Introduction
Arsenic, a toxicant of natural occurrence in mineral deposits, is used in many human activities such as manufacturing, agriculture, and medicine [
1]. Arsenical compounds are transported into the environment mainly by water from wells drilled into the arsenic-rich geologic strata or by ambient air during smelting and burning of coal [
1,
2]. The main route of arsenic exposure for the general population is via drinking water. After absorption, inorganic arsenic is accumulated in the liver, spleen, kidneys, lungs and gastrointestinal tract. During metabolism, most of the inorganic arsenic such as As(III) and As(V) are metabolized to dimethylarsinic acid and monomethylarsonic acid and then rapidly cleared from the tissues through urine [
3]. However, this biomethylation process can easily become saturated and lead to the excess inorganic arsenic being deposited in the skin, hair and nails, where it binds tightly to keratin [
4]. The lethal dose of inorganic arsenic for human is estimated to be between 10-20 mg/Kg [
3]. In attempting to eliminate water-borne diseases caused by drinking contaminated surface water, millions of shallow wells were drilled in Bangladesh during the last 30 years that brought geological arsenic to the surface. This caused an estimated 40 million people in this country suffering from arsenic poisoning-related diseases [
5].
It has been found that the trivalent form of arsenic is able to bind to sulfhydryl groups of enzymes in the pyruvate dehydrogenase system and glyceraldehyde-3-phosphate dehydrogenase. Arsenic is able to bind to enzymes specially bound with lipoic acid in the tricarboxylic acid cycle and therefore can interfere with oxidative phosphorylation in cells. In the pentavalent form, arsenic can also exert toxicity by competitively substituting its ions for the body's phosphate ions. This can lead to breaking down by hydrolysis of high energy bonds in compounds such as ATP resulting in a marked depletion of cellular ATP and eventually death of the metabolizing cells. Arsenic is also a potent modulator of antioxidant defense system by decreasing glutathione-S-transferase activity in the liver and by increasing the activity of glutathione peroxidase both in the liver and kidney [
6]. Arsenic has inhibitory effect on choline esterase [
7], but no effect has been found in the activities of glutathione reductase and glucose-6-phosphate dehydrogenase [
6,
8].
It has been reported that arsenic can lead to skin lesions, atherosclerotic diseases and cancers. Associations of long-term arsenic exposure and peripheral vascular disease [
9], and ischemic heart disease in arseniasis-hyperendemic villages in Taiwan have been documented [
10]. Also increased mortality from ischemic heart disease has been reported among copper smelter workers exposed to arsenic [
11]. Our recent publication reports an association of clinical complications of arsenic toxicity with the nutritional status of the intoxicated subjects [
12]. Study carried out in Taiwan concluded that patients affected with black foot disease, a unique peripheral vascular disease caused by long-term arsenic exposure via drinking water, have a significantly higher mortality due to ischemic heart disease [
13]. A dose-response relationship between carotid atherosclerosis and long-term exposure to arsenic in drinking water has been reported in a population with black foot disease [
14]. Prevalence studies carried out in Bangladesh and Taiwan revealed that arsenic exposure might induce hypertension [
15,
16]. The prevalence of respiratory effects of drinking water arsenic has been reported in West Bengal, India [
17] and in Bangladesh [
18]. Further, epidemiological evidence of diabetogenic effect of arsenic has been found among copper smelters and glass workers in Sweden, and from arsenic exposure through drinking water in Bangladesh [
19,
20]. Since the toxic effects of arsenic have given variable results among populations in different parts of the world, this study has been undertaken to find out the relationship between drinking water arsenic toxicity and the possible biochemical changes in Bangladeshi patients.
Materials and Methods
Study Area
The study area of this investigation was the northwestern district of Chapainowabganj where arsenic contamination in drinking water was first detected in Bangladesh in 1993. Samples were collected during November 2001 to August 2003 from patients living in the arsenic endemic rural villages of Rajarampur, Achinpara, Chandnai, and Bottola.
