Post-Katrina Fecal Contamination in Violet Marsh near New Orleans
Abstract
:Introduction
Materials and Methods
Sediment Sampling
Bacterial Indicators of Pathogens in Sewage
Fecal Sterol Analyses
Results
Fecal Bacteria Indicator Culture Data
Fecal Sterol Data
Discussion


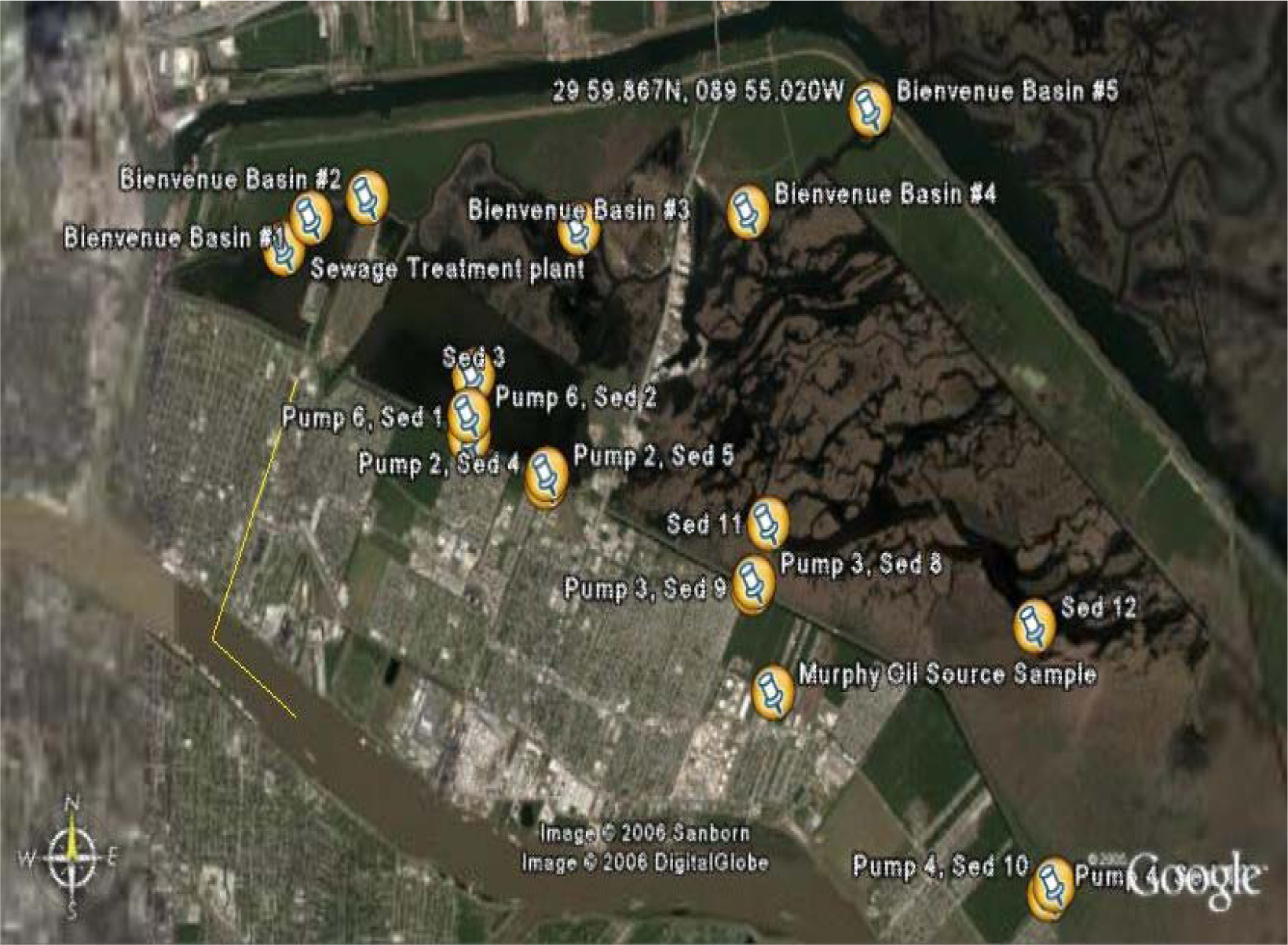
| Sample Name | Latitude | Longitude | Description |
|---|---|---|---|
| Sewage Plant | 29.984166 | −90.001866 | Northwest of treatment plant in marsh |
| Murphy Oil Site | 29.940866 | −89.931083 | Munster Ln, North of Judge Perez, intersection of drainage canal running N.W. |
| Pump 2 Sed 4 | 29.961400 | −89.963983 | Before pump #2 |
| Pump 2 Sed 5 | 29.962183 | −89.963783 | After pump #2 |
| Pump 3 Sed 8 | 29.951633 | −89.933833 | After pump #3 |
| Pump 3 Sed 9 | 29.951050 | −89.934100 | Before pump #3 |
| Pump 4 Sed 10 | 29.922100 | −89.890416 | After pump #4 |
| Pump 4 Sed 13 | 29.921133 | −89.891266 | Before pump #4 |
| Pump 6 Sed 1 | 29.965925 | −89.975072 | Before pump #6 |
| Pump 6 Sed 2 | 29.967916 | −89.975088 | After pump #6 |
| Sed 3 | 29.971766 | −89.974433 | Due north of pump #6, middle of marsh |
| Sed 11 | 29.957350 | −89.931783 | NNE of pump #3, middle of marsh |
| Sed 12 | 29.947333 | −89.893266 | Due north of pump #4 middle of marsh |
| Bienvenue Basin 1 | 29.987200 | −89.997950 | Adjacent to treatment plant aerator within discharge canal |
| Bienvenue Basin 2 | 29.989166 | −89.989816 | Beginning of treatment plant discharge canal |
| Bienvenue Basin 3 | 29.986166 | −89 959183 | Towards the end of treatment plant discharge canal |
| Bienvenue Basin 4 | 29.987733 | −89.934683 | North shore of marsh between discharge canal and intra-coastal waterway lock |
| Bienvenue Basin 5 | 29.997783 | −89.917000 | Adjacent to intra-coastal waterway canal lock |
| Table plate count results from Top of Soil Core | |||||
|---|---|---|---|---|---|
| Sample | Location | Total | Fecal | Fecal | 40 CFR 503 |
