Genotoxicity of Silver Nanoparticles in Vicia faba: A Pilot Study on the Environmental Monitoring of Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles
2.2. Test System and Treatment
2.3. Plant as Test Material-Vicia faba

2.3.1. Storage of Seeds:
2.3.2. Growing of Lateral Roots from Seeds
2.3.3. Chromosomal Aberration Assay
2.3.4. Fixation and Staining of Root Tips
2.3.5. Scoring of Slides
2.3.6. Micronucleus Test
2.3.7. Statistical Analysis
3. Results
3.1. Nanomaterial Characterization
| Concentration (mg/L) | Release of Ag+ ion: | |
|---|---|---|
| Exposure to Ag | Exposure to Ag NPs | |
| Control | n | n |
| 12.5 | 1.2 | 1.2 |
| 25 | 30.4 | 49.2 |
| 50 | 60.5 | 121.8 |
| 100 | 228.6 | 403.9 |
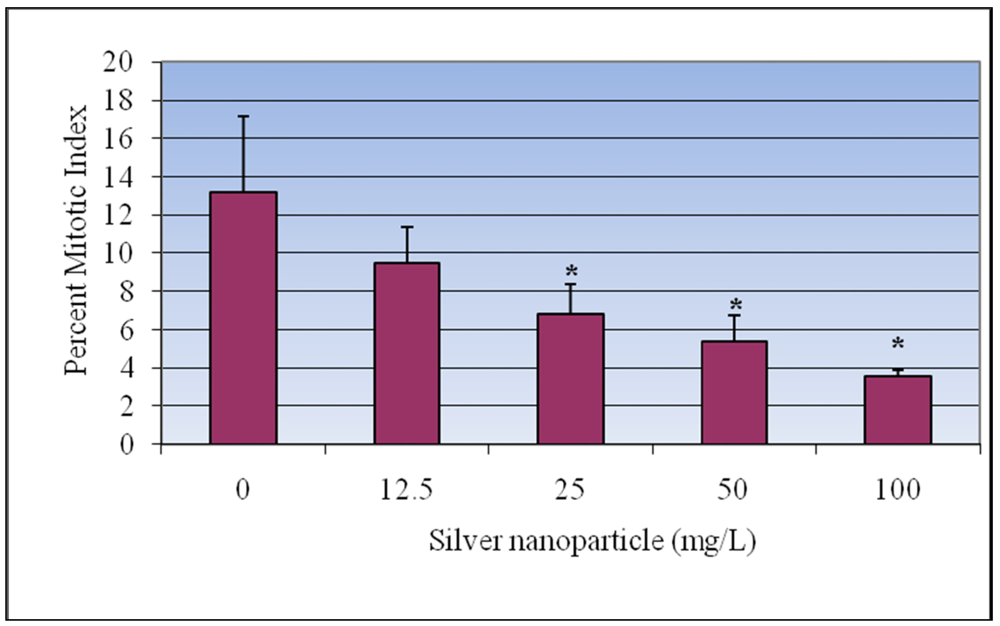
3.2. Mitotic Index
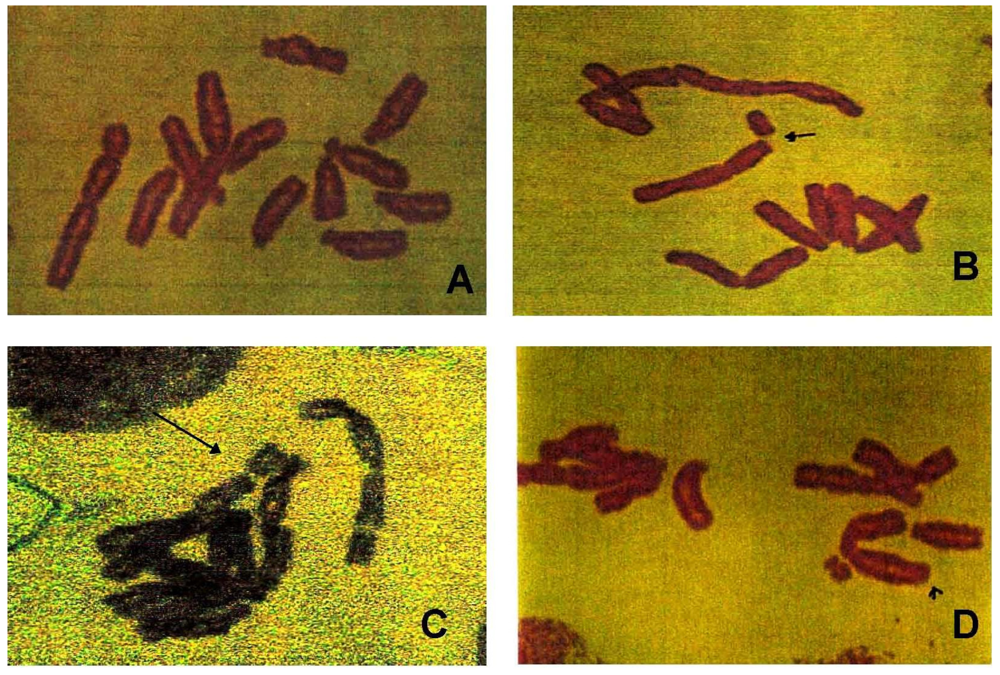
3.3. Chromosomal Aberrations
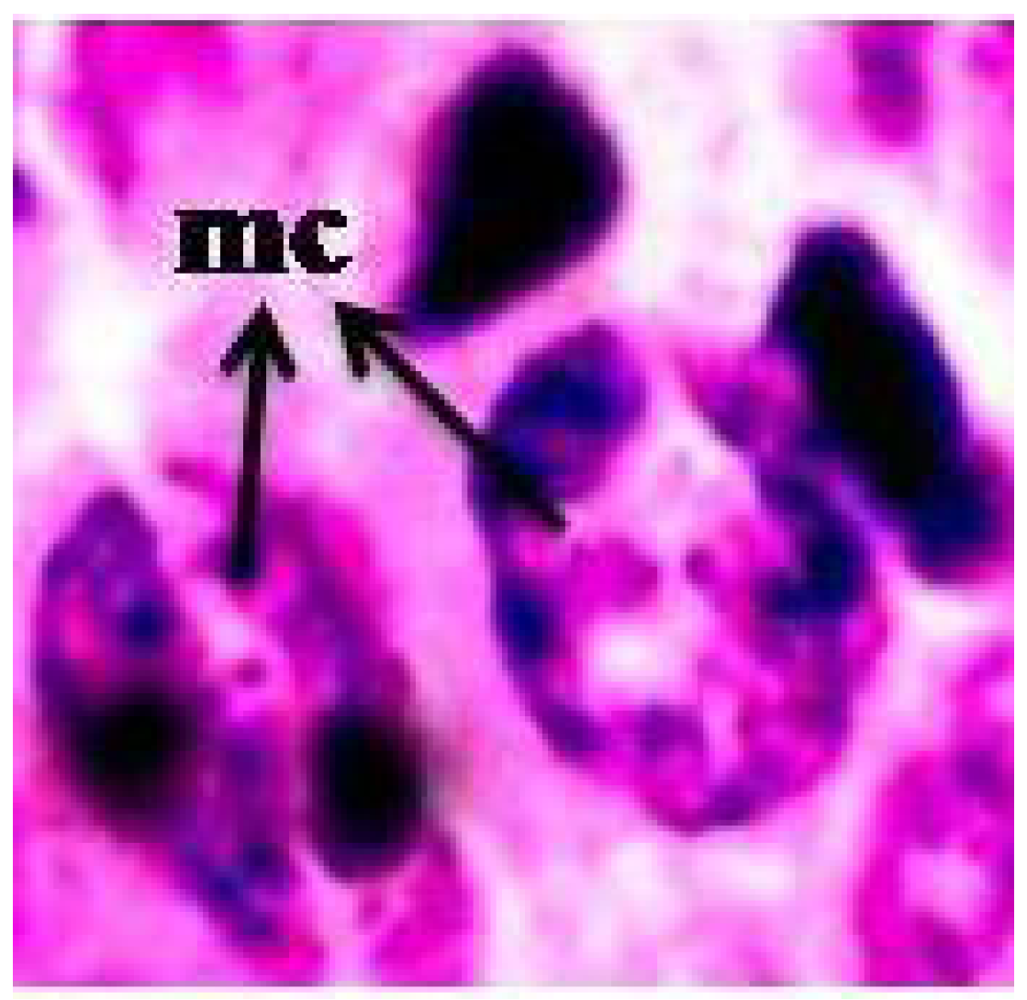
3.4. Micronucleus Assay



4. Discussion
5. Conclusions
Acknowledgements
References
- Lam, C.W.; James, J.T.; McCluskey, R.; Hunter, R.L. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004, 77, 126–134. [Google Scholar]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Hummel, S.; Technau, A.; Froelich, K.; Ginzkey, C.; Koehler, C.; Hagen, R.; Kleinsasser, N. Silver nanoparticles: Evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol. Lett. 2011, 201, 27–33. [Google Scholar] [CrossRef]