Arsenicosis Patient Inclusion Criteria
Subjects consuming drinking water that contained arsenic levels greater than the tolerable limit recommended by WHO (50 µg/L), and having signs of arsenic toxicity were enrolled in this study as the patients. Their source of drinking water was tubewell or artisan well. The prevalence of arsenicosis was based on appearance of skin lesions. After informed consent, the researchers with the help of a public health nurse interviewed all the patients personally. The information on symptoms and complications of arsenic toxicity, duration of exposure, anthropometric parameters including age, height, and body weight of the patients were recorded on prepared questionnaires.
Sample Collection
Blood samples (about 3 mL) were collected from the subjects with their full consent to participate in this study. A total of 235 blood samples were collected of which 115 were from patients with arsenic toxicity (arsenicosis), 80 from unexposed subjects living in the same area as the patients but drinking safe water and with no sign of arsenic toxicity, and the remaining from unexposed subjects living in the city. Spot urine and drinking water samples were also collected from each individual. All samples were collected in fresh, sterile universals. The blood sample was allowed to clot and serum was separated immediately after collection. The frozen sera were transported to the laboratory in ice-packed containers and stored at –80°C until analyzed.
Preservation of Water and Urine Samples and Analyses of Arsenic
Concentrated nitric acid was mixed with the drinking water samples at 1 mL per liter and stored at 4°C until analyzed. The urine samples were frozen immediately after collection. Arsenic content in the water and urine samples were measured following the standard procedure by using a Flow Injection - Hydride Generation - Atomic Absorption Spectrophotometer and the results were expressed in µg/L.
Reagents
All reagents used for the analyses of biochemical parameters in this study were purchased from Randox Laboratories Ltd., Diamond Road, Crumin, UK.
Determination of Serum Glucose
Glucose was determined after enzymatic oxidation by glucose oxidase. A standard graph was prepared and glucose concentrations present in the serum samples were determined from the graph and the results were expressed in mg/dL.
Determination of Serum Creatinine
To determine creatinine, 100 µL of serum sample was added with 1000 µL of the working reagent and then mixed. Readings were taken at standard time intervals and creatinine concentrations in the samples were determined and expressed in mg/dL.
Determination of Total Proteins in Serum
Serum sample (20 µL) was added to 1000 µL of the working biuret reagent, mixed and then incubated at 37°C for 30 minutes. The absorbance was taken at 546nm against the reagent blank. The total protein concentration of the serum samples was determined and the results were expressed in g/dL.
Determination of Alkaline Phosphatase Activity
For the determination of alkaline phosphatase (ALP) activity, 20 µL of the serum samples were added to 1000 µL of the working reagent, mixed together and waited for 10 seconds. The absorbance of the solutions was taken against air at 405 nm at 0, 1, 2, and 3 minutes. ALP activity was expressed in U/L.
Measurement of Alanine Transaminase Activity
For the determination of alanine transaminase (ALT) activity in serum, 100 µL of the sample was added with 1000 µL of the working reagent and mixed. The initial absorbance was taken after 1 minute at 365 nm and then after 2 and 3 minutes interval. ALT activity in serum was expressed in U/L.
Measurement of Aspartate Transaminase Activity
Aspartate transaminase (AST) activity in serum was determined by adding 100 µL of the sample with 1000 µL of the working reagent. The initial absorbance was taken after 1 minute at 365 nm and then at 2 and 3 minutes interval. AST activity in serum was expressed in U/L.
Determination of Triacylglycerol Levels in Serum
The levels of triacylglycerol (TAG) in serum were determined after enzymatic hydrolysis with lipases to glycerol and free fatty acids. For the determination, 10 µL of the serum sample was mixed with 1000 µL of the working reagent, incubated at 37°C for 5 minutes and then the absorbance was taken at 546 nm and the values were calculated and expressed in mg/dL.
Determination of Cholesterol Levels in Serum Samples
To determine the level of cholesterol in serum, 10 µL of the sample was mixed with the supplied buffered enzyme reagent, shaken carefully and incubated at 37°C for 5 minutes. The O.D. was taken at 546 nm and the values were calculated and expressed in mg/dL.
