Importance of Geospatial Heterogeneity in Chronic Disease Burden for Policy Planning in an Urban Setting Using a Case Study of Singapore
Abstract
:1. Introduction
2. Materials and Methods
- Seed random initial physical locations for the synthetic population;
- Calculate the chi-square statistic for each census table using the synthetic population as the observed values and the census as the expected values;
- Select two random individuals in the population and swap their physical locations;
- Calculate the new chi-square statistic for each census table. If the new chi-square statistic is lower than the previous one, accept the swap. If not, reject the swap and revert the physical locations to their original values;
- Repeat steps 2–4 until improvement in the chi-square statistic is marginal, implying optimal convergence.
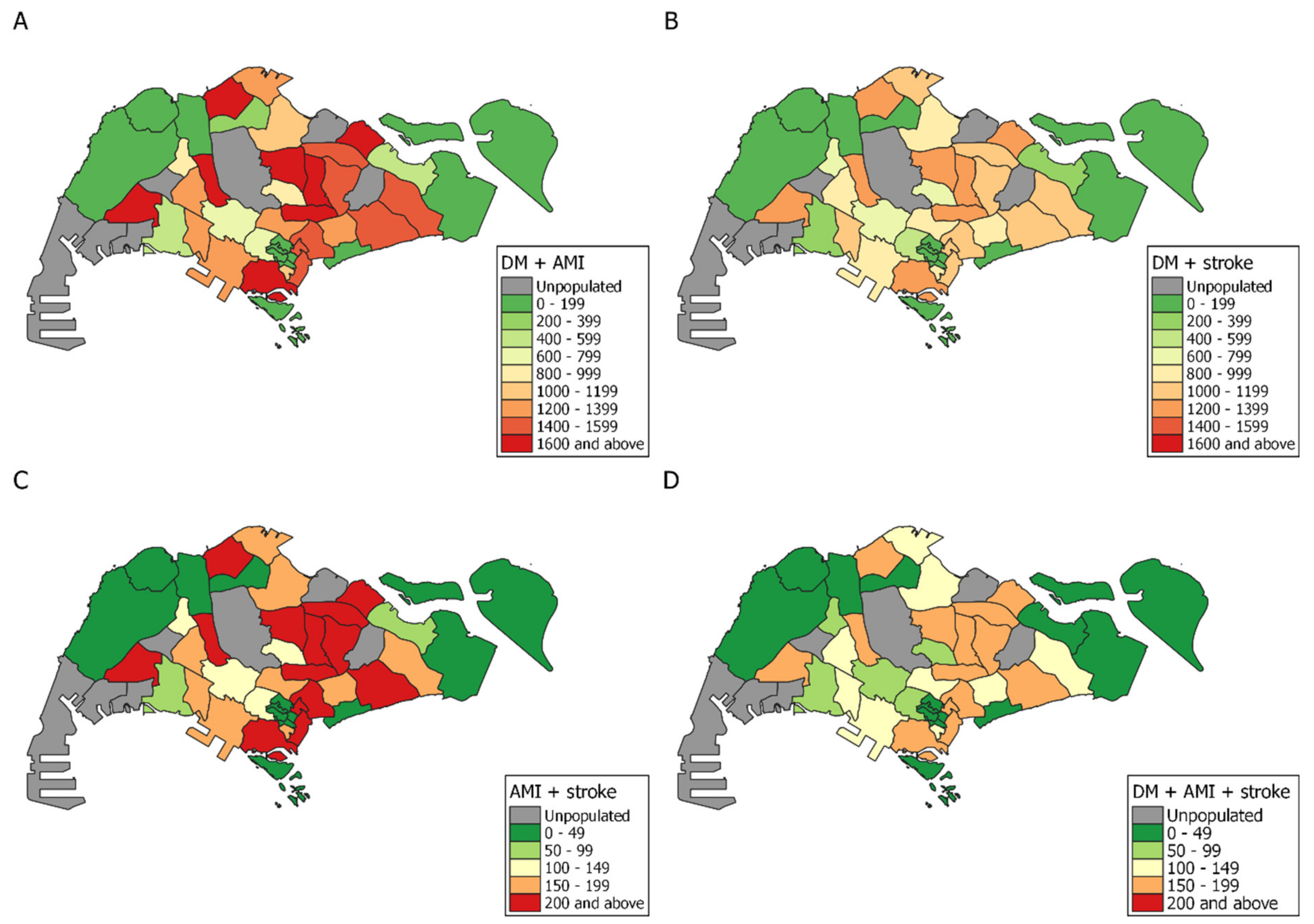
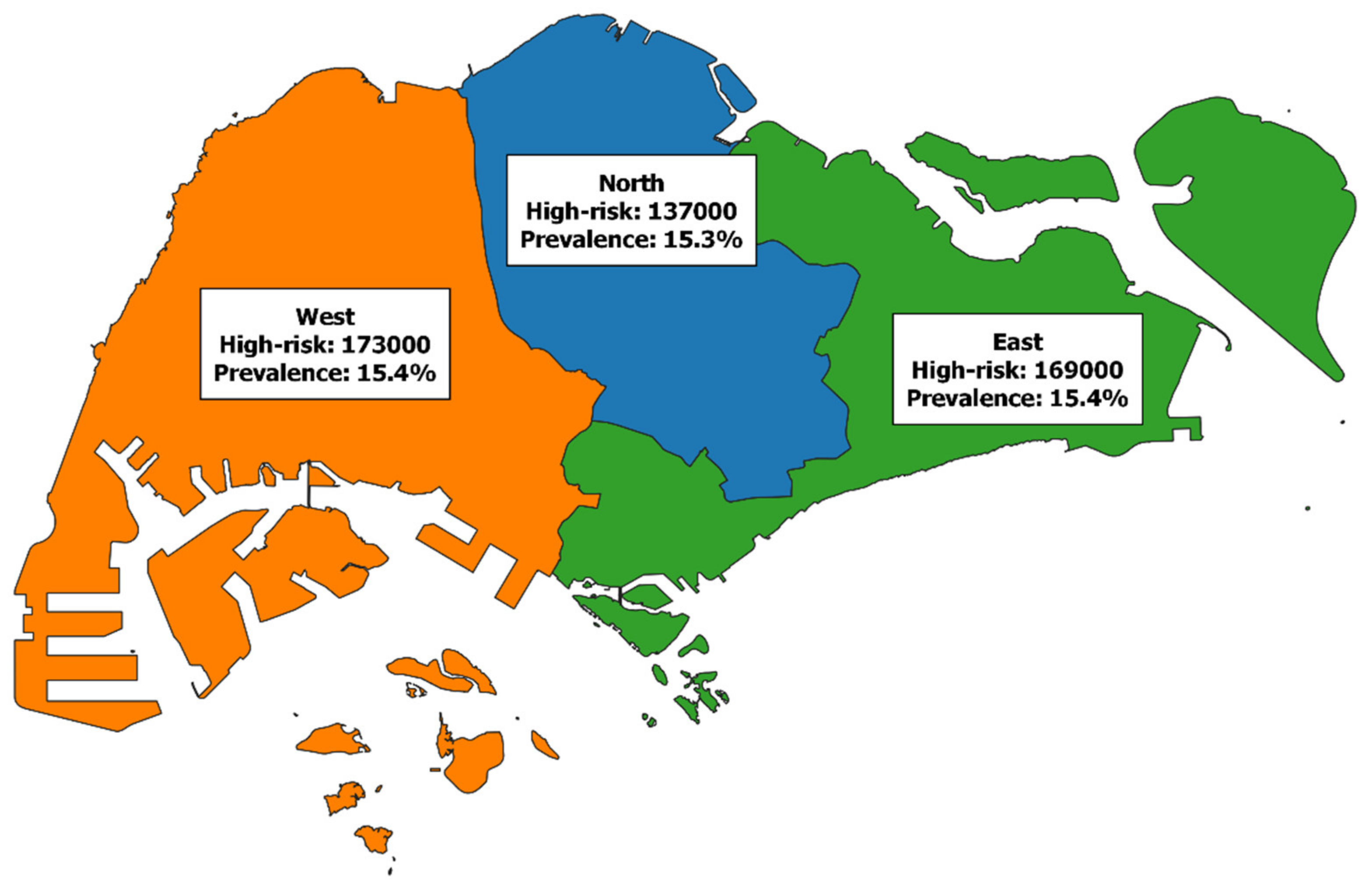
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Planning Area | DM Cases | AMI Cases | Stroke Cases | DM Prevalence (%) | AMI Prevalence (%) | Stroke Prevalence (%) | Population |
|---|---|---|---|---|---|---|---|
| ANG MO KIO | 16,144 | 3807 | 2424 | 12.68 | 2.97 | 1.91 | 127,072 |
| BEDOK | 15,692 | 3705 | 2341 | 12.85 | 3.02 | 1.91 | 122,710 |
| BISHAN | 8365 | 1954 | 1261 | 12.79 | 3.03 | 1.91 | 65,059 |
| BOON LAY | 6 | 2 | 1 | 19.31 | 5.16 | 1.44 | 40 |
| BUKIT BATOK | 12,753 | 2962 | 1930 | 12.87 | 3 | 1.95 | 100,088 |
| BUKIT MERAH | 18,123 | 4274 | 2709 | 12.79 | 3 | 1.92 | 141,752 |
| BUKIT PANJANG | 18,412 | 4372 | 2744 | 12.64 | 2.98 | 1.88 | 144,840 |
| BUKIT TIMAH | 8029 | 1871 | 1208 | 12.96 | 3.03 | 1.94 | 62,103 |
| CENTRAL WATER CATCHMENT | 3 | 1 | 0 | 15.78 | 5.09 | 1.99 | 21 |
| CHANGI | 263 | 62 | 37 | 12.49 | 2.98 | 1.76 | 2083 |
| CHOA CHU KANG | 8890 | 2067 | 1338 | 12.81 | 2.97 | 1.92 | 70,095 |
| CLEMENTI | 13,738 | 3256 | 2052 | 12.82 | 3 | 1.9 | 106,644 |
| DOWNTOWN CORE | 15,890 | 3717 | 2384 | 12.63 | 2.94 | 1.9 | 125,886 |
| GEYLANG | 12,921 | 3020 | 1942 | 12.88 | 3 | 1.94 | 100,403 |
| HOUGANG | 15,640 | 3670 | 2361 | 12.72 | 2.96 | 1.92 | 122,791 |
| JURONG EAST | 4911 | 1147 | 742 | 12.91 | 3.04 | 1.93 | 38,102 |
| JURONG WEST | 17,695 | 4163 | 2650 | 12.54 | 2.92 | 1.89 | 140,248 |
| KALLANG | 15,593 | 3650 | 2338 | 12.76 | 2.98 | 1.92 | 122,948 |
| LIM CHU KANG | 41 | 10 | 6 | 14.21 | 3.99 | 1.85 | 323 |
| MANDAI | 2386 | 561 | 355 | 12.89 | 3.08 | 1.86 | 18,524 |
