Human Blood Concentrations of Cotinine, a Biomonitoring Marker for Tobacco Smoke, Extrapolated from Nicotine Metabolism in Rats and Humans and Physiologically Based Pharmacokinetic Modeling
Abstract
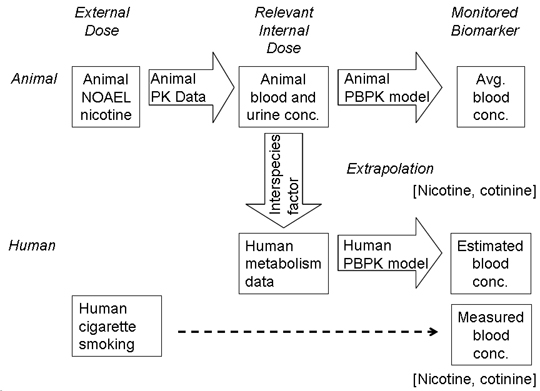
:1. Introduction
2. Experimental Section
2.1. Chemicals, Animals, and Enzyme Preparations
2.2. Nicotine and Cotinine Determinations in Biological Samples from Rats and Human Smokers
2.3. Human Metabolic Study
2.4. Estimation of Nicotine/Cotinine Concentrations by PBPK Modeling with Suitable Parameters
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Collins, FS; Gray, GM; Bucher, JR. Toxicology. Transforming environmental health protection. Science 2008, 319, 906–907. [Google Scholar]
- Toxicity Testing in the 21st Century: A Vision and a Strategy; The National Academies Press: Washington, DC, USA, 2007.
- Hayes, KR; Bradfield, CA. Advances in toxicogenomics. Chem. Res. Toxicol 2005, 18, 403–414. [Google Scholar]
- Edwards, SW; Preston, RJ. Systems biology and mode of action based risk assessment. Toxicol. Sci 2008, 106, 312–318. [Google Scholar]
- Wild, CP. Complementing the genome with an “exposome”: The outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidem. Biomarker. Prev 2005, 14, 1847–1850. [Google Scholar]
- Clewell, HJ; Gentry, PR; Covington, TR; Sarangapani, R; Teeguarden, JG. Evaluation of the potential impact of age- and gender-specific pharmacokinetic differences on tissue dosimetry. Toxicol. Sci 2004, 79, 381–393. [Google Scholar]
- Guengerich, FP. Cytochrome P450 and chemical toxicology. Chem. Res. Toxicol 2008, 21, 70–83. [Google Scholar]
- Robinson, DE; Balter, NJ; Schwartz, SL. A physiologically based pharmacokinetic model for nicotine and cotinine in man. J. Pharmacok. Biopharm 1992, 20, 591–609. [Google Scholar]
- Benowitz, NL; Hukkanen, J; Jacob, P, III. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol 2009, 192, 29–60. [Google Scholar]
- Benowitz, NL. Pharmacology of nicotine: addiction and therapeutics. Annu. Rev. Pharmacol. Toxicol 1996, 36, 597–613. [Google Scholar]
- Kume, A; Kume, T; Masuda, K; Shibuya, F; Yamazaki, H. Dose-dependent effects of cigarette smoke on blood biomarkers in healthy Japanese volunteers: Observations from smoking and non-smoking. J. Health Sci 2009, 55, 259–264. [Google Scholar]
- Rose, JE; Behm, FM; Westman, EC; Coleman, RE. Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: Implications for addiction. Drug Alcohol Depend 1999, 56, 99–107. [Google Scholar]
- Hukkanen, J; Jacob, P, III; Benowitz, NL. Metabolism and disposition kinetics of nicotine. Pharmacol. Rev 2005, 57, 79–115. [Google Scholar]
- Kwon, JT; Nakajima, M; Chai, S; Yom, YK; Kim, HK; Yamazaki, H; Shon, DR; Yamamoto, T; Kuroiwa, Y; Yokoi, T. Nicotine metabolism and CYP2A6 allele frequencies in Koreans. Pharmacogenetics 2001, 11, 317–323. [Google Scholar]
- Nakajima, M; Kwon, JT; Tanaka, N; Zenta, T; Yamamoto, Y; Yamamoto, H; Yamazaki, H; Yamamoto, T; Kokoiwa, Y; Yokoi, T. Relationship between interindividual differences in nicotine metabolism and CYP2A6 genetic polymorphism in humans. Clin. Pharmacol. Ther 2001, 69, 72–78. [Google Scholar]
- Feng, S; Kapur, S; Sarkar, M; Muhammad, R; Mendes, P; Newland, K; Roethig, HJ. Respiratory retention of nicotine and urinary excretion of nicotine and its five major metabolites in adult male smokers. Toxicol. Lett 2007, 173, 101–106. [Google Scholar]
- Roethig, HJ; Munjal, S; Feng, S; Liang, Q; Sarkar, M; Walk, RA; Mendes, PE. Population estimates for biomarkers of exposure to cigarette smoke in adult U.S. cigarette smokers. Nicotine Tob. Res 2009, 11, 1216–1225. [Google Scholar]
- Nagano, T; Shimizu, M; Kiyotani, K; Kamataki, T; Takano, R; Murayama, N; Shono, F; Yamazaki, H. Biomonitoring of urinary cotinine concentrations associated with plasma levels of nicotine metabolites after daily cigarette smoking in a male Japanese population. Int. J. Environ. Res. Public Health 2010, 7, 2953–2964. [Google Scholar]