| Conforms | Coliforms | Streptococci | BioSolid Res Std FecColif | ||
| CFU/gm | CFU/gm | CFU/gm | 1000 | ||
| Bienvenue Basin 1 | TOP | 17,000 | < 1,000 | <1,000 | - |
| Bienvenue Basin 2 | TOP | 12,000 | < 1,000 | <1,000 | - |
| Bienvenue Basin 3 | TOP | <1000 | < 1,000 | <1,000 | - |
| Bienvenue Basin 4 | TOP | <1000 | < 1,000 | <1,000 | - |
| Bienvenue Basin 5 | TOP | 3,000 | < 1,000 | <1,000 | - |
| Sewage Plant | TOP | 10,000 | < 1,000 | <1,000 | - |
| Murphy Oil Site | TOP | 1,600,000 | 630,000 | 100 | > |
| Pump 2 Sed4 | TOP | 57,000 | 14,000 | <100 | > |
| Pump 2 Sed 5 | TOP | 133,000 | 25,000 | <100 | > |
| Pump 3 Sed 8 | TOP | 84,000 | 5,000 | <100 | > |
| Pump 3 Sed 9 | TOP | 630,000 | 70,000 | <100 | > |
| Pump 4 Sed 10 | TOP | 77,000 | 10,000 | <100 | > |
| Pump 4 Sed 13 | TOP | 128,000 | 15,000 | <100 | > |
| Pump 6 Sed 1 | TOP | 30,000 | 8,000 | <100 | > |
| Pump 6 Sed 2 | TOP | 65,000 | 2,000 | <100 | > |
| Sed 11 | TOP | 33,000 | 3,000 | <100 | > |
| Sed 12 | TOP | >200000 | 4,000 | <1,000 | > |
| Sed 3 | TOP | 2,100 | 3,000 | <100 | > |
| Mean | 192,073 | 65,750 | |||
| Standard Deviation | 419,233 | 178,681 | |||
| Median | 57,000 | 9,000 | |||
| Table X: Fecal sterol content of sediment from the tops and bottoms of cores. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Location | A | B | C | D | Ratio | Ratio | Ratio | Ratio |
| Coprostanol nmol/gm dw | Epicoprostanol nmol/gm dw | Cholesterol nmol/gm dw | Cholestanol nmol/gm dw | A/D | B/A | A/C | A/A+D | ||
| Bienvenue Basin 1 | Top | 28.3 | 1.6 | 43.5 | 3.8 | 7.37 | 0.06 | 0.65 | 0.88 |
| Bienvenue Basin 2 | Top | 28.5 | 41.4 | 355.2 | 41.0 | 0.70 | 1.45 | 0.08 | 0.41 |
| Bienvenue Basin 3 | Top | 9.2 | 0.8 | 43.6 | 7.9 | 1.16 | 0.08 | 0.21 | 0.54 |
| Bienvenue Basin 4 | Top | 9.1 | 2.6 | 42.7 | 5.0 | 1.81 | 0.29 | 0.21 | 0.64 |
| Bienvenue Basin 5 | Top | 4.2 | 0.4 | 110.9 | 5.1 | 0.82 | 0.10 | 0.04 | 0.45 |
