Tobacco Smoke: Involvement of Reactive Oxygen Species and Stable Free Radicals in Mechanisms of Oxidative Damage, Carcinogenesis and Synergistic Effects with Other Respirable Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cigarette Smoking Methods: The standard puff protocol
2.3. Measurements of Stable Free Radical in Cigarette Tar by EPR
2.4. Spin-Trapping and Measurements of O2•− and HO• Generated by Aqueous Cigarette Tar (ACT) Solutions at Physiological pH
2.5. Spin-Trapping Measurements of the Gaseous Phase of Mainstream Smoke
2.6. HPLC/UV-EC Quantitative Measurements of the 8-OHdG from Mixtures of ACT with 2’-dG in Buffered Solutions
2.7. Synergistic Effect of ACT with Various Fibres and Respirable Particles
2.8. Comparison of Conventional Cellulose Acetate Filters and “Bio-Filters” (these experimental results were presented in our previous paper) [61]
3. Results and Discussion
4. Conclusions
Acknowledgments
References and Notes
- Vineis, P; Alavanja, M; Buffler, P; Fontham, E; Franceschi, S; Gao, YT; Gupta, PC; Hackshaw, A; Matos, E; Samet, J; Sitas, F; Smith, J; Stayner, L; Straif, K; Thun, MJ; Wichmann, HE; Wu, AH; Zaridze, D; Peto, R; Doll, R. Tobacco smoke and cancer: recent epidemiological evidence. J. Natl. Cancer Inst 2004, 96, 99–105. [Google Scholar]
- Ezzati, M; Lopez, AD. Estimates of global mortality attributable to smoking in 2000. Lancet 2003, 362, 847–852. [Google Scholar]
- Peto, R; Lopez, AD. Future worldwide health effects of current smoking patterns. In Critical Issues in Global Health; Koop, CE, Pearson, CE, Schwartz, MR, Eds.; Jossey-Bass: San Francisco, CA, USA, 2001; pp. 150–167. [Google Scholar]
- Hecht, SS. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst 1999, 91, 1194–1210. [Google Scholar]
- Hoffman, D; Hoffmann, I. The changing cigarette, 1950–1995. J. Toxicol. Environ. Health 1997, 50, 307–364. [Google Scholar]
- Hoffmann, D; Wynder, EL. Chemical constituents and bioactivity of tobacco smoke. In Tobacco: A Major International Hazard; 74, Zaridze, DG, Peto, R, Eds.; International Agency for Research on Cancer, IARC Scientific Publications: Lyon, France, 1986; pp. 145–166. [Google Scholar]
- Pryor, WA; Stone, K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate and peroxynitrite. Ann. NY Acad. Sci 1993, 686, 28. [Google Scholar]
- Norman, V. An overview of the vapor phase, semivolatile and nonvolatile components of cigarette smoke. Recent Adv. Tob. Sci 1977, 3, 58. [Google Scholar]
- Brunnemann, KD; Hoffmann, D. The pH of tobacco smoke. J. Food Cosmet. Toxicol 1974, 12, 5–124. [Google Scholar]
- Pryor, WA; Doodley, MM; Church, DF. Mechanisms of cigarette smoke toxicity: the inactivation of human alpha-1-proteinase inhibitor by nitric oxide/isoprene mixtures in air. Chem.-Biol. Interact 1985, 54, 71–183. [Google Scholar]
- Church, DF; Pryor, WA. Free radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect 1985, 64, 1–126. [Google Scholar]
- Eiserich, JP; van der Vliet, A; Handelman, GJ; Halliwell, B; Cross, CE. Dietary antioxidants and cigarette smoke-induced biomolecular damage: a complex interaction. Am. J. Clin. Nutr 1996, 62, 90S–150S. [Google Scholar]
- Pryor, WA; Curch, DF; Evans, MD; Rice, WY; Hayes, JR. A comparison of the free radical chemistry of tobacco-burning cigarettes and cigarettes that only heat tobacco. Free Radic. Biol. Med 1990, 8, 275–279. [Google Scholar]
- Environmental Protection Agency, Respiratory Health Effects of Passive Smoking: Lung Cancer and Other Disorders; EPA Publications: Washington DC, USA, 1992.