Determination of HDL-Cholesterol in Serum
To determine the high-density lipoprotein (HDL)-cholesterol, 200 µL of the supplied precipitation reagent was added to 100 µL of the serum sample, left undisturbed for 10 minutes and then centrifuged. The clear supernatant (100 µL) was taken, mixed with 1000 µL of the buffered enzyme reagent and incubated at 37°C for 5 minutes. The absorbance was taken at 546 nm and the values were calculated and expressed in mg/dL.
Determination of Inorganic Phosphorus
Inorganic phosphate in serum was reacted with molybdic acid to form a phosphomolybdic acid complex that was reduced by ferrous ammonium sulfate to molybdenum blue, which was measured at 690 nm. The levels of inorganic phosphorus in serum were calculated and expressed in mg/dL.
Statistical Analysis of the Data
Data analyses were carried out using the Statistical Package for Social Sciences (version 10.0 for Windows, SPSS Inc., Chicago, USA). The methods used were independent t– test for comparison of two groups (control and patients), correlations and simple statistics. The results were considered significant when p was ≤ 0.05. Mean ± S.D. values were calculated for each parameter.
Results
Anthropometrical Data of the Patients
Of the total 115 patients enrolled in this study, there were 36 males and 79 females. The whole population of patients had the mean age of 35.4 ± 13.7 yrs. The mean age of the male patients was 35.5 ± 16.1 yrs that varied from 14 to 85 yrs, and that of the female patients was 35.3 ± 12.7 yrs ranging from 14 to 75. The male patients had the mean height of 1.67 meters, body weight of 56.4 ± 8.1 Kg and body mass index (BMI) of 20.4 ± 3.1. On the other hand, the female patients had the mean height of 1.49 meters, body weight of 49.2 ± 8.1 Kg and BMI of 21.4 ± 3.3.
Anthropometrical Data of the Controls
Of the total 120 unexposed control subjects, there were 81 males and 39 females. The mean age of the control subjects was 30.9 ± 12.1 yrs that varied from 14 to 72. The control males had the mean height of 1.70 meters, body weight of 63.2 ± 8.0 Kg and BMI of 22.0 ± 2.3. The control females had the mean height of 1.60 meters, body weight of 54.1 ± 7.8 Kg and BMI of 21.2 ± 2.5.
Clinical Symptoms and Duration of Arsenic Exposure
The clinical symptoms based on skin manifestations, complications and duration of arsenic exposure were recorded in the questionnaire. The clinical symptoms included diffused and spotted melanoses with black and white appearances, rough and mottled skin, keratosis or hardening of the skin with the formation of nodules. Spotted melanosis was more often seen on the throat, chest, back, or limbs. Many of the patients suffered from severe skin irritations. Prolonged exposure caused the skin to become rough and thickened due to diffused keratosis that usually developed on the soles of feet and palms of both hands with occasional formation of cracks on these areas. Non-specific symptoms such as gastric and abdominal pain, respiratory problems, weakness, headache, palpitation, itchy rash and anaemia were found to be more prevalent in the arsenic-exposed subjects. The whole population of patients had the mean duration of exposure for 7.6 ± 5.2 yrs that varied from 1 – 25 yrs with a median of 6.5 yrs.
Levels of Arsenic in the Drinking Water and Urine Samples
It was found that a total of 13 patients drank water that contained more than 600 µg/L of arsenic. There were 21 patients included in this study who switched to safe drinking water (from 2 - 3 months before sample collection) following the onset of arsenical skin lesions. The mean level of arsenic in the drinking water of the patients was 218.1 ± 218.4 µg/L, with a median value of 156 µg/L and the levels varied from 3.0 - 875.0 µg/L. On the other hand, the mean arsenic concentrations in the drinking water and urine samples of the unexposed (control) populations were 11.3 and 29.4 µg/L, respectively. The mean level of arsenic in the urine of the patients was 234.6 ± 311.5 µg/L while the median value was 129.7 µg/L and the levels varied from 20.0 – 1764.0 µg/L. Statistical analysis showed the levels of arsenic in the drinking water and urine samples of the patients was positively correlated at 1% level.