| Sewage Plant | Top | 27.3 | 18.1 | 29.2 | 6.5 | 4.20 | 0.66 | 0.93 | 0.81 |
| Murphy Oil Site | Top | 20.8 | 0.6 | 17.2 | 1.3 | 15.58 | 0.03 | 1.21 | 0.94 |
| Pump 2 Sed 4 | Top | 3.0 | 3.7 | 67.7 | 3.8 | 0.79 | 1.24 | 0.04 | 0.44 |
| Pump 2 Sed 5 | Top | 61.3 | 4.6 | 344.7 | 30.3 | 2.02 | 0.07 | 0.18 | 0.67 |
| Pump 3 Sed 8 | Top | 20.6 | 1.8 | 145.8 | 10.0 | 2.06 | 0.09 | 0.14 | 0.67 |
| Pump 3 Sed 9 | Top | 39.1 | 2.2 | 90.0 | 9.1 | 4.31 | 0,06 | 0.44 | 0.81 |
| Pump 4 Sed 10 | Top | 28.1 | 2.0 | 32.4 | 5.9 | 4.72 | 0,07 | 0.87 | 0.83 |
| Pump 4 Sed 13 | Top | 13.4 | 1.0 | 68.6 | 6.3 | 2.11 | 0.08 | 0.20 | 0.68 |
| Pump 6 Sed 1 | Top | 22.0 | 1.7 | 117.4 | 10.5 | 2.09 | 0.08 | 0.19 | 0.68 |
| Pump 6 Sed 2 | Top | 9.5 | 0.8 | 44.3 | 6.8 | 1.39 | 0.08 | 0.21 | 0.58 |
| Sed 11 | Top | 21.5 | 6.0 | 90.3 | 7.0 | 3.06 | 0.28 | 0.24 | 0.75 |
| Sed 12 | Top | 4.3 | 0.7 | 40.6 | 7.3 | 0.58 | 0.17 | 0.10 | 0.37 |
| Sed 3 | Top | 14.3 | 1.1 | 67.5 | 11.0 | 1.31 | 0.07 | 0.21 | 0.57 |
| Mean | 20.2 | 5.1 | 97.3 | 9.9 | 3.1 | 0.3 | 0.3 | 0.7 | |
| Standard Deviation | 14.4 | 10.0 | 98.0 | 9.8 | 3.6 | 0.4 | 0.3 | 0.2 | |
| Median | 20.7 | 1.8 | 67.6 | 6.9 | 2.0 | 0.1 | 0.2 | 0.7 | |
| Bienvenue Basin 1 | Bottom | 61.2 | 2.5 | 80.2 | 6.5 | 9.38 | 0.04 | 0.76 | 0.90 |
| Bienvenue Basin 2 | Bottom | 87.8 | 4.6 | 115.4 | 11.3 | 7.78 | 0.05 | 0.76 | 0.89 |
| Bienvenue Basin 3 | Bottom | 3.4 | 0.5 | 23.4 | 3.0 | 1.15 | 0.14 | 0.15 | 0.53 |
| Bienvenue Basin 4 | Bottom | 6.0 | 0.5 | 33.2 | 7.0 | 0.86 | 0.09 | 0.18 | 0.46 |
| Bienvenue Basin 5 | Bottom | 3.4 | 0.5 | 22.0 | 5.0 | 0.68 | 0.14 | 0.15 | 0.40 |
| Sewaqe Plant | Bottom | 20.3 | 2.7 | 91.8 | 19.8 | 1.02 | 0.13 | 0.22 | 0.51 |
| Murphy Oil Site | Bottom | 23.9 | 1.2 | 15.3 | 4.6 | 5.18 | 0.05 | 1.56 | 0.84 |
| Pump 2 Sed 4 | Bottom | 8.1 | 0.7 | 84.5 | 4.8 | 1.67 | 0.09 | 0.10 | 0.63 |
| Pump 2 Sed 5 | Bottom | 32.8 | 3.2 | 99.2 | 19.1 | 1.72 | 0.10 | 0.33 | 0.63 |