- Hackshaw, AK; Law, MR; Wald, NJ. The accumulated evidence on lung cancer and environmental tobacco smoke. Br Med J 1997, 315, 980–988. [Google Scholar]
- Eisner, MD; Balmes, J; Katz, PP; Trupin, L; Yelin, EH; Blanc, PD. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ. Health 2005, 4, 7–9. [Google Scholar]
- Heidrich, J; Wellmann, J; Heuschmann, PU; Kraywinkel, K; Keil, U. Mortality and morbidity from coronary heart disease attributable to passive smoking. Eur. Heart J 2007, 28, 2498–2502. [Google Scholar]
- Hecht, SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem. Res. Toxicol 1998, 11, 559–603. [Google Scholar]
- Philips, DH. DNA adducts in human tissues: biomarkers of exposure to carcinogens in tobacco smoke. Environ Health Perspect 1996, 104, 453–458. [Google Scholar]
- Pryor, WA; Stone, K; Zang, LY; Bermudez, E. Fractionation of aqueous cigarette tar extracts: fractions that contain the tar radical cause DNA damage. Chem. Res. Toxicol 1998, 11, 441–448. [Google Scholar]
- Leaderson, P; Tagesson, C. Cigarette smoke-induced DNA damage in cultured human cells: role of hydroquinone and catechol in the formation of oxidative DNA-adduct, 8-hydroxydeoxyguanosine. Chem.-Biol. Interact 1990, 75, 71–81. [Google Scholar]
- Asami, S; Manabe, H; Miyake, J; Tsurudone, Y; Hirano, T. Cigarette smoking induces an increase in oxidative DNA damage, 8-hydroxydeoxyguanosine, in a central site of human lung. Carcinogenesis 1997, 18, 1763–1766. [Google Scholar]
- Huang, MF; Lin, WL; Ma, YC. A study of reactive oxygen species in mainstream of cigarette. Indoor Air 2005, 15, 135–140. [Google Scholar]
- Pryor, WA. Biological effects of cigarette smoke, wood smoke, and the smoke from plastics: the use of Electron spin resonance. Free Radic. Biol. Med 1992, 13, 659–676. [Google Scholar]
- Pryor, WA; Hales, BJ; Premovic, PI; Church, DF. The radicals in cigarette tar: their nature and suggested physiological implications. Science 1983, 220, 425–427. [Google Scholar]
- Bermudez, E; Stone, K; Carter, KM; Pryor, WA. Environmental tobacco smoke is just as damaging to DNA as mainstream smoke. Environ. Health Perspect 1994, 102, 870–874. [Google Scholar]
- Nakayama, T; Church, DF; Pryor, WA. Quantitative analysis of hydrogen peroxide formed in aqueous cigarette tar extracts. Free Radic. Biol. Med 1989, 7, 9–15. [Google Scholar]
- Moreno, JJ; Foroozesh, M; Church, DF; Pryor, WA. Release of iron from ferritin by aqueous extracts of cigarette smoke. Chem. Res. Toxicol 1992, 5, 116–123. [Google Scholar]
- Lapenna, D; de Gioia, S; Mezzeti, A; Ciofani, G; Consoli, A; Marzio, L; Cuccurullo, F. Cigarette smoke, ferritin, and lipid peroxidation. Am. J. Respir. Crit. Care Med 1995, 151, 431–435. [Google Scholar]