Levels of Serum Glucose
The levels of random blood glucose in the serum of 10% of the patients were above the normal range (˃140 mg/dL). In comparison, only 3.6% of the controls had serum glucose level higher than this value. This observation showed that the incidence of diabetes mellitus was 2.8 times higher (10% vs. 3.6%) in the arsenic patients than the control subjects. The mean level of random glucose in serum of the whole population of patients was 95.2 ± 32.8 and that of the control subjects was 99.2 ± 20.8 mg/dL.
Levels of Serum Creatinine
Of the total patients studied, 67% had serum creatinine level that was within the normal range of 0.8 - 1.4 mg/dL; 5% had elevated and 28% had lower values. In the control subjects, 67% had normal creatinine level in serum; whereas 22% had elevated and 11% had lower levels. The mean serum creatinine level in the whole population of patients was 0.97 ± 0.47 mg/dL and that in the control subjects was 1.15 ± 0.38 mg/dL. Statistical analysis revealed that serum creatinine level in the arsenic-affected patients was significantly lower than the control subjects (p = 0.007).
Study of Organ-Specific Enzymes in Arsenicosis Patients
To evaluate the status of liver and cardiac muscle functions in human arsenic toxicity, activities of ALT and AST, and that for kidney functions ALP were assayed in the serum of the patients.
Activity of Serum ALT
The activities of the liver-specific enzyme ALT in the serum of only 1% of the patients were elevated while the remaining were found within the normal range of 0 - 40 U/L. On the other hand, about 3% of the control subjects had an elevated activity of ALT in serum while the rest showed normal activity. The mean ALT activity in the patients was 14.5 ± 9.3 U/L and that in the controls was 16.8 ± 13.5 U/L. The results are shown in
Table 1.
Table 1.
Activities of ALP, ALT and AST in the controls and arsenicosis patients
Table 1.
Activities of ALP, ALT and AST in the controls and arsenicosis patients
| Study Subjects | Enzyme Activity in Serum (U/L): Mean ± SD (Range) |
| Alkaline Phosphatase (ALP) | Alanine Transaminase (ALT) | Aspartate Transaminase (AST) |
| Patients | 196.6 ± 79.5 | 14.5 ± 9.3 | 18.0 ±9.0 |
| n = 112 | (82.8 – 521.6) | (3.2 – 71.2) | (0 – 59.8) |
| Controls | 149.1 ± 63.3 | 16.8 ± 13.5 | 19.8 ± 8.1 |
| n = 90 | (41.4 – 421.4) | (0 – 98.0) | (0.9 – 56.6) |
Statistics
(Controls vs.
Patients) | P < 0.0001 | * | * |
Activity of Serum AST
The activities of the liver- and cardiac muscle-specific enzyme AST in the serum of only about 2% of the patients and controls were elevated (normal range: 0 - 37 U/L). The rest of the patients and controls had normal activity. The mean AST activity in serum of the whole population of patients was 18.0. ± 9.0 U/L and that in the control subjects was 19.8 ± 8.1 U/L. The results are shown in
Table 1.
Activity of Serum ALP
The activities of the kidney-, liver-, and bone cells-specific enzyme alkaline phosphatase in serum showed 67% of the patients had normal activity (range: 73 - 207 U/L), and 33% had higher activity. Compared to the patient group, 83% of the control subjects showed normal activity; 13% had higher activity, and about 4% had serum ALP activity that was below the normal level. The mean ALP activity in the whole population of patients was 196.6 ± 79.5 U/L, and that in the control subjects was 149.1 ± 63.3 U/L. Statistical analysis revealed that serum ALP activity in the arsenicosis patients was significantly higher than the control subjects (p < 0.0001,
Table 1).
