Villa-Vicente, J.G.; González-Gross, M.; Ara, I.; Casajús, J.A.; et al. Associations between Daily Movement Distribution, Bone Structure, Falls, and Fractures in Older Adults: A Compositional Data Analysis Study. Int. J. Environ. Res. Public Health 2021, 18, 3757. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18073757
Moradell A, Rodríguez-Gómez I, Fernández-García ÁI, Navarrete-Villanueva D, Marín-Puyalto J, Pérez-Gómez J, Villa-Vicente JG, González-Gross M, Ara I, Casajús JA, et al. Associations between Daily Movement Distribution, Bone Structure, Falls, and Fractures in Older Adults: A Compositional Data Analysis Study. International Journal of Environmental Research and Public Health. 2021; 18(7):3757. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18073757
Chicago/Turabian StyleMoradell, Ana, Irene Rodríguez-Gómez, Ángel Iván Fernández-García, David Navarrete-Villanueva, Jorge Marín-Puyalto, Jorge Pérez-Gómez, José Gerardo Villa-Vicente, Marcela González-Gross, Ignacio Ara, José Antonio Casajús, and et al. 2021. "Associations between Daily Movement Distribution, Bone Structure, Falls, and Fractures in Older Adults: A Compositional Data Analysis Study" International Journal of Environmental Research and Public Health 18, no. 7: 3757. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18073757