| MARINE PARADE | 1067 | 253 | 156 | 12.82 | 3.05 | 1.86 | 8218 |
| MUSEUM | 540 | 130 | 75 | 12.38 | 2.92 | 1.72 | 4300 |
| NEWTON | 1489 | 352 | 220 | 13.08 | 3.13 | 1.88 | 11,378 |
| NORTH-EASTERN ISLANDS | 19 | 5 | 2 | 16.79 | 4.63 | 2.05 | 138 |
| NOVENA | 13,007 | 3030 | 1954 | 12.51 | 2.9 | 1.87 | 103,267 |
| ORCHARD | 173 | 42 | 24 | 12.66 | 3.05 | 1.71 | 1389 |
| OUTRAM | 12,003 | 2812 | 1797 | 12.87 | 3.01 | 1.93 | 93,343 |
| PASIR RIS | 4341 | 1008 | 654 | 12.85 | 3.04 | 1.88 | 33,857 |
| PAYA LEBAR | 9 | 2 | 1 | 14.47 | 4.22 | 2 | 62 |
| PIONEER | 9 | 3 | 1 | 16.01 | 4.72 | 2.44 | 62 |
| PUNGGOL | 16,953 | 3993 | 2546 | 12.69 | 2.96 | 1.91 | 132,833 |
| QUEENSTOWN | 13,008 | 3027 | 1965 | 12.64 | 2.93 | 1.9 | 102,433 |
| RIVER VALLEY | 232 | 56 | 33 | 12.58 | 3.16 | 1.74 | 1858 |
| ROCHOR | 15,692 | 3690 | 2364 | 12.74 | 2.98 | 1.92 | 123,289 |
| SELETAR | 10 | 2 | 1 | 14.79 | 4.29 | 2.12 | 66 |
| SEMBAWANG | 13,519 | 3170 | 2030 | 12.63 | 2.95 | 1.9 | 107,035 |
| SENGKANG | 15,015 | 3550 | 2256 | 12.89 | 3.03 | 1.94 | 116,560 |
| SERANGOON | 16,727 | 3947 | 2488 | 12.68 | 2.97 | 1.89 | 131,887 |
| SINGAPORE RIVER | 394 | 96 | 57 | 12.2 | 2.86 | 1.74 | 3172 |
| SOUTHERN ISLANDS | 103 | 25 | 14 | 12.76 | 3.11 | 1.68 | 832 |
| SUNGEI KADUT | 87 | 21 | 12 | 13.87 | 3.27 | 1.91 | 692 |
| TAMPINES | 14,065 | 3318 | 2108 | 12.76 | 3 | 1.92 | 110,510 |
| TANGLIN | 7460 | 1732 | 1126 | 12.83 | 2.98 | 1.93 | 58,295 |
| TENGAH | 11 | 2 | 1 | 16.2 | 3.71 | 2.1 | 67 |
| TOA PAYOH | 17,297 | 4071 | 2577 | 12.41 | 2.9 | 1.85 | 138,303 |
| TUAS | 9 | 2 | 1 | 15.45 | 4.13 | 1.93 | 60 |
| WESTERN WATER CATCHMENT | 103 | 25 | 14 | 12.89 | 3.12 | 1.76 | 809 |
| WOODLANDS | 16,492 | 3906 | 2472 | 12.7 | 2.98 | 1.91 | 129,993 |
References
- Cheah, J. Chronic disease management: A Singapore perspective. BMJ 2001, 323, 990–993. [Google Scholar] [CrossRef] [Green Version]
- Chapel, J.M.; Ritchey, M.D.; Zhang, D.; Wang, G. Prevalence and Medical Costs of Chronic Diseases Among Adult Medicaid Beneficiaries. Am. J. Prev. Med. 2017, 53, S143–S154. [Google Scholar] [CrossRef] [Green Version]
- Cost of Managing Complications Resulting from Type 2 diabetes Mellitus in Canada. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/pmc/articles/PMC153533/ (accessed on 19 September 2019).