- Frei, B; Forte, TM; Ames, BN; Cross, CE. Gas phase oxidants of cigarette smoke induce lipid peroxidation and changes in lipoprotein properties in human blood plasma. Biochem. J 1991, 277, 133–138. [Google Scholar]
- Santanam, N; Sanchez, R; Hendler, S; Parthasarathy, S. Aqueous extracts of cigarette smoke promote the oxidation of low density lipoprotein by peroxidases. FEBS Lett 1997, 414, 549–551. [Google Scholar]
- Ambrose, JA; Barua, RS. The pathophysiology of cigarette smoking and cardiovascular disease. An update. J. Am. Coll. Cardiol 2004, 43, 1731–1737. [Google Scholar]
- Cross, CE; Traber, M; Eiserich, J; van der Vliet, A. Micronutrient antioxidants and smoking. Br. Med. Bull 1999, 55, 691–704. [Google Scholar]
- Panta, K; Chattopadyay, R; Chattopadyay, DJ; Chatterjee, IB. Vitamin C prevents cigarette smoke-induced oxidative damage in vivo. Free Radic. Biol. Med 2000, 29, 115–124. [Google Scholar]
- Traber, MG; van der Vliet, A; Reznick, AZ; Cross, CE. Tobacco-related diseases. Is there a role for antioxidant micronutrient supplementation? Clin. Chest Med 2000, 21, 173–187. [Google Scholar]
- Henneken, CH; Buring, JE; Manson, JE; Stampfer, M; Rosner, B; Cook, NR; Belanger, C; LaMotte, F; Gaziano, JM; Ridker, PM; Willett, W; Peto, R. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med 1996, 334, 1145–1149. [Google Scholar]
- Prieme, H; Loft, S; Nyyssonen, K; Salonen, JT; Poulsen, HE. No effect of supplementation with vitamin E, ascorbic acid, or coenzyme Q10 on oxidative DNA damage estimated by 8-oxo-7,8-dihydro-2’-deoxyguanosine excretion in smokers. Am. J. Clin. Nutr 1997, 65, 503–507. [Google Scholar]
- Saracci, R. The interaction of tobacco smoking and other agents in cancer etiology. Epidemiol. Rev 1987, 9, 175–193. [Google Scholar]
- Reif, AE; Heeren, T. Consencus on synergism between cigarette smoke and other environmental carcinogens in the causation of lung cancer. Adv. Cancer Res 1999, 76, 161–186. [Google Scholar]
- Kamp, DW; Graceffa, P; Pryor, WA; Weitzman, SA. The role of free radicals in asbestos-induced disease. Free Radic. Biol. Med 1992, 12, 293–315. [Google Scholar]
- Jackson, JH; Schraufstatter, IU; Hyslop, PA; Vosbeck, K; Sauerheber, R; Weitzman, SA; Cochrane, CG. Role of oxidants in DNA damage: hydroxyl radical mediates the synergistic DNA damaging effects of asbestos and cigarette smoke. J. Clin. Invest 1987, 80, 1090–1095. [Google Scholar]