| Pump 3 Sed 8 | Bottom | 0.9 | 0.1 | 4.9 | 0.4 | 2.16 | 0.08 | 0.19 | 0.68 |
| Pump 3 Sed 9 | Bottom | 12.6 | 0.5 | 20.3 | 5.1 | 2.50 | 0.04 | 0.62 | 0.71 |
| Pump 4 Sed 10 | Bottom | 0.0 | 0.0 | 2.7 | 0.9 | 0.00 | - | 0.00 | 0.00 |
| Pump 4 Sed 13 | Bottom | 0.0 | 0.0 | 2,1 | 0.4 | 0.00 | - | 0.00 | 0.00 |
| Pump 6 Sed 1 | Bottom | 5.0 | 0.5 | 24.0 | 3.5 | 1.41 | 0.10 | 0.21 | 0.59 |
| Pump 6 Sed 2 | Bottom | 8.0 | 1.1 | 56.5 | 10.0 | 0.79 | 0.13 | 0.14 | 0.44 |
| Sed 11 | Bottom | 14.2 | 1.4 | 84.5 | 12.3 | 1.15 | 0.10 | 0.17 | 0.54 |
| Sed 12 | Bottom | 6.0 | 1.3 | 55.6 | 9.8 | 0.61 | 0.22 | 0.11 | 0.38 |
| Sed 3 | Bottom | 11.2 | 1.1 | 63.3 | 18.4 | 0.61 | 0.10 | 0.18 | 0.38 |
| Mean | 16.9 | 1.2 | 48.8 | 7.9 | 2.1 | 0.1 | 0.3 | 0.5 | |
| Standard Deviation | 23.1 | 1.2 | 36.8 | 6.2 | 2.6 | 0.0 | 0.4 | 0.3 | |
| Median | 8.0 | 0.9 | 44.4 | 5.8 | 1.2 | 0.1 | 0.2 | 0.5 | |
Acknowledgments
References
- U.S. Environmental Protection Agency. U.S. EPA Hurricane Response 2005; U.S. Environmental Protection Agency: Washington, DC, 2005; http://www.epa.gov/katrina/index.html.
- Pardue, JH; Moe, WM; McInnis, D; Thibodeaux, LJ; Valsaraj, KT; Maciasz, E; VanHeerden, I; Korevec, N; Yuan, QZ. Chemical and microbiological parameters in New Orleans floodwater following Hurricane Katrina. Environmental Science & Technology 2005, 39, 8591–8599. [Google Scholar]
- Presley, SM; Rainwater, TR; Austin, GP; Platt, SG; Zak, JC; Cobb, GP; Marsland, EJ; Tian, K; Zhang, B; Anderson, TA; Cox, SB; Abel, MT; Leftwich, BD; Huddleston, JR; Jeter, RM; Kendall, RJ. Assessment of pathogens toxicants in New Orleans, LA following Hurricane Katrina. Environmental Science & Technology 2006, 40, 468–474. [Google Scholar]
- Interagency Performance Evaluation Task Force. Performance Evaluation of the New Orleans and Southeast Louisiana Hurricane Protection System; U.S. Army Corps of Engineers: Vicksburg, MS, 2006; https://ipet.wes.army.mil/.