- Shibayama, T.; Noguchi, H.; Takahashi, H.; Tamiya, N. Relationship between social engagement and diabetes incidence in a middle-aged population: Results from a longitudinal nationwide survey in Japan. J. Diabetes Investig. 2018, 9, 1060–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Huang, N.; Chou, Y.-J. Trends in the Prevalence of Multiple Chronic Conditions in Taiwan from 2000 to 2010: A Population-Based Study. Prev. Chronic Dis. 2014, 11, E187. [Google Scholar] [CrossRef] [Green Version]
- Ministry of Health. Executive Summary on National Population Health Survey; Ministry of Health: Singapore, 2016. [Google Scholar]
- Health Promotion Board. Singapore Myocardial Infarction Registry Annual Report; Health Promotion Board: Singapore, 2016. [Google Scholar]
- Health Promotion Board. Singapore Stroke Registry Annual Report; Health Promotion Board: Singapore, 2016. [Google Scholar]
- Bagley, S.C.; Altman, R.B. Computing disease incidence, prevalence and comorbidity from electronic medical records. J. Biomed. Inform. 2016, 63, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Setia, M.S. Methodology series module 3: Cross-sectional studies. Indian J. Dermatol. 2016, 61, 261–264. [Google Scholar] [CrossRef]
- Goldner, E.M.; Jones, W.; Waraich, P. Using Administrative Data to Analyze the Prevalence and Distribution of Schizophrenic Disorders. Psychiatr. Serv. 2003, 54, 1017–1021. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, R.; Ringold, S.; Khanna, D.; Neogi, T.; Johnson, S.R.; Miller, A.; Brunner, H.I.; Ogawa, R.; Felson, D.; Ogdie, A.; et al. Distinctions Between Diagnostic and Classification Criteria? Arthritis Rheum. 2015, 67, 891–897. [Google Scholar] [CrossRef]
- Bradley, C.J.; Penberthy, L.; Devers, K.J.; Holden, D.J. Health Services Research and Data Linkages: Issues, Methods, and Directions for the Future. Heal. Serv. Res. 2010, 45, 1468–1488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harron, K.L.; Doidge, J.C.; Knight, H.; Gilbert, R.; Goldstein, H.; Cromwell, D.; Van Der Meulen, J.H. A guide to evaluating linkage quality for the analysis of linked data. Int. J. Epidemiology 2017, 46, 1699–1710. [Google Scholar] [CrossRef] [Green Version]
- Safdar, N.; Abbo, L.M.; Knobloch, M.J.; Seo, S.K. Research Methods in Healthcare Epidemiology: Survey and Qualitative Research. Infect. Control. Hosp. Epidemiology 2016, 37, 1272–1277. [Google Scholar] [CrossRef] [Green Version]