- Valavanidis, A; Balomenou, H; Macropoulou, I; Zarodimos, I. A study of the synergistic interaction of asbestos fibers with cigarette tar extracts for the generation of hydroxyl radicals in aqueous buffer solution. Free Radic. Biol. Med 1996, 20, 853–858. [Google Scholar]
- Leanderson, P; Tagesson, C. Mineral fibers, cigarette smoke, and oxidative DNA damage. In DNA and Free Radicals; Halliwell, B, Aruoma, OI, Eds.; Ellis Harwood: Chichester, UK, 1993; pp. 293–314. [Google Scholar]
- Vainio, H; Boffetta, P. Mechanisms of the combined effect of asbestos and smoking in the etiology of cancer. Review. Scand J. Work Environ. Health 1994, 20, 235–242. [Google Scholar]
- Jung, M; Davis, WP; Taatjes, DJ; Churg, A; Mossman, BT. Asbestos and cigarette smoke cause increased DNA strand breaks and neutrophils in bronchial epithelial cells in vivo. Free Radic. Biol. Med 2000, 28, 1295–1299. [Google Scholar]
- Band, P; Feldstein, M; Saccomanno, G; Watson, L; King, G. Potentiation of cigarette smoking and radiation. Evidence from a sputum cytology survey among uranium miners and controls. Cancer 1980, 45, 1273–1277. [Google Scholar]
- Damber, L; Larsson, LG. Combined effects of mining and smoking in the causation of lung carcinoma: a case-control study in northern Sweden. Acta Radiol. Oncol 1982, 21, 305–13. [Google Scholar]
- Dalal, NS; Newman, J; Rack, D; Leonard, S; Vallyathan, V. Hydroxyl radical generation by coal mine dust: possible implications to coal worker’s pneumoconiosis. Free Radic. Biol. Med 1995, 18, 11–20. [Google Scholar]
- Liu, X; Lu, J; Liu, S. Synergistic induction of hydroxyl radical-induced DNA single-strand breaks by chromium(VI) compounds and cigarette smoke solution. Mutat. Res 1999, 440, 109–117. [Google Scholar]
- Pershagen, G; Walls, S; Taube, A; Linnman, L. On the interaction between occupational arsenic exposure and smoking and its relationship to cancer. Scand. J. Work Environ. Health 1981, 7, 302–307. [Google Scholar]
- Welch, K; Higgins, I; Oh, M; Burchfield, C. Arsenic exposure, smoking, and respiratory cancer in copper smelter workers. Arch. Environ. Health 1982, 37, 325–329. [Google Scholar]
- Molyneux, MJ; Davies, MJ. Direct evidence for hydroxyl-induced damage to nuclei acids by chromium(VI)-derived species: implications for chromium carcinogenesis. Carcinogenesis 1995, 16, 875–882. [Google Scholar]
- Costa, D; Guignard, J; Pezerat, H. Production of free radicals arising from the surface activity of minerals and oxygen. Part II. Arsenites, sulfides, and sulfoarsenites of iron, nickel and copper. Toxicol. Ind. Health 1989, 5, 1079–1097. [Google Scholar]
- Landolph, JR. Role of free radicals in metal-induced carcinogenesis. Metal Ion Biol. Syst 1999, 36, 445–483. [Google Scholar]
