Figure 8.
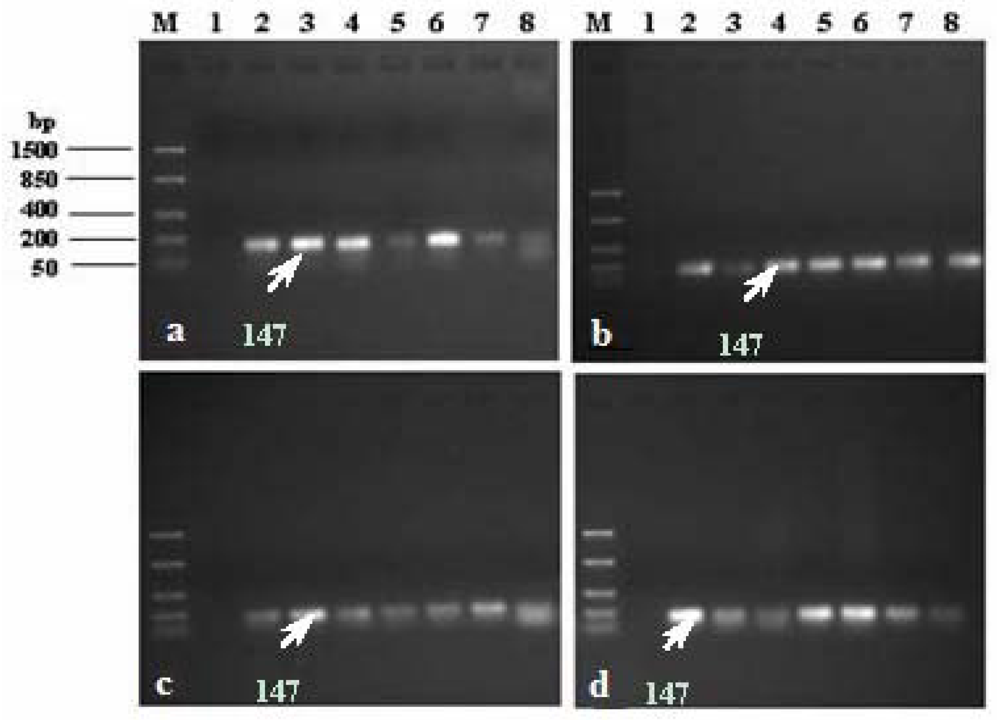
Amplification of uidA gene in E. coli. Lane M: DNA ladder, Lane 1: negative control, Lane 2: positive control: E. coli ATCC 25922 (10 μL), Lane 3: positive control: E. coli ATCC 25922 (5 μL), Lanes 4 to 8: presumptive E. coli colonies 1 to 5 isolated from raw water samples. Presumptive E. coli colonies isolated from (a) Apies River, (b) Hartbeespoort Dam, (c) Delmas borehole, and (d) Wallmannsthal borehole.
3.3.2. Bacterial Removal by Biosand Filter-Zeolite (BSF-Z)
Poor removal efficiency was observed when filtering synthetic water source through the BSF-Z, as
E. coli concentrations after treatment ranged between 2 Log
10 and 6 Log
10. However, complete removal of
E. coli and faecal coliforms was observed during the first and third trials, respectively, after filtering SWL of total volumes of 800 L and 760 L (
Figure 10a,b). During the filtration of highly turbid surface water, no faecal coliforms were detected during the first, second and sixth trials after filtering total volumes of 1120 L, 1140 L and 1220 L, respectively, while
E. coli were completely removed during the first three trials (
Figure 10a,b).
Trials with low-turbidity groundwater sources indicated no faecal coliforms during the first two and last two trials after adding total volumes of 880 L, 900 L and 960 L, 980 L, respectively, to the BSF-Z (
Figure 10a). Total removal of
E. coli was noted during the second, third and fourth trials after filtering total volumes of 900 L, 920 L and 940 L of low-turbidity groundwater, respectively, as well as in the second and third hours of the first trial (
Figure 10b). Adding up to 1060 L, 1080 L and 1100 L of highly turbid groundwater to the filter resulted in the total removal of faecal coliforms during the last three trials. As indicated in
Figure 10a, this bacterial group could also not be detected during specific hours of the first three trials after filtration of up to 1000 L, 1020 L and 1040 L. No
E. coli were detected during the last four trials after filtering total volumes of 1040 L, 1060 L, 1080 L and 1100 L, as well as during the second and third hours of the first two trials (
Figure 10b).