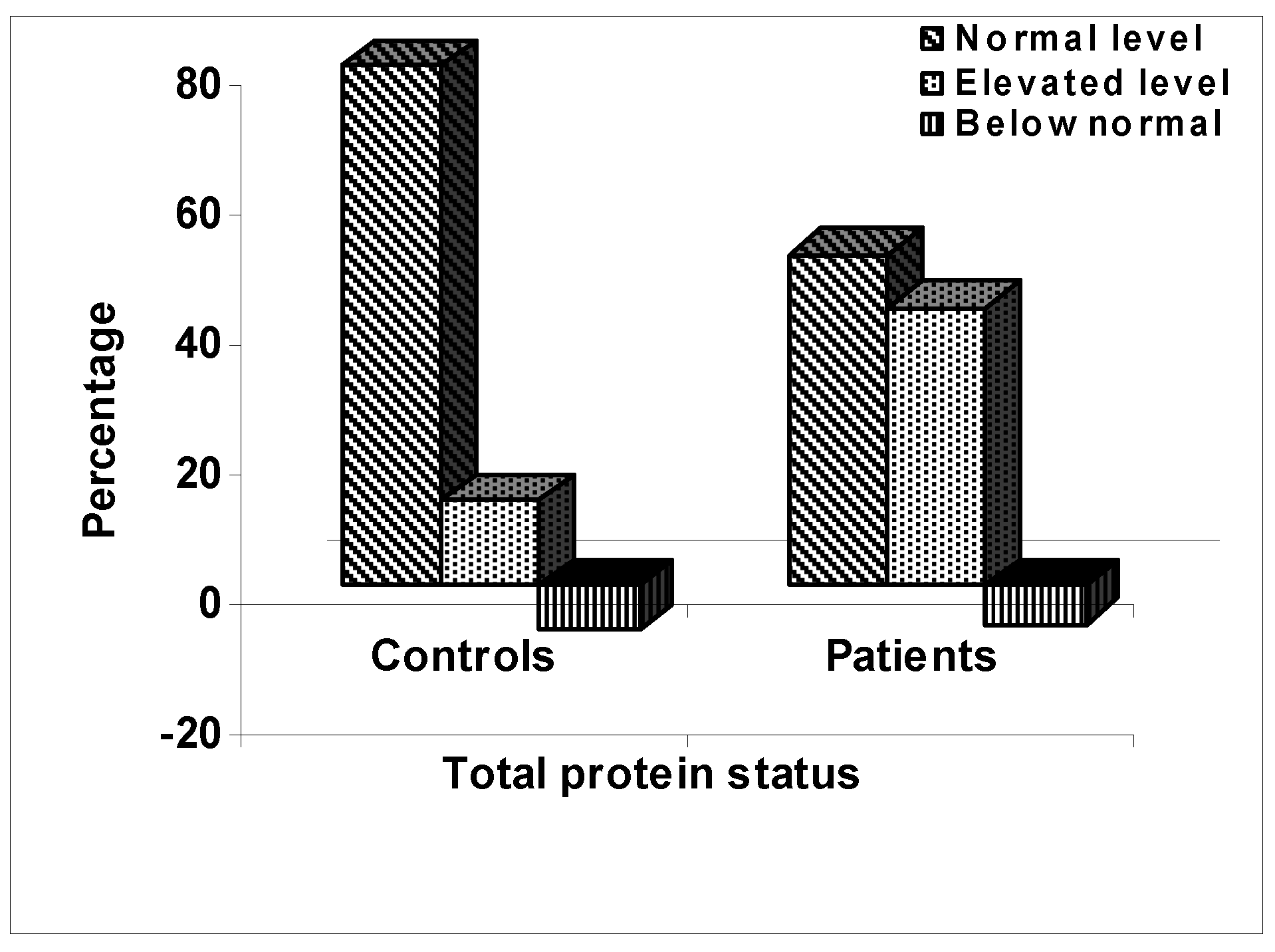
Levels of Total Protein
It was found that about 51% of the patients had total protein level in serum that was within the normal range of 66 - 87 g/L; while 43% had higher and only 6% had lower levels. On the other hand, 80% of the control subjects had normal levels of protein in serum; while 13% had higher and 7% had lower values. The patients had the mean level of total protein in serum of 84.0 ± 14.6 g/L and the controls had 77.3 ± 9.0 g/L. Statistical analysis showed that the levels of total protein in the serum of the arsenicosis patients was significantly higher than the control subjects (p < 0.0001). The results are shown in
Figure 1.
Figure 1.
Percentages of the populations of the control subjects and arsenicosis patients with normal, elevated, and below normal levels of total protein in serum.
Figure 1.