- Elwood, JM; Pearson, JCG; Skippen, DH. Alcohol, smoking, social and occupational factors in the aetiology of cancer of the oral cavity, pharynx and larynx. Int. J. Cancer 1984, 34, 603–612. [Google Scholar]
- Franceschi, S; Levi, F; La Vecchia, C; Conti, E; Dal Maso, L; Barzan, L; Talamini, R. Comparison of the effect of smoking and alcohol drinking between oral and pharyngeal cancer. Int. J. Cancer 1999, 83, 1–4. [Google Scholar]
- Moncada, S; Higgs, EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur. J. Clin. Invest 1991, 21, 361–374. [Google Scholar]
- Beckman, JS; Crow, JP. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem. Soc. Trans 1993, 21, 330–334. [Google Scholar]
- Ischiropoulos, H; Zhu, L; Chen, J; Tsai, M; Martin, JC; Smith, CD; Beckman, JS. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch. Biochem. Biophys 1992, 298, 431–437. [Google Scholar]
- Lala, PK; Chakraborty, C. Role of nitric oxide in carcinogenesis and tumour progression. Review. Lancet Oncol 2001, 2, 149–156. [Google Scholar]
- Valavanidis, A; Haralambous, E. A comparative study by electron paramagnetic resonance of free radical species in the mainstream and sidestream smoke of cigarettes with conventional acetate filters and “bio-filters”. Redox Report 2001, 6, 161–171. [Google Scholar]
- Deliconstantinos, G; Villiotou, V; Stavrides, J. Scavenging effects of hemoglobin and related heme containing compounds on nitric oxide, reactive and carcinogenic volatile compounds of cigarette smoke. A new method for protection against the dangerous cigarette constituents. Anticancer Res 1994, 14, 2717–2726. [Google Scholar]
- Pryor, WA; Tamura, M; Church, DF. ESR spin trapping study of the radicals produced in NOx/olefin reactions: A mechanism for the production of the apparently long-lived radicals in gas-phase cigarette smoke. J. Am. Chem. Soc 1984, 106, 5073–5079. [Google Scholar]
- Cueto, R; Church, DF; Pryor, WA. Quantitative fourier transform infrared analysis of gas phase cigarette smoke and other gas mixtures. Anal. Lett 1989, 22, 751–763. [Google Scholar]
- Schreiber, J; Mottley, C; Sinha, BK; Kalyanaraman, B; Mason, RP. One-electron reduction of dianomycin, daunomycinone, and 7-deoxydaumycinone by the xanthine/xanthine oxidase system: detection of semiquinone free radicals by electron spin resonance. J. Am. Chem. Soc 1987, 109, 348–351. [Google Scholar]
- Pryor, WA; Prier, DG; Church, DF. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and cigarette tar. Environ. Health Perspect 1983, 47, 345–355. [Google Scholar]
- Ross, MM; Chedekel, MR; Risby, TH. Electron paramagnetic resonance spectrometry of diesel particulate matter. Environ. Int 1982, 7, 325–329. [Google Scholar]