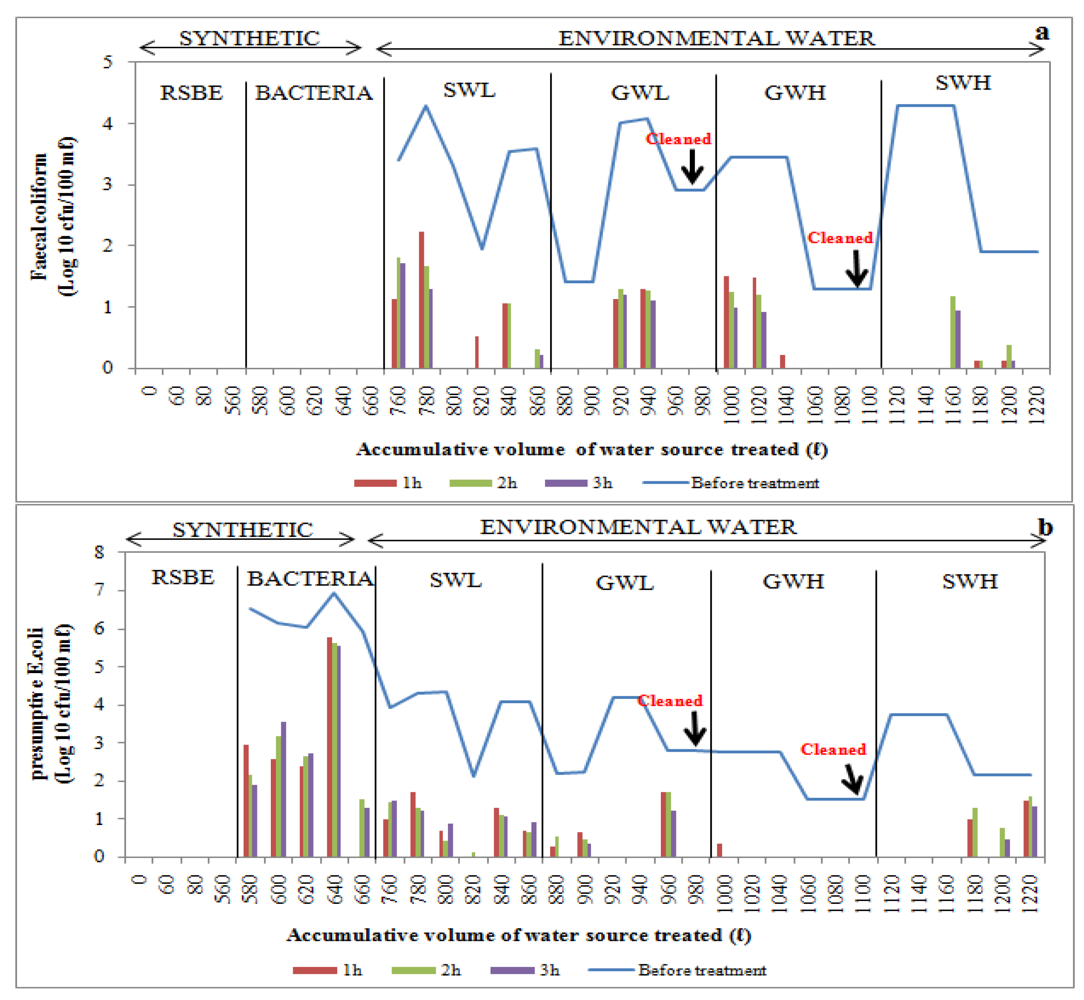
Figure 10.