- Marathe, M.V.; Ramakrishnan, N. Recent Advances in Computational Epidemiology. IEEE Intell. Syst. 2013, 28, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Barhak, J.; Isaman, D.J.; Ye, W.; Lee, D. Chronic disease modeling and simulation software. J. Biomed. Inform. 2010, 43, 791–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, M.; Alexander, C.M.; Mavros, P.; Lopez, V.A.; Malik, S.; Phatak, H.M.; Wong, N.D. Use of the UKPDS Outcomes Model to predict all-cause mortality in U.S. adults with type 2 diabetes mellitus: Comparison of predicted versus observed mortality. Diabetes Res. Clin. Pr. 2011, 91, 121–126. [Google Scholar] [CrossRef]
- Hennessy, D.A.; Flanagan, W.M.; Tanuseputro, P.; Bennett, C.J.; Tuna, M.; Kopec, J.; Wolfson, M.C.; Manuel, D.G. The Population Health Model (POHEM): An overview of rationale, methods and applications. Popul. Heal. Metrics 2015, 13, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.C.; Yi, S.S.; Athens, J.K.; Vinson, A.J.; Wall, S.P.; Ravenell, J.E. Using Geospatial Analysis and Emergency Claims Data to Improve Minority Health Surveillance. J. Racial Ethn. Heal. Disparities 2017, 5, 712–720. [Google Scholar] [CrossRef]
- Phan, T.P.; Alkema, L.; Tai, E.S.; Tan, K.H.X.; Yang, Q.; Lim, W.-Y.; Teo, Y.Y.; Cheng, C.-Y.; Wang, X.; Wong, T.Y.; et al. Forecasting the burden of type 2 diabetes in Singapore using a demographic epidemiological model of Singapore. BMJ Open Diabetes Res. Care 2014, 2, e000012. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.W.; Dickens, B.S.L.; Cook, A.R. Projected burden of type 2 diabetes mellitus-related complications in Singapore until 2050: A Bayesian evidence synthesis. BMJ Open Diabetes Res. Care 2020, 8, e000928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Department of Statistics. General Household Survey 2015; Department of Statistics: Singapore, 2016; ISBN 978-981-09-8924-8. [Google Scholar]
- National Academies of Sciences. Protecting Privacy and Confidentiality While Providing Access to Data for Research Use. Innovations in Federal Statistics: Combining Data Sources While Protecting Privacy; National Academies Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Gale, E.A.M.; Gillespie, K.M. Diabetes and gender. Diabetologia 2001, 44, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Health. National Health Survey; Ministry of Health: Singapore, 2010. [Google Scholar]
- Ministry of Health. Singapore Stroke Registry Annual Report; Ministry of Health: Singapore, 2017. [Google Scholar]
- Ministry of Health. Singapore Myocardial Infarction Registry Annual Report; Ministry of Health: Singapore, 2017. [Google Scholar]