Percentages of the populations of the control subjects and arsenicosis patients with normal, elevated, and below normal levels of total protein in serum.
Study of the Lipid Profiles in Arsenicosis Patients
To evaluate whether chronic arsenic exposure in human could develop the risk of cardiovascular as well as peripheral vascular diseases, the biochemical markers related to these diseases such as lipid profiles in the serum of arsenicosis patients were analyzed and the values were compared to those of the control subjects.
Table 2.
Serum lipid profiles in the controls and arsenicosis patients
Table 2.
Serum lipid profiles in the controls and arsenicosis patients
Study
Subjects | Lipids in Serum (mg/dL): Mean ± SD
(Range) |
| Cholesterol | Tria-cylglycerol | HDL- Cholesterol | LDL- Cholesterol |
| Patients | 163.7 ± 40.2 | 173.0 ± 87.9 | 36.4 ± 15.7 | 94.4 ± 39.2 |
| n = 110 | (82.6-286.7) | (24.0-531.0) | (4.7-108.0) | (16.3-213.2) |
| Controls | 186.2 ± 54.4 | 164.3 ± 88.6 | 40.9 ± 15.1 | 113.2 ± 46.9 |
| n = 85 | (85.0-370.8) | (34.0-458.1) | (13.6 -87.9) | (28.8- 294.3) |
Statistics
(controls
vs.
patients) | P < 0.005 | * | P < 0.05 | P < 0.005 |
Levels of Serum Triacylglycerol
Of the total patients studied for this test, 52% had serum TAG level that was within the normal range of 50 - 165 mg/dL; about 47% had higher and only 1% had lower levels. In comparison, a normal TAG level was found in 56% of the total control subjects; while 40% had higher and about 4% had lower values. The mean level of TAG in the patients was 173.0 ± 87.9 mg/dL while that in the control subjects was 164.3 ± 88.6 mg/dL. The results are shown in
Table 2.
Levels of Serum Cholesterol
About 54% of the patients had serum cholesterol level that was within the normal range of 150 - 250 mg/dL; whereas 45% had lower and only 1% had higher values. On the other hand, normal cholesterol level was found in about 67% of the control subjects; whereas 12% had higher and 21% had lower than the normal levels. The mean level of serum cholesterol in the patients was 163.7 ± 40.2 mg/dL and that in the control subjects was 186.2 ± 54.4 mg/dL. Statistical analysis revealed that serum cholesterol level in the arsenicosis patients was significantly lower than the unexposed control subjects (p < 0.005,
Table 2).
Levels of Serum HDL-Cholesterol
Evaluation of the levels of HDL-cholesterol in the serum showed 46% of the patients had the normal values (above 35 mg/dL); whereas the remaining 54% had lower values. In the control subjects, normal HDL-cholesterol levels were found in about 63% and the remaining 37% had lower values. The patients had the mean value of serum HDL-cholesterol level of 36.4 ± 15.7 mg/dL. Similarly, the control subjects had the mean value of 40.9 ± 15.1 mg/dL. The results are shown in
Table 2.
Levels of Serum LDL-Cholesterol
Of the total patients studied, 79% had serum LDL-cholesterol level that was within the normal range of 60 - 180 mg/dL; the remaining 20% had lower and 1% had higher values. Normal LDL-cholesterol level was found in about 83% of the total control subjects; whereas 7% had higher and 10% had lower values. The mean level of serum LDL-cholesterol in the patients was 94.4 ± 39.2 mg/dL; whereas that in the control subjects was 113.2 ± 46.9 mg/dL. Statistical analysis revealed that serum LDL-cholesterol level in the arsenicosis patients was significantly lower than the control subjects (p < 0.005). The results are shown in
Table 2.
Study of the Lipid Profiles with Respect to Gender
To evaluate whether gender difference had any effect on serum lipid profiles, the values in the male and female patients as well as male and female control subjects were compared separately. It was found that gender difference had no significant effects on the levels of serum TAG but the levels of cholesterol and LDL-cholesterol were significantly higher in the female patients than the male patients. In comparison, the levels of cholesterol and LDL-cholesterol were significantly higher in the control males than the control females. Among the males, the levels of serum cholesterol, LDL-cholesterol and HDL-cholesterol were significantly higher in the controls than the patients. In the females, although the profiles of cholesterol, TAG, and LDL-cholesterol were slightly higher in the patients, they were not significantly different than the controls. The results are shown in
Table 3.