Log10 faecal coliform (a) and E.coli (b) concentrations in biosand filter-zeolite before and after treatment of over 1 220 L of source water: synthetic water and environmental water samples (SWL, GWL, GWH and SWH). RSBE is the running of the system before the experiment, using deionised water.
Figure 10.
Log10 faecal coliform (a) and E.coli (b) concentrations in biosand filter-zeolite before and after treatment of over 1 220 L of source water: synthetic water and environmental water samples (SWL, GWL, GWH and SWH). RSBE is the running of the system before the experiment, using deionised water.
In general, the trend in the removal of target bacterial pathogens in BSF-S and BSF-Z was towards increased bacterial removal with repeated filtration of raw water sources. This shows that either the development of the biological layer (
Schmutzdecke) or the increase in the retention time with the filter media as the filtration rate decreases due to head-loss accumulation has an impact on bacterial removal [
40,
41,
42,
43]. Studies by Kaiser
et al. [
38] and Campos and his colleagues [
40] have also shown that bacterial removal efficiency improves with repeated filter use and increasing time of filtration, due to filter maturation.
3.3.3. Bucket Filter (BF)
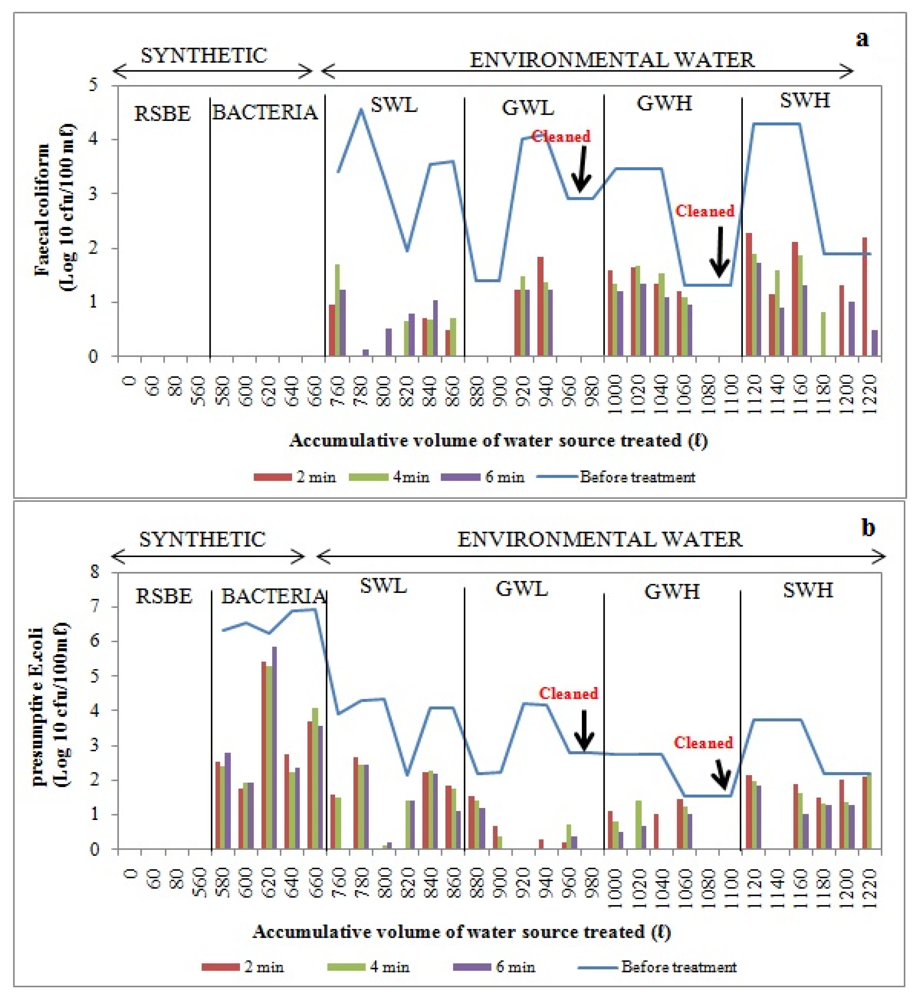
The removal of
E. coli from synthetic water source by BF was found to be poor, as can be seen in
Figure 11. With surface water sources, total removal of faecal coliforms was observed in the second and fourth hours of the second and third trials after filtering total volumes of 780 L and 800 L, respectively (
Figure 11a). No
E. coli were detected during the second and third trials after filtering up to 800 L and 820 L. The complete removal of
E. coli and faecal coliforms was achieved during the second trial and in the first and third hours of the fourth trial, respectively, after filtering total volumes of 1140 L and 1180 L (
Figure 11a,b).