- Benowitz, NL. Clinical pharmacology of nicotine: Implications for understanding, preventing, and treating tobacco addiction. Clin. Pharmacol. Ther 2008, 83, 531–541. [Google Scholar]
- Tricker, AR. Nicotine metabolism, human drug metabolism polymorphisms, and smoking behaviour. Toxicology 2003, 183, 151–173. [Google Scholar]
- Ribeiro, EB; Bettiker, RL; Bogdanov, M; Wurtman, RJ. Effects of systemic nicotine on serotonin release in rat brain. Brain Res 1993, 621, 311–318. [Google Scholar]
- Yamazaki, H; Shimizu, M; Nagashima, T; Minoshima, M; Murayama, N. Rat cytochrome P450 2C11 in liver microsomes involved in oxidation of anesthetic agent propofol and deactivated by prior treatment with propofol. Drug Metab. Dispos 2006, 34, 1803–1905. [Google Scholar]
- Robinson, DE; Balter, NJ; Schwartz, SL. A physiologically based pharmacokinetic model for nicotine and cotinine in man. J. Pharmacok. Biopharm 1992, 20, 591–609. [Google Scholar]
- Omura, T; Sato, R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem 1964, 239, 2370–2378. [Google Scholar]
- Murayama, N; Kaneko, N; Horiuchi, K; Ohyama, K; Shimizu, M; Ito, K; Yamazaki, H. Cytochrome P450-dependent drug oxidation activity of liver microsomes from Microminipigs, a possible new animal model for humans in non-clinical studies. Drug Metab. Pharmacokinet 2009, 24, 404–408. [Google Scholar]
- Yamazaki, H; Mimura, M; Sugahara, C; Shimada, T. Catalytic roles of rat and human cytochrome P450 2A enzymes in testosterone 7α- and coumarin 7-hydroxylations. Biochem. Pharmacol 1994, 48, 1524–1527. [Google Scholar]
- Yamazaki, H; Nakamura, M; Komatsu, T; Ohyama, K; Hatanaka, N; Asahi, S; Shimada, N; Guengerich, FP; Shimada, T; Nakajima, M; Yokoi, T. Roles of NADPH-P450 reductase and apo- and holo-cytochrome b5 on xenobiotic oxidations catalyzed by 12 recombinant human cytochrome P450s expressed in membranes of Escherichia coli. Protein Expr. Purif 2002, 24, 329–337. [Google Scholar]
- Yamanaka, H; Nakajima, M; Nishimura, K; Yoshida, R; Fukami, T; Katoh, M; Yokoi, T. Metabolic profile of nicotine in subjects whose CYP2A6 gene is deleted. Eur. J. Pharm. Sci 2004, 22, 419–425. [Google Scholar]
- Yamazaki, H; Inoue, K; Hashimoto, M; Shimada, T. Roles of CYP2A6 and CYP2B6 in nicotine C-oxidation by human liver microsomes. Arch. Toxicol 1999, 73, 65–70. [Google Scholar]
- Plowchalk, DR; Andersen, ME; deBethizy, JD. A physiologically based pharmacokinetic model for nicotine disposition in the Sprague-Dawley rat. Toxicol. Appl. Pharmacol 1992, 116, 177–188. [Google Scholar]
- Kato, M; Shitara, Y; Sato, H; Yoshisue, K; Hirano, M; Ikeda, T; Sugiyama, Y. The quantitative prediction of CYP-mediated drug interaction by physiologically based pharmacokinetic modeling. Pharm. Res 2008, 25, 1891–1901. [Google Scholar]
- Emoto, C; Murayama, N; Rostami-Hodjegan, A; Yamazaki, H. Utilization of estimated physicochemical properties as an integrated part of predicting hepatic clearance in the early drug-discovery stage: Impact of plasma and microsomal binding. Xenobiotica 2009, 39, 227–235. [Google Scholar]
- Howard, LA; Micu, AL; Sellers, EM; Tyndale, RF. Low doses of nicotine and ethanol induce CYP2E1 and chlorzoxazone metabolism in rat liver. J. Pharmacol. Exp. Ther 2001, 299, 542–550. [Google Scholar]
- Yue, J; Khokhar, J; Miksys, S; Tyndale, RF. Differential induction of ethanol-metabolizing CYP2E1 and nicotine-metabolizing CYP2B1/2 in rat liver by chronic nicotine treatment and voluntary ethanol intake. Eur. J. Pharmacol 2009, 609, 88–95. [Google Scholar]
- Naritomi, Y; Terashita, S; Kimura, S; Suzuki, A; Kagayama, A; Sugiyama, Y. Prediction of human hepatic clearance from in vivo animal experiments and in vitro metabolic studies with liver microsomes from animals and humans. Drug Metab. Dispos 2001, 29, 1316–1324. [Google Scholar]
- Kyerematen, GA; Taylor, LH; deBethizy, JD; Vesell, ES. Pharmacokinetics of nicotine and 12 metabolites in the rat. Application of a new radiometric high performance liquid chromatography assay. Drug Metab. Dispos 1988, 16, 125–129. [Google Scholar]