Table 3.
Comparison of serum lipid profiles in the male and female controls and arsenicosis patients
Table 3.
Comparison of serum lipid profiles in the male and female controls and arsenicosis patients
| Study Subjects | Lipids in Serum (mg/dL): Mean ± SD (Range) |
| Cholesterol | Triacylglycerol | HDL- Cholesterol | LDL- Cholesterol |
| Patients: | |
| Male | 152.1± 38.8 | 192.6 ± 109.1 | 35.1± 15.4 | 80.4 ± 37.9 |
| Female | 170.1± 40.3 | 164.5 ± 76.1 | 36.9 ± 15.9 | 100.4 ± 38.5 |
| | P < 0.05 | * | * | P < 0.02 |
| Controls: | |
| Male | 201.3 ± 58.6 | 174.5 ± 94.6 | 41.7 ± 14.2 | 125.1± 49.6 |
| Female | 162.3 ± 35.5 | 147.1± 75.9 | 39.6 ± 16.7 | 92.2 ± 33.1 |
| | P < 0.0001 | * | * | P < 0.005 |
| Males: | |
| Patients | 152.1± 38.8 | 192.6 ± 109.1 | 35.1± 15.4 | 80.4 ± 37.9 |
| Controls | 201.3 ± 58.6 | 174.5 ± 94.6 | 41.7 ± 14.2 | 125.1± 49.6 |
| | P < 0.0001 | * | P < 0.05 | P < 0.0001 |
| Females: | |
| Patients | 170.1± 40.3 | 164.5 ± 76.1 | 36.9 ± 15.9 | 100.4 ± 38.5 |
| Controls | 162.3 ± 35.5 | 147.1± 75.9 | 39.6 ± 16.7 | 92.2 ± 33.1 |
| | * | * | * | * |
Figure 2.
Levels of inorganic phosphate in the serum of the controls and arsenicosis patients. Each symbol indicates the value in one subject and the horizontal line shows the upper limit for normal values (4.97 mg/dL).
Figure 2.
Levels of inorganic phosphate in the serum of the controls and arsenicosis patients. Each symbol indicates the value in one subject and the horizontal line shows the upper limit for normal values (4.97 mg/dL).
Levels of Inorganic Phosphorus
It was found that the levels of inorganic phosphorus in the serum of arsenicosis patients were significantly higher than the controls (normal range: 2.5 – 4.97 mg/dL). While the mean level of serum phosphorus in the patients was 6.4 ± 1.6 mg/dL the corresponding level in the controls was 4.6 ± 1.4 mg/dL (p < 0.0001). The results are shown in
Figure 2.
Discussion
Endemic arsenic exposure emerged as a single catastrophe affecting millions of people living in different parts of the world. According to a report published in 2001, at least 25 million people in Bangladesh have been drinking arsenic contaminated water [
21], and the number is ever increasing. Biochemical changes caused by chronic arsenic poisoning have not been investigated in such a huge population. It has long been known that arsenic is able to exert its toxic effects on the metabolic pathways by modulating the antioxidant defense system, interrupting the glycolytic pathway and citric acid cycle, and thus inhibits oxidative phosphorylation. Arsenic can alter activities of different enzymes such as transaminases, alkaline phosphatase, gyIceralehyde-3-phosphate dehydrogenase, pyruvate decarboxylase, glutathione peroxidase etc. involved in normal functioning of different organs in human body [
22,
23,
24].
It has been found that competition with phosphorus in the oxidative phosphorylation process is caused by inorganic compounds [
25]; mainly in the pentavalent form [
24]. Arsenic can compete with phosphorus in the oxidative phosphorylation process by replacing phosphorus in the cells thereby causing an increase in the levels of unutilized inorganic phosphate in serum. Previous workers have reported similar observations [
22,
26]. This explains why many of the patients suffer from weakness, as phosphate becomes unavailable inside the cells to be utilized for the synthesis of high-energy compounds.