During the first two trials and last two trials with low-turbidity groundwater, there was total removal of faecal coliforms after filtering up to 880 L, 900 L and 960 L, 980 L respectively of this water source through the bucket filter (
Figure 11a). Complete removal of
E. coli was observed during the fourth and sixth trials after filtering up to total volumes of 920 L and 980 L, respectively. No
E. coli were detected in the second and third hours of the fourth trial (
Figure 11b). During the treatment of highly turbid groundwater, faecal coliforms and
E. coli were totally removed during the last two trials after filtering total volumes of 1080 and 1100 L (
Figure 11a,b).
Figure 11.
Log10 faecal coliform (a) and E.coli (b) concentrations in bucket filter before and after treatment of over 1220 L of source water: synthetic water and environmental water samples (SWL, GWL, GWH and SWH). RSBE is the running of the system before the experiment, using deionised water.
Figure 11.
Log10 faecal coliform (a) and E.coli (b) concentrations in bucket filter before and after treatment of over 1220 L of source water: synthetic water and environmental water samples (SWL, GWL, GWH and SWH). RSBE is the running of the system before the experiment, using deionised water.
Overall, the bucket filter showed the poorest removal of faecal coliforms [2–3 log
10 (99–99.9%)] and
E. coli [1–3 log
10 (90–99.9%)], as no biological layer develops on the top surface of the sand bed in a rapid sand filter to enhance the removal of bacterial pathogens (
Table 6). The bucket filter does not have a resting water level as the biological sand filters have, and dries up. Thus, conditions in a bucket filter are not favourable for the development of a biological layer [
9,
34]. This clearly explains its poor performance in bacterial removals compared to the biosand filters. As Sobsey [
9] reported that bucket filters are not expected to reduce bacteria by more than 90%, approximately 50% reduction was expected. However, the BF filter in this study removed upto 99.9%. The high performance of this rapid sand filter can be attributed to the particle size of sand used (0.3 mm), which is smaller than the size reported by Sobsey (1–3 mm) [
9].
Table 6.
Average percentage bacterial removal efficiency of HWTS from test water samples.
Table 6.
Average percentage bacterial removal efficiency of HWTS from test water samples.
| Devices | Faecal coliforms, Log10 cfu/100 mL removal efficiency (%) n = 18 |
|---|
| SWL | SWH | GWL | GWH |
|---|
| BSF-Standard | 2.7 (99.7) | 3.7 (>99.9) | 2.2 (99.2) | 3.4 (>99.9) |
| BSF-Zeolite | 3.0 (99.9) | >4.0 (100) | 2.7 (99.7) | >3.6 (100) |
| BF | 2.7 (99.7) | 2.3 (99.3) | 2.0 (99) | 2.4 (99.4) |
| CCF | 3.3 (>99.9) | 3.2 (>99.9) | 2.5 (99.5) | 2.6 (99.6) |
| SIPP | >4.0 (100) | >4.0 (100) | >3.0 (100) | >3.6 (100) |
| Devices | E. coli |
| Synthetic water n = 15 | Environmental water samples n = 18 |
| SWL | SWH | GWL | GWH |
| BSF-Standard | 3.6 (99.9) | 2.4 (99.4) | 3.1 (>99.9) | 1.6 (96) | >3.7 (100) |
| BSF-Zeolite | 3.2 (>99.9) | 2.4 (99.4) | 3.0 (99.9) | 1.3 (93) | >3.7 (100) |
| BF | 3.3 (>99.9) | 1.5 (95) | 2.2 (99.2) | 1.8 (98) | 3.0 (99.9) |
| CCF | 3.2 (>99.9) | 1.8 (98) | 3.2 (>99.9) | >2.5 (99.5) | 2.9 (99.8) |
| SIPP | >6.6 (100) | >3.5 (100) | >4.0 (100) | >2.5 (100) | >3.7 (100) |
3.3.5. Silver-Impregnated Porous Pot (SIPP)
This device consistently produced high-quality water that did not contain any traces of faecal coliform
or E. coli during each hourly interval for each day of the filter run throughout the study period (
Table 7). The device removed between 2.5 Log
10 and 6.6 Log
10 of these coliform bacteria from contaminated water. The results for SIPP are presented in
Table 7, as no bacteria were detected in any of the treated water samples.