- Sagai, M; Saito, H; Ichinose, T; Kodama, M; Mori, Y. Biological effects of diesel exhaust particles. I. In vivo production of superoxide and in vivo toxicity in mouse. Free Radic. Biol. Med 1993, 14, 37–47. [Google Scholar]
- Stone, KK; Bermudez, E; Pryor, WA. Aqueous extracts of cigarette tar containing the tar free radical cause DNA nicks in mammalian cells. Environ. Health Perspect 1994, 102, 173–178. [Google Scholar]
- Donaldson, K; Brown, DM; Mitchell, C; Dineva, M; Beswick, PH; Gilmour, P; MacNee, W. Free radical activity of PM10: iron-mediated generation of hydroxyl radicals. Environ. Health Perspect 1997, 105, 1285–1289. [Google Scholar]
- Shi, T; Schins, RPF; Knaapen, AM; Kuhlbusch, T; Pitz, M; Heinrich, J; Borm, PJ. Hydroxyl radical generation by electron paramagnetic resonance as a new method to monitor ambient particulate matter composition. J. Environ. Monitor 2003, 5, 550–556. [Google Scholar]
- Flicker, TM; Green, SA. Detection and separation of gas-phase carbon-centered radicals from cigarette smoke and diesel exhaust. Anal. Chem 1998, 70, 2208–2212. [Google Scholar]
- Ichinose, T; Yajima, Y; Nagashima, M; Takenoshita, S; Nagamachi, Y; Sagai, M. Lung carcinogenesis and formation of 8-hydroxy-deoxyguanosine in mice by diesel exhaust particles. Carcinogenesis 1997, 18, 185–192. [Google Scholar]
- Kim, JY; Mukherjee, S; Ngo, LC; Christiani, DC. Urinary 8-hydroxy-2’0-deoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to fine particulates. Environ. Health Perspect 2004, 112, 666–671. [Google Scholar]
- Prahalat, AK; Inmon, J; Dailey, LA; Madden, MC; Ghio, AJ; Gallagher, JE. Air pollution particles mnediated oxidative DNA base damage in a cell free system and in human airway epithelial cells in relation to particulate metal content and bioreactivity. Chem. Res. Toxicol 2001, 14, 879–887. [Google Scholar]
- Squadrito, GL; Cueto, R; Dellinger, B; Pryor, WA. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radic. Biol. Med 2001, 31, 1132–1138. [Google Scholar]
- Shi, T; Knaapen, AM; Begerow, J; Birmili, W; Borm, PJ; Schins, RP. Temporal variation of hydroxyl radical generation and formation of 8-hydroxy-2’-deoxyguanosine formation by coarse and fine particulate matter. Occup. Environ. Med 2003, 60, 315–321. [Google Scholar]
- Valavanidis, A; Vlachogianni, T; Fiotakis, K. Comparative study of the formation of oxidative damage marker 8-hydroxy-2’-deoxyguanosine (8-OHdG) adduct from the nucleoside 2’-deoxyguanosine by transition metals and suspensions of particulate matter in relation to metal content and redox reactivity. Free Radic. Res 2005, 39, 1071–1081. [Google Scholar]