Available reports suggest that the kidney is less sensitive to chronic arsenic exposure relative to other organs [
27]; while acute intoxication by arsenic can be associated with tubuloninterstitial nephritis [
28]. In this study, creatinine levels in the serum have been found significantly lower in the arsenic patients compared to the normal healthy subjects. Although the average value of serum creatinine in the patients is within the normal range, long-term arsenic toxicity may cause loss of muscle mass that is reflected by their lower BMI compared to the controls.
We found the prevalence of chronic infection or inflammation possibly due to arsenical skin lesions that could be the reasons for the patients having significantly higher levels of total protein in serum. This is supported with the finding of a strong direct correlation between the levels of total protein in serum and that of the inorganic phosphate. Also there has been an association of hyperglycemia with arsenic toxicity. We found the prevalence of diabetes mellitus to be 2.8 times higher among the arsenicosis patients compared to the controls. This observation supports the previous reports that show a significant relationship between arsenic toxicity and the prevalence of diabetes mellitus [
20,
29]. It has been suggested that trivalent arsenicals inhibit insulin-dependent glucose uptake, and thereby cause arsenic-induced diabetes [
29].
Previous workers found that chronic exposure to arsenic could develop the risk of cardiovascular as well as peripheral vascular diseases among residents of arseniasis-hyperendemic villages in Taiwan and other countries [
10,
15,
16,
30,
31]. To find out the biochemical markers related to these diseases among the arsenicosis patients in Bangladesh, the lipid profiles of serum samples have been analyzed. The levels of total cholesterol, HDL-cholesterol and LDL-cholesterol show significantly lower values. These observations can be explained as the patients are from different geographical location, subjected to different socioeconomic and environmental conditions, and have low BMI, indicating that they have been suffering from nutritional deficiency. Further, while the levels of serum cholesterol and LDL-cholesterol were significantly higher in the control males than the control females, the values were significantly higher in the female patients than the male patients. Also, the control males had significantly higher levels of serum cholesterol, HDL-cholesterol, and LDL-cholesterol than the male patients. These differences could be due to higher BMI of the control males than control females, and male patients; and female patients than male patients.
There have been some evidences suggesting of changes in cholestatic function of the liver as shown by conjugated hyperbilirubinemia and elevated alkaline phosphatase activity, which is directly related to the concentration of total arsenic in urine [
32]. In another observation [
33], it has been reported that more than 50% of the total patients studied with arsenic toxicity in West Bengal, India, have an abnormally high ALP activity (>200 U/L); while about 12% and 28% have elevated AST and ALT activities, respectively. The results of the present study show for the first time that about one-third (33%) of the arsenic patients in Bangladesh have an elevated ALP activity in serum, and the mean activity of the whole population is significantly higher than that of the unexposed subjects. These observations suggested that the activities of the above mentioned enzymes should be studied in other populations of arsenicosis patients.
It is possible that to meet the ever-increasing demand of inorganic phosphate for intracellular utilization that is affected by chronic arsenic toxicity, the terminal phosphate groups from phosphate compounds are continuously being cleaved by the action of ALP, thereby causing its high activity. The similar effects of arsenic can enhance the activities of liver enzymes AST and ALT since both of them are pyridoxal phosphate-linked enzymes. The other possibility may be due to the direct effect of arsenic on liver cells, since histological studies of samples of liver showed enlargement and fibrosis of varying degrees in the portal regions [
34]. However, very few of the patients enrolled in the present study showed elevated AST and ALT activities in serum. Following correlation analysis of the data, we found the ALP activity to be directly correlated with the levels of total protein in serum of the patients (p
< 0.01) as well as with the levels of phosphate (p
< 0.02).
In conclusion, it has been revealed from the results of this study that chronic exposure to high levels of arsenic in drinking water causes a significant elevation of inorganic phosphate in serum while the levels of creatinine and lipid profiles are significantly lowered; the total protein and ALP activities in serum are significantly elevated and a considerable diabetogenic effect of arsenic has been detected among Bangladeshi patients.