Table 7.
Average bacterial removal efficiency by SIPP from various water samples.
Table 7.
Average bacterial removal efficiency by SIPP from various water samples.
| Water source | Number of trials | Bacterial species | Log10 before treatment | Log10 removal |
|---|
| Synthetic water | 5 | E. coli | 6.6 Log | >6.6 Log |
| SWL | 6 | E. coli | 4.1 Log | >4.1 Log |
| Faecal coliforms | 3.9 Log | >3.9 Log |
| SWH | 6 | E. coli | 3.5 Log | >3.5 Log |
| Faecal coliforms | 4.0 Log | >4.0 Log |
| GWL | 6 | E. coli | 3.7 Log | >3.7 Log |
| Faecal coliforms | 3.6 Log | >3.6 Log |
| GWH | 6 | E. coli | 2.5 Log | >2.5 Log |
| Faecal coliforms | 3.1 Log | >3.1 Log |
The average faecal coliform and
E. coli removal and percentage removal efficiency by HWTS devices from the various water sources are summarised in
Table 6. The SIPP was the only device that consistently produced drinking water complying with the recommended limits set by the
South African National Standard (SANS) 241 Drinking Water Specification [
16], regardless of the quality of the source water treated. The percentage removal of
E. coli observed in the other four devices when treating synthetic water was >99.9%. The removal of target bacteria from the environmental water samples ranged between 2–4 Log
10 (99.9% to >99.99%), 1–4 Log
10 (90% to >99.99%), 1–3 Log
10 (90% to 99.9%) and 2–3 Log
10 (90% to 99.9%) for the BSF-S, BSF-Z, BF and CCF, respectively (
Table 7). All devices removed
E. coli within similar ranges or in some cases above the ranges reported by Elliott
et al. [
35], Baumgartner [
42] and Laurent [
43]. Biosand filters have been reported to remove
E. coli in the rate ranging between 85% and 98% [
35,
40,
41]. Chemically modified rapid sand filters may remove between 80% and 95% [
43]. The removal of bacteria by the SIPP and CCF was observed to be >99% for the duration of the study. The performance of these two filters was within the ranges reported by previous investigators. The ceramic candle filter and silver-coated ceramic pot filters have been shown to remove
E. coli in ranges between 80% and 99%, and between 95% and 100%, respectively [
44,
45].
As stated above, the preliminary investigation on the performance of SIPP in removing pathogenic organisms from synthetic water sources was conducted in our laboratory by Momba and co-authors [
29].
Escherichia coli spiked in synthetic water were used as a prototype organism and the authors included a control porous pot that was not impregnated with AgNO
3. It was revealed that only the Ag impregnated pot was significantly effective in removing this organism [
30]. The more efficient bacterial performance of the SIPP as compared to the control porous pot was attributed to the imbedded Ag nanoparticles in the micropores [
29]. The silver leached from the SIPP pot was at a level of between 0.5 and 0.6 mg/L, higher than the WHO recommendation of 0.1 mg/L [
31]. The Ag elution was greatest in the early stages (within the first 5 L) but appeared to begin to stabilize after filtering up to 10 L of contaminated synthetic water. The SIPP, however, displayed a more gradual reduction for Ag released into the water [
29].