- U.S. Environmental Protection Agency. U.S. EPA Surface Water Treatment Rule. National Primary Drinking Water Regulations. Code of Federal Regulations, Title 40, Part 141–142; U.S. Environmental Protection Agency: Washington, DC, 2002. [Google Scholar]
- National Research Council. Indicators of waterborne pathogens; The National Academies Press: Washington, DC, 2004. [Google Scholar]
- U.S. Environmental Protection Agency. Standardized analytical methods for use during homeland security events; U.S. Environmental Protection Agency: Washington, DC, 2004. [Google Scholar]
- International Life Sciences Institute. Revised framework for microbial risk assessment; International Life Sciences Institute: Washington, DC, 2000. [Google Scholar]
- Isobe, KO; Tarao, M; Chiem, NH; Minh, LY; Takada, H. Effect of environmental factors on the relationship between concentrations of coprostanol and fecal indicator bacteria in tropical (Mekong Delta) and temperate (Tokyo) freshwaters. Applied and Environmental Microbiology, 2004, 70(2), 814–821. [Google Scholar]
- Jin, G; Englande, AJ; Bradford, H; Jeng, H. Comparison of E. coli, enterococci, and fecal coliform as indicators for brackish water quality assessment. Water Environment Research 2004, 76(3), 245–255. [Google Scholar]
- Walker, RW; Wun, C; Litsky, W. Coprostanol as an indicator of fecal pollution. CRC Critical Reviews in Environmental Control 1982, 12, 91–112. [Google Scholar]
- Nichols, PD; Leeming, R; Rayner, MS; Latham, V. Use of capillary gas chromatography for measuring fecal-derived sterols application to stormwater, the sea-surface microlayer, beach greases, regional studies, and distinguishing algal blooms and human and non-human sources of sewage pollution. Journal of Chromatography A 1996, 733(1–2), 497–509. [Google Scholar]
- Eneroth, P; Hellström, K; Ryhage, R. Identification and quantification of neutral fecal steroids by gas-liquid chromatography and mass spectrometry: studies of human excretion during two dietary regimens. Journal of Lipid Research 1964, 5, 245–262. [Google Scholar]
- Murtaugh, JJ; Bunch, RL. Sterol as a measure of fecal pollution. J. Water Pollut. Control Fed. 1967, 39, 404–409. [Google Scholar]
- Ogura, K. Fate of coprostanol, an index of fecal pollution, in Tokyo Bay. Geochemistry 1983, 17, 68–75. [Google Scholar]
- Takada, H; Farrington, JW; Bothner, MH; Johnson, CG; Tripp, BW. Transport of sludge-derived organic pollutants to deep sea sediments at deep water dump site 106. Environmental Science & Technology 1994, 28, 1062–1072. [Google Scholar]
- Nishimura, M; Koyama, T. The occurrence of stanols in various living organisms and the behaviour of sterols in contemporary sediments. Geochimica et Cosmochimica Acta 1977, 41, 379–385. [Google Scholar]
- Arscott, DB; Aufdenkampe, AK; Bott, TL; Dow, CL; Jackson, JK; Kaplan, LA; Newbold, JD; Sweeney, BW. Water quality monitoring in the source water areas for New York City: An integrative watershed approach; Stroud Water Research Center: Avondale, PA, 2004. [Google Scholar]
- Kirchmer, CJ. 5β-Cholestan-3β-ol: an indicator of fecal pollution; Ph.D. thesis; University of Florida: Gainesville, FL, 1971. [Google Scholar]
- Dutka, BJ; Chau, ASY; Coburn, J. Relationship between bacterial indicators of water pollution and fecal sterols. Water Res. 1974, 8, 1047–1055. [Google Scholar]
- Leeming, R; Coleman, R. Bayside drains faecal origins study: Sterol/bacterial sampling 1999–2000; CSIRO Marine Research Report No. FPP- 02; Commonwealth Scientific and Industrial Research Organisation: Clayton, Victoria, Australia, 2000. [Google Scholar]
- Grimalt, JO; Fernández, P; Bayona, JM; Albaigés, J. Assessment of fecal sterols and ketones as indicators of urban sewage inputs to coastal waters. Environmental Science & Technology, 1990, 24(3), 357–363. [Google Scholar]
- Centers for Disease Control and Prevention. Surveillance for Illness and Injury after Hurricane Katrina--Three Counties, Mississippi, September 5–October 11, 2005. Journal of the American Medical Association 2006, 295, 1994–1996. [Google Scholar]
- Hou, A; Laws, EA; Gambrell, RP; Bae, H-S; Tan, M; Delaune, RD; Li, Y; Roberts, H. Pathogen Indicator Microbes and Heavy Metals in Lake Pontchartrain following Hurricane Katrina. Environmental Science & Technology 2006, 40, 5904–5910. [Google Scholar]
- Eaton, ED; Clesceri, LS; Rice, EW; Greenberg, AE; Franson, MAH (Eds.) Standard Methods for the Examination of Water and Wastewater, 21st Ed; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, 2005.