- Valavanidis, A; Balomenou, H; Macropoulou, I; Zarodimos, I. A study of the synergistic interaction of asbestos fibers with cigarette tar ectracts for the generation of hydroxyl radicals in aqueous buffer solutions. Free Radic. Biol. Med 1996, 20, 853–858. [Google Scholar]
- Lund, LG; Aust, AE. Iron-catalyzed reactions may be responsible for the biochemical and biological effects of asbestos. Biofactors 1991, 3, 83–89. [Google Scholar]
- Shukla, A; Gulumian, M; Hei, TK; Kamp, DW; Rahman, Q; Mossman, BT. Multiple roles of oxidants in the pathogenesis of asbestos-induced diseases. Review. Free Radic. Biol. Med 2003, 34, 1117–1129. [Google Scholar]
- Kamp, DW; Greenberger, MJ; Sbalchierro, JS; Preusen, SE; Weitzman, SA. Cigarette smoke augments asbestos-induced alveolar epithelial cell injury: role of free radicals. Free Radic. Biol. Med 1998, 25, 728–739. [Google Scholar]

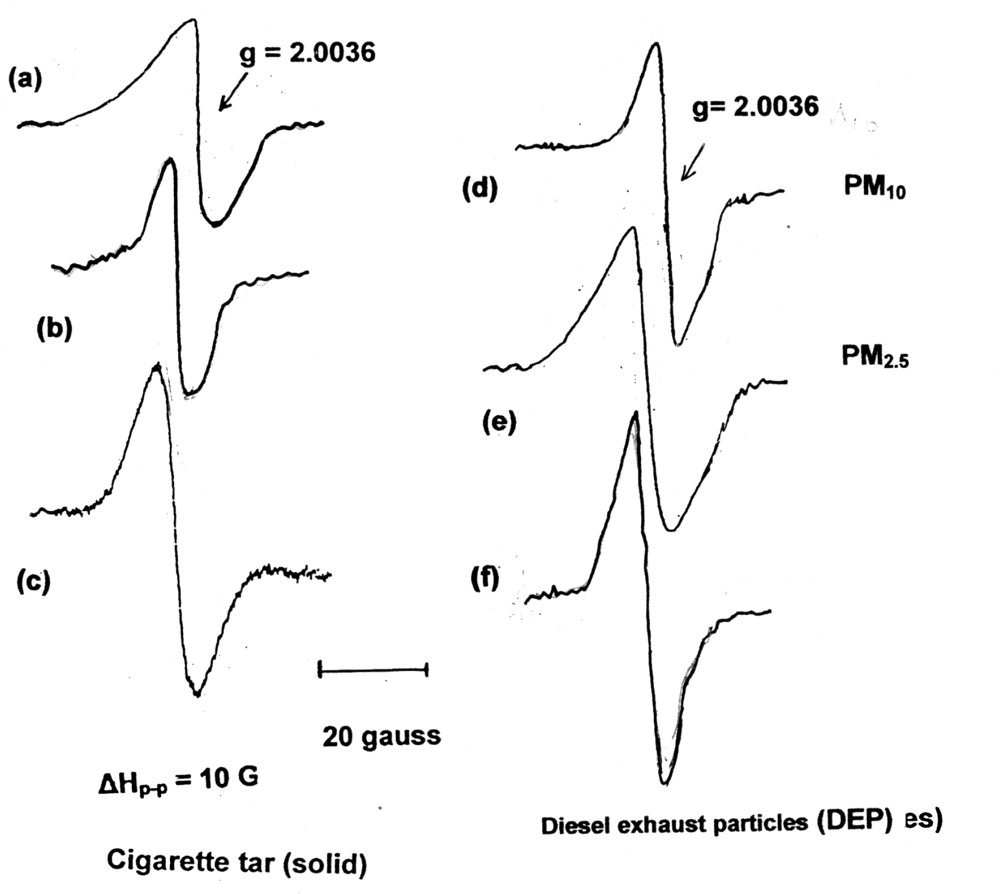
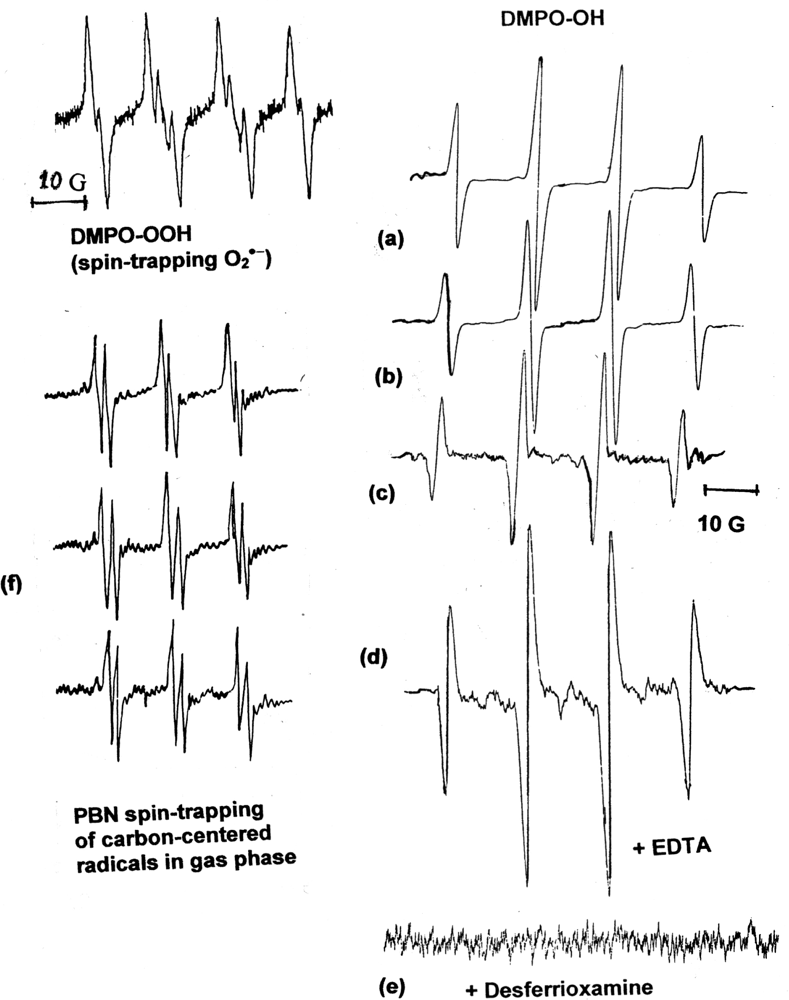

| Material | Mixture | 8-OHdG (μg/106 dG) |
|---|---|---|
| ACT (low tar) | 50 mg | 120 ± 10 |
| ACT (middle tar) | 50 mg | 145 ± 12 |
| ACT (high tar) | 50 mg | 170 ± 20 |
| PM10 + H2O2 | 50 mg + 0.001 H2O2 | 85 ± 4 |
| PM2.5 + H2O2 | 50 mg + 0.001 H2O2 | 100 ± 6 |
| DEP + H2O2(diesel exhaust particles) | 50 mg + 0.001 H2O2 | 115 ± 10 |
| Asbestos fibres (fresh grinding) +ACT | 10 mg +50 mg | 250 ± 35 |
Share and Cite
Valavanidis, A.; Vlachogianni, T.; Fiotakis, K. Tobacco Smoke: Involvement of Reactive Oxygen Species and Stable Free Radicals in Mechanisms of Oxidative Damage, Carcinogenesis and Synergistic Effects with Other Respirable Particles. Int. J. Environ. Res. Public Health 2009, 6, 445-462. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph6020445
Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco Smoke: Involvement of Reactive Oxygen Species and Stable Free Radicals in Mechanisms of Oxidative Damage, Carcinogenesis and Synergistic Effects with Other Respirable Particles. International Journal of Environmental Research and Public Health. 2009; 6(2):445-462. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph6020445
Chicago/Turabian StyleValavanidis, Athanasios, Thomais Vlachogianni, and Konstantinos Fiotakis. 2009. "Tobacco Smoke: Involvement of Reactive Oxygen Species and Stable Free Radicals in Mechanisms of Oxidative Damage, Carcinogenesis and Synergistic Effects with Other Respirable Particles" International Journal of Environmental Research and Public Health 6, no. 2: 445-462. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph6020445