Similar observations were also noted in the current study. The SIPP filter was found to be the most efficient device in removing faecal coliforms and
E. coli from all test water source samples, as it reduced the maximum
E. coli concentration of up to 6.6 log
10 in spiked water to 0 cfu/100 mL (
Table 6,
Table 7). This corroborated the findings of Van Halem and co-workers [
44], who determined that the highest possible reduction of
E. coli by the silver-impregnated ceramic pot filter was 7 log
10. The SIPP filter showed elevated silver concentrations in the filtrate from the first and the second run with synthetic water (
Table 8). Although the concentration of the Ag in the filtrate gradually dropped to 0.22 mg/L after filtration of groundwater and surface water sources up to a total volume of 320 L, this value was still above the WHO recommended limits [
31]. Though SIPP filter displayed high levels of Ag elution above the level recommended by the WHO, independent studies have indicated the relative non-toxicity of Ag and its health benefits [
46].
Table 8.
Silver concentration in filtered water.
Table 8.
Silver concentration in filtered water.
| Filter runs | Silver concentration in filtered water (mg/L) |
|---|
| Time intervals |
|---|
| 1 h | 2 h | 3 h |
|---|
| Run 1: Deionised water | 0.982 | 0.812 | 0.537 |
| Run 2: Groundwater | 0.247 | 0.230 | 0.218 |
| Run 3: Surface water | 0.240 | 0.256 | 0.280 |
| WHO Limit [31] | 0.1 mg/L |
The bacterial removal efficiency of the SIPP filter in the present study can be due to straining of bacteria through the fine pores of the clay pot [
22,
23]. It is also possible that the removal efficiency could have been enhanced by the AgNO
3 that was embedded in the clay during the manufacturing process. The longer disinfection capacity of the SIPP could be attributed to the presence of the silver ion since independent studies have corroborated the effect of Ag in a water purification application, irrespective of substrate [
47,
48,
49,
50]. Silver has bacteriostatic properties and inactivates bacteria by disrupting the disulphide bonds of proteins in the cell membrane or by inhibiting DNA replication [
23,
44,
51]. It is thus possible that the silver is contributing to the removal of bacteria. However, in future a study involving the comparison of the performance of the SIPP to a non-silver clay pot should be included to give a clear indication of whether the silver enhances the performance of the clay pot filter. Future studies should also include a long-term study whereby the concentration of silver in water filtered by SIPP is monitored on a weekly basis over 12 months or more. This would also indicate how the SIPP performs as the silver in leached out.
Table 9.
Comparing the performance of devices.
Table 9.
Comparing the performance of devices.
| Filter | Water source | Flow rate | Turbidity | Faecal coliforms | E. coli |
|---|
| SIPP vs. CCF | SWL | 0.0002 | 0.0004 | 0.0000 | 0.0000 |
| SWH | 0.0061 | 0.0000 | 0.0000 | 1.0000 |
| GWL | 0.0000 | 0.0804 | 0.0012 | 0.0000 |
| GWH | 0.0000 | 0.0000 | 0.0000 | 0.0012 |
| SIPP vs. BSF-S | SWL | 0.0000 | 0.5150 | 0.0000 | 0.5950 |
| SWH | 0.0000 | 0.0050 | 1.0000 | 1.0000 |
| GWL | 0.0035 | 0.0260 | 0.0340 | 0.8980 |
| GWH | 0.0000 | 0.0000 | 0.9890 | 1.0000 |
| SIPP vs. BSF-Z | SWL | 0.0000 | 0.8720 | 0.2890 | 0.9730 |
| SWH | 0.0000 | 1.0000 | 1.0000 | 1.0000 |
| GWL | 0.0000 | 0.9940 | 1.0000 | 0.9930 |
| GWH | 0.0000 | 0.0000 | 1.0000 | 1.0000 |
| SIPP vs. BF | SWL | 0.0000 | 0.0000 | 0.0010 | 0.0000 |
| SWH | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| GWL | 0.0000 | 0.0000 | 0.0020 | 0.0000 |
| GWH | 0.0000 | 0.0000 | 0.0000 | 0.8410 |
| BSF-S vs. BSF-Z | SWL | 0.0000 | 0.0002 | 0.1872 | 0.2005 |
| SWH | 0.0000 | 0.0000 | 0.6774 | 1.0000 |
| GWL | 0.0000 | 0.0267 | 0.1391 | 0.0020 |
| GWH | 0.0000 | 0.0000 | 0.5526 | 0.4004 |
Due to its highly effective performance, the SIPP was statistically compared to the other selected devices. The removal of faecal coliforms and
E. coli by the CCF and BF was found to be significantly lower than that of the SIPP (
p < 0.05) (
Table 9). This was expected, as the first two devices lack a disinfectant that is able to inactivate bacteria at a rate comparable to that of the SIPP device. In the CCF, bacterial pathogens are removed through mechanical trapping and adsorption [
45,