- Ringelberg, DB; Talley, J; Perkins, E; Bouwer, E; Luthy, R; Fredrickson, HL. Succession of phenotypic, genotypic and metabolic community characteristics during in vitro bioslurry treatment of PAH-contaminated sediments. Applied and Environmental Microbiology 2001, 67(4), 1542–1550. [Google Scholar]
- Gomez, D; Page, RW; Holsing, N; Gassman, N; Riley, GF. Implementation of a Chemical Method for Differentiating Human and Animal Fecal Impacts in Surface Waters and Sediments; Florida Department of Natural Resource Protection: Fort Lauderdale, FL, 1998. [Google Scholar]
- Burton, GA; Gunnison, D; Lanza, GR. Survival of pathogenic bacteria in various freshwater sediments. Applied and Environmental Microbiology 1987, 53(4), 633–638. [Google Scholar]
- Hill, DD; Owens, WE; Tchounwou, PB. The Impact of Rainfall on Fecal Coliform Bacteria in Bayou Dorcheat (North Louisiana). Int. J. Environ. Res. Public Health 2006, 3(1), 114–117. [Google Scholar]
- U.S. Environmental Protection Agency. Bacterial water quality standards for recreational waters (freshwater and marine waters); U.S. Environmental Protection Agency: Washington, DC, 2003. [Google Scholar]
- Louisiana Department of Environmental Quality. Post-hurricane water quality assessments: Katrina monitoring; Louisiana Department of Environmental Quality: Baton Rouge, LA, 2005. [Google Scholar]
- U.S. Environmental Protection Agency. The incidence and severity of sediment contamination in surface waters of the United States: National Sediment Quality Survey; U.S. Environmental Protection Agency: Washington, DC, 2004. [Google Scholar]
- Dortch, MS; Zakikhani, M; Kim, S-C. Contaminant Fate/Transport Modeling for Environmental Consequences of IPET Task 9. ERDC/EL TR-06-9; U.S. Army Engineer Research and Development Center: Vicksburg, MS, 2006. [Google Scholar]
© 2007 MDPI All rights reserved.
Share and Cite
Furey, J.S.; Fredrickson, H.; Foote, C.; Richmond, M. Post-Katrina Fecal Contamination in Violet Marsh near New Orleans. Int. J. Environ. Res. Public Health 2007, 4, 84-92. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph2007040001
Furey JS, Fredrickson H, Foote C, Richmond M. Post-Katrina Fecal Contamination in Violet Marsh near New Orleans. International Journal of Environmental Research and Public Health. 2007; 4(2):84-92. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph2007040001
Chicago/Turabian StyleFurey, John S., Herbert Fredrickson, Chris Foote, and Margaret Richmond. 2007. "Post-Katrina Fecal Contamination in Violet Marsh near New Orleans" International Journal of Environmental Research and Public Health 4, no. 2: 84-92. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph2007040001