- Benowitz, NL; Jacob, P, III. Nicotine and cotinine elimination pharmacokinetics in smokers and nonsmokers. Clin. Pharmacol. Ther 1993, 53, 316–323. [Google Scholar]
- Fagerstrom, K. The nicotine market: An attempt to estimate the nicotine intake from various sources and the total nicotine consumption in some countries. Nicotine Tob. Res 2005, 7, 343–350. [Google Scholar]
- Rose, JE; Mukhin, AG; Lokitz, SJ; Turkington, TG; Herskovic, J; Behm, FM; Garg, S; Garg, PK. Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarettes containing 11C-nicotine. Proc. Natl. Acad. Sci. USA 2010, 107, 5190–5195. [Google Scholar]
- Poulin, P; Theil, FP. Prediction of pharmacokinetics prior to in vivo studies. 1. Mechanism-based prediction of volume of distribution. J. Pharm. Sci 2002, 91, 129–156. [Google Scholar]
- Amidon, GL; Sinko, PJ; Fleisher, D. Estimating human oral fraction dose absorbed: A correlation using rat intestinal membrane permeability for passive and carrier-mediated compounds. Pharm. Res 1988, 5, 651–654. [Google Scholar]
Appendix A
Appendix B
Appendix C






| Parameter | Symbol | Nicotine | Cotinine | Unit |
|---|---|---|---|---|
| Octanol-water partition coefficient | logP | 0.930 | 0.040 | |
| Hepatic intrinsic clearance | CLh,int | 5.44 | 0.208 | L/h |
| Liver-plasma concentration ratio | Kp,h | 0.797 | 0.680 | - |
| Renal clearance | CLr | 0.0994 | 0.00421 | L/h |
| Plasma unbound fraction | fu,p | 0.688 | 0.743 | - |
| Ratio of the blood to plasma concentration | Rb | 1.00 | 1.00 | - |
| Volume of systemic circulation | V1 | 0.746 | 0.451 | L |
| Hepatic volume | Vh | 0.00850 | 0.00850 | L |
| Hepatic blood flow rate of systemic circulation to the tissue compartment | Qh | 0.853 | 0.853 | L/h |
| Absorption rate constant | ka | 1.07 | - | h−1 |
| Fraction absorbed × intestinal availability | FaFg | 1.00 | - | - |
| Dose | Dose | 0.25 | - | mg |
| Parameter | Symbol | Nicotine | Cotinine | Unit |
|---|---|---|---|---|
| Hepatic intrinsic clearance | CLh,int | 755 | 20.6 | L/h |
| Renal clearance | CLr | 4.25 | 0.180 | L/h |
| Volume of systemic circulation | V1 | 209 | 127 | L |
| Hepatic volume | Vh | 1.50 | 1.50 | L |
| Hepatic blood flow rate systemic circulation to the tissue compartment | Qh | 96.6 | 96.6 | L/h |
| Absorption rate constant | ka | 0.795 | - | h−1 |
| Dose | Dose | 70 | - | mg |
| Enzyme source | Clearance, μL/min/mg protein | L/h a |
|---|---|---|
| Rat livers, untreated a | 7.9 ± 1.4 | 0.142 |
| Rat livers, treated with nicotine b | 9.6 ± 1.9 | 0.173 |
| Pooled human livers | 6.7 | 24.0 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamazaki, H.; Horiuchi, K.; Takano, R.; Nagano, T.; Shimizu, M.; Kitajima, M.; Murayama, N.; Shono, F. Human Blood Concentrations of Cotinine, a Biomonitoring Marker for Tobacco Smoke, Extrapolated from Nicotine Metabolism in Rats and Humans and Physiologically Based Pharmacokinetic Modeling. Int. J. Environ. Res. Public Health 2010, 7, 3406-3421. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph7093406
Yamazaki H, Horiuchi K, Takano R, Nagano T, Shimizu M, Kitajima M, Murayama N, Shono F. Human Blood Concentrations of Cotinine, a Biomonitoring Marker for Tobacco Smoke, Extrapolated from Nicotine Metabolism in Rats and Humans and Physiologically Based Pharmacokinetic Modeling. International Journal of Environmental Research and Public Health. 2010; 7(9):3406-3421. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph7093406
Chicago/Turabian StyleYamazaki, Hiroshi, Kana Horiuchi, Ryohji Takano, Taku Nagano, Makiko Shimizu, Masato Kitajima, Norie Murayama, and Fumiaki Shono. 2010. "Human Blood Concentrations of Cotinine, a Biomonitoring Marker for Tobacco Smoke, Extrapolated from Nicotine Metabolism in Rats and Humans and Physiologically Based Pharmacokinetic Modeling" International Journal of Environmental Research and Public Health 7, no. 9: 3406-3421. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph7093406