52]. Mechanical trapping takes place in two ways. The first is through the surface filtration, whereby particles that are too large to pass through the pore are trapped on the surface of the candle. The second is through the depth filtration, where particles that penetrate the candle filter are trapped throughout the depth of the candle wall [
52]. In the BF, the removal of particles, including bacteria, occurs through physical trapping with deeper penetration into the sand bed [
9]. However, the microbiological quality of water filtered through the BF (rapid sand filter) has been revealed to be poor, as no biological layer develops on the top surface of the sand bed [
9,
53] to remove bacterial pathogens. The statistical analysis showed that both the BSF-S and BSF-Z performed similarly to the SIPP in removing the indicator bacteria from environmental water samples (
p > 0.05). However, the experimental data indicated that SIPP was the best device overall, as the average removal of these bacteria from environmental water samples by the BSF-S and BSF-Z devices ranged between 96% to 99.9% while removal of bacteria by the SIPP was >99.99% throughout the study. The average removal of faecal coliforms ranged between 2.2 to 3 log
10 or 99.2% to >99.9% for BSF-S and 3 to >3.7 log
10 or 99.9% to 100% for BSF-Z, whereas the average removal of
E. coli from raw water ranged between 1.6 to 4 log
10 (96% to 100%) for BSF-S and between 1.3 to 4 log
10 (93% to 100%) for BSF-Z (
Table 6).
The removal efficiencies obtained from this study are much higher than those reported by Stauber
et al. [
41] and Ngai
et al. [
53] for biosand filters, namely between 60% and 100%. This implies that the modified biosand filters BSF-S and BSF-Z are more efficient. The more efficient performance of the BSF-S can be attributed to the fine sand (0.15 mm) used which enhanced straining of particles due to the formation of finer pores as compared to conventional biosand filters [
13,
17]. The high performance of the BSF-Z can be a result of the antimicrobial properties of zeolites reported by Princz
et al. [
18], Kallo and Ming [
19] and Widiastuti
et al. [
20]. Statistical analysis showed that there was a significant difference (
p < 0.05) in the removal of
E. coli from surface water of high turbidity by BSF-S and BSF-Z. The BSF-Z showed higher removal efficiency than BSF-S (
Figure 9 and
Figure 10). However these biosand filters performed similarly in removing
E. coli from all groundwater samples and from surface water of low turbidity (
p > 0.05) and faecal coliforms from all test water samples (
Table 9). The removal of bacteria from raw water by biosand filters occurs by a combination of physical and biological processes [
54]. The biological process involves a predation process, whereby algae and protozoa that develop on the surface of the top layer of sand within four to six weeks of filter use consume incoming bacteria; these organisms, along with slime or biofilm, comprise the
Schmutzdecke or biological layer [
41,
53,
54]. A great improvement in the removal of indicator bacteria was noted in both the BSF-S and BSF-Z as contaminated raw water was progressively treated. The bacterial removal efficiency was higher during filtration of surface water of high turbidity (SWH) compared to surface water of low turbidity (SWL) which was the first environmental water sample to be treated (
Figure 9 and
Figure 10). This can be attributed to the maturation of the biological layer that occurs with increased filter use [
35,
38]. The biological layer is also responsible for the decline in flow rate as the pores become clogged due to the accumulation of dirt in the sand bed. This increases the contact time between filter media and contaminated water thereby enhancing the performance of biosand filters over time [
54]. Statistical analysis was also performed to compare the bacterial removal efficiency of the one-, two- and three-hour intervals of filtration for each filter. No significant differences (
p > 0.05) were found between these hourly intervals across all five devices and the multiple water sources filtered. This finding implies that water for drinking can be collected at any time from these devices. However, it should be kept in mind that only water from the SIPP can be collected and consumed directly after filtration. The Water from the CCF, BSF-S, and BSF-Z filters would require a disinfection step to render their water safe for consumption.