- Venketasubramanian, N.; Chen, C.L.H. Burden of Stroke in Singapore. Int. J. Stroke 2008, 3, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Smolina, K.; Wright, F.L.; Rayner, M.; Goldacre, M.J. Long-Term Survival and Recurrence After Acute Myocardial Infarction in England, 2004 to 2010. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 532–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobkin, B.H.; Dorsch, A. New Evidence for Therapies in Stroke Rehabilitation. Curr. Atheroscler. Rep. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Leon, B.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.H.X.; Tan, L.W.L.; Sim, X.; Tai, E.S.; Lee, J.J.-M.; Chia, K.S.; Van Dam, R.M. Cohort Profile: The Singapore Multi-Ethnic Cohort (MEC) study. Int. J. Epidemiology 2018, 47, 699–699j. [Google Scholar] [CrossRef] [Green Version]
- Teh, W.L.; Abdin, E.; Vaingankar, J.A.; Seow, E.; Sagayadevan, V.; Shafie, S.; Shahwan, S.; Zhang, Y.; Chong, S.A.; Ng, L.L.; et al. Prevalence of stroke, risk factors, disability and care needs in older adults in Singapore: Results from the WiSE study. BMJ Open 2018, 8, e020285. [Google Scholar] [CrossRef] [Green Version]
- Yeo, K.K.; Zheng, H.; Chow, K.Y.; Ahmad, A.; Chan, B.P.; Chang, H.M.; Chong, E.; Chua, T.S.J.; Foo, D.C.G.; Low, L.P.; et al. Comparative analysis of recurrent events after presentation with an index myocardial infarction or ischaemic stroke. Eur. Hear. J. Qual. Care Clin. Outcomes 2017, 3, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Morley, C.; Unwin, M.; Peterson, G.M.; Stankovich, J.; Kinsman, L. Emergency department crowding: A systematic review of causes, consequences and solutions. PLoS ONE 2018, 13, e0203316. [Google Scholar] [CrossRef] [PubMed]
- Dummer, T.J. Health geography: Supporting public health policy and planning. Can. Med Assoc. J. 2008, 178, 1177–1180. [Google Scholar] [CrossRef] [Green Version]
- Brownson, R.C.; Chriqui, J.F.; Stamatakis, K.A. Understanding Evidence-Based Public Health Policy. Am. J. Public Heal. 2009, 99, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Musa, G.J.; Chiang, P.-H.; Sylk, T.; Bavley, R.; Keating, W.; Lakew, B.; Tsou, H.-C.; Hoven, C.W. Use of GIS Mapping as a Public Health Tool—From Cholera to Cancer. Heal. Serv. Insights 2013, 6, HSI.S10471–6. [Google Scholar] [CrossRef]
- National Healthcare Group. The Straits Times Proportion of Older Adults with Multiple Chronic Diseases Surges; National Healthcare Group: Singapore, 2019. [Google Scholar]