Turbidity is a measure of the clarity of a liquid and was once considered mainly an aesthetic characteristic of drinking water [
36,
55]. However, some studies have shown that controlling turbidity is a key step in eradicating pathogens from drinking water, as microorganisms can be embedded or attached to the surface of larger suspended particles. Therefore, the removal of the larger particles could result in the removal of pathogenic microorganisms [
56]. Correlations between turbidity reduction and bacterial reductions were performed to determine the relationship between the turbidity and the bacterial concentration of a water sample. The results from our study show that there is a direct relationship (as the turbidity was reduced, the indicator bacteria were also reduced) between turbidity and bacterial removal as strong positive correlations were observed between the removal of
E. coli and turbidity from groundwater of low turbidity by the BSF-S (
r = 0.761) and BSF-Z (
r = 0.780). LeChevallier and Norton [
55] and Fox [
56] who reported that water samples with a lower turbidity are associated with lower microbial counts and vice versa substantiate these findings. However, strong negative correlations were observed between the removal of turbidity and removal of faecal coliforms and
E. coli from SWH and GWH by the BSF-S (
r = −0.721), BSF-Z (
r = −0.796) and CCF (
r = −0.784), respectively (
Table 10). These findings imply that an indirect relationship existed between the turbidity and bacterial concentration (as the turbidity was reduced by these devices, the concentration of faecal coliforms or
E. coli increased and
vice versa). However, from the graphs it can be seen that 1–3 log (90%–99.9%) reduction of the microorganisms was achieved (
Figure 9a,
Figure 10a). It is possible that the turbidity value remained high even though high bacterial removal efficiencies were observed. This can be explained by the fact that organic and inorganic materials, slit, clay and soluable coloured organic particles largely contribute to the turbidity of a water source, as opposed to bacteria or viruses, which are small and translucent [
55].
Table 10.
Pearson’s correlation values show relationship between removal of source water turbidity and bacterial removal efficiency of HWTS devices.
Table 10.
Pearson’s correlation values show relationship between removal of source water turbidity and bacterial removal efficiency of HWTS devices.
| Organism | Devices | Environmental water samples (correlation coefficient: r) |
| SWL | SWH | GWL | GWH |
| R | p | R | P | r | p | R | p |
| Faecal coliforms | BSF-S | −0.294 | 0.002 | −0.721 | 0.000 | −0.041 | 0.001 | 0.481 | 0.003 |
| BSF-Z | −0.044 | 0.043 | −0.796 | 0.023 | 0.541 | 0.000 | 0.255 | 0.001 |
| BF | −0.187 | 0.001 | 0.538 | 0.000 | −0.126 | 0.000 | 0.037 | 0.000 |
| CCF | −0.486 | 0.000 | - | - | 0.452 | 0.000 | −0.784 | 0.000 |
| SIPP | - | - | - | - | - | - | - | - |
| Organism | Devices | Environmental water samples (correlation coefficient: r) |
| SWL | SWL | SWL | SWL |
| R | p | R | P | r | p | R | p |
| E. coli | BSF-S | −0.099 | 0.000 | 0.041 | 0.000 | 0.761 | 0.001 | - | 0.002 |
| BSF-Z | 0.285 | 0.000 | 0.315 | 0.000 | 0.780 | 0.000 | - | - |
| BF | −0.102 | 0.000 | 0.328 | 0.000 | −0.021 | 0.000 | −0.231 | 0.000 |
| CCF | 0.267 | 0.000 | −0.453 | 0.000 | −0.192 | 0.000 | −0.877 | 0.001 |
| SIPP | - | - | - | - | - | - | - | - |