- Barton, H.; Grant, M. Urban Planning for Healthy Cities. J. Hered. 2012, 90, 129–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulman, D.M.; Vijan, S.; Omenn, G.S.; Hayward, R.A. The Relative Merits of Population-Based and Targeted Prevention Strategies. Milbank Q. 2008, 86, 557–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northridge, M.E.; Sclar, E. A Joint Urban Planning and Public Health Framework: Contributions to Health Impact Assessment. Am. J. Public Heal. 2003, 93, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Flacke, J.; Schüle, S.A.; Köckler, H.; Bolte, G. Mapping Environmental Inequalities Relevant for Health for Informing Urban Planning Interventions—A Case Study in the City of Dortmund, Germany. Int. J. Environ. Res. Public Heal. 2016, 13, 711. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Feng, Z.; Xue, D.; Liu, Y.; Wu, R. Exploring the links between population density, lifestyle, and being overweight: Secondary data analyses of middle-aged and older Chinese adults. Heal. Qual. Life Outcomes 2019, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Brook, J.R.; CANUE—The Canadian Urban Environmental Health Research Consortium; Setton, E.M.; Seed, E.; Shooshtari, M.; Doiron, D. The Canadian Urban Environmental Health Research Consortium—A protocol for building a national environmental exposure data platform for integrated analyses of urban form and health. BMC Public Heal. 2018, 18, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moutselos, K.; Maglogiannis, I. Evidence-based Public Health Policy Models Development and Evaluation using Big Data Analytics and Web Technologies. Med Arch. 2020, 74, 47–53. [Google Scholar] [CrossRef]
- Schuurman, N.; Fiedler, R.S.; Grzybowski, S.C.W.; Grund, D. Defining rational hospital catchments for non-urban areas based on travel-time. Int. J. Heal. Geogr. 2006, 5, 43. [Google Scholar] [CrossRef] [Green Version]

| Demographic Group | DM Cases (thousands) | AMI Cases (thousands) | Stroke Cases (thousands) | DM Prevalence | Past AMI Prevalence | Stroke Prevalence |
|---|---|---|---|---|---|---|
| Chinese males | 152.3 (151.7, 153.0) | 41.2 (40.8, 41.5) | 28.0 (27.7, 28.4) | 13.2 (13.2, 13.3) | 3.6 (3.5, 3.6) | 2.4 (2.4, 2.5) |
| Chinese females | 87.6 (87.0, 88.1) | 6.2 (6.1, 6.3) | 12.9 (12.6, 13.1) | 7.8 (7.7, 7.8) | 0.6 (0.5, 0.6) | 1.1 (1.1, 1.2) |
| Malay males | 38.8 (38.5, 39.1) | 18.1 (17.8, 18.3) | 7.2 (7.0, 7.3) | 20.0 (19.9, 20.2) | 9.3 (9.2, 9.5) | 3.7 (3.6, 3.8) |
| Malay females | 32.6 (32.3, 33.0) | 3.9 (3.8, 4.0) | 4.1 (4.0, 4.3) | 17.1 (16.9, 17.2) | 2.0 (2.0, 2.1) | 2.2 (2.1, 2.2) |
| Indian males | 43.6 (43.2, 43.9) | 15.5 (15.2, 15.7) | 3.9 (3.8, 4.1) | 23.2 (23.1, 23.4) | 8.3 (8.1, 8.4) | 2.1 (2.1, 2.2) |
| Indian females | 29.9 (29.6, 30.2) | 2.9 (2.8, 3.0) | 1.8 (1.8, 1.9) | 20.3 (20.2, 20.5) | 2.0 (1.9, 2.0) | 1.3 (1.2, 1.3) |
| Other males | 8.2 (8.0, 8.4) | 4.9 (4.8, 5.0) | 1.1 (1.0, 1.1) | 14.1 (13.9, 14.5) | 8.5 (8.3, 8.7) | 1.9 (1.7, 2.0) |
| Other females | 3.9 (3.7, 4.0) | 0.6 (0.6, 0.7) | 0.4 (0.4, 0.5) | 6.7 (6.5, 6.9) | 1.1 (1.0, 1.2) | 0.8 (0.7, 0.8) |
| Age Group | DM Cases (thousands) | AMI Cases (thousands) | Stroke Cases (thousands) | DM Prevalence | Past AMI Prevalence | Stroke Prevalence |
|---|---|---|---|---|---|---|
| 18–29 | 10.2 (10.0, 10.4) | 2.8 (2.7, 2.9) | 1.6 (1.5, 1.7) | 1.5 (1.5, 1.5) | 0.4 (0.4, 0.4) | 0.2 (0.2, 0.2) |
| 30–39 | 34.4 (34.1, 34.7) | 7.7 (7.6, 7.9) | 4.2 (4.0, 4.3) | 5.3 (5.3, 5.4) | 1.2 (1.2, 1.2) | 0.7 (0.6, 0.7) |
| 40–49 | 73.0 (72.6, 73.4) | 13.7 (13.5, 13.9) | 7.6 (7.4, 7.8) | 11.3 (11.3, 11.4) | 2.1 (2.1, 2.2) | 1.2 (1.2, 1.2) |
| 50–59 | 124.5 (124.0, 125.0) | 27.3 (26.9, 27.6) | 16.7 (16.5, 16.9) | 19.9 (19.8, 20.0) | 4.4 (4.3, 4.4) | 2.7 (2.6, 2.7) |
| 60–69 | 154.8 (154.2, 155.4) | 41.7 (41.4, 42.0) | 29.5 (29.1, 29.8) | 29.8 (29.6, 29.9) | 8.0 (8.0, 8.1) | 5.7 (5.6, 5.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, K.W.; Koo, J.R.; Lim, J.T.; Cook, A.R.; Dickens, B.L. Importance of Geospatial Heterogeneity in Chronic Disease Burden for Policy Planning in an Urban Setting Using a Case Study of Singapore. Int. J. Environ. Res. Public Health 2021, 18, 4406. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18094406
Tan KW, Koo JR, Lim JT, Cook AR, Dickens BL. Importance of Geospatial Heterogeneity in Chronic Disease Burden for Policy Planning in an Urban Setting Using a Case Study of Singapore. International Journal of Environmental Research and Public Health. 2021; 18(9):4406. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18094406
Chicago/Turabian StyleTan, Ken Wei, Joel R. Koo, Jue Tao Lim, Alex R. Cook, and Borame L. Dickens. 2021. "Importance of Geospatial Heterogeneity in Chronic Disease Burden for Policy Planning in an Urban Setting Using a Case Study of Singapore" International Journal of Environmental Research and Public Health 18, no. 9: 4406. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph18094406