- Oberdörster, G. Effects of Ultrafine Particles in the Lung and Potential Relevance to Environmental Particles. In Aerosol Inhalation; Marijnissen, J.M.C., Gradon, L., Eds.; Kluwer Academic: Dordrecht, The Netherland, 1996; p. 165. [Google Scholar]
- Arora, S.; Jain, J.; Rajwade, J.; Paknikar, K. Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol. Lett. 2009, 179, 93–100. [Google Scholar]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol. In Vitro 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar]
- Lam, C.W.; James, J.T.; McCluskey, R.; Arepalli, S.; Hunter, R.L. A review of carbon nanotube toxicity and assessment of potential and environmental health risks. Crit. Rev. Toxicol. 2006, 36, 189–217. [Google Scholar]
- Patlolla, A.; Patlolla, B.; Tchounwou, P. Evaluation of cell viability, DNA damage, and cell death in normal human dermal fibroblast cells induced by functionalized multiwalled carbon nanotube. Mol. Cell Biochem. 2010, 338, 225–232. [Google Scholar] [CrossRef]
- Patlolla, A.K.; Hussain, S.M.; Schlager, J.J.; Patlolla, S.; Tchounwou, P.B. Comparative study of the clastogenicity of functionalized and non-functionalized multi-walled carbon nanotubes in bone marrow cells of Swiss-Webster mice. Environ. Toxiciol. 2010, 25, 608–621. [Google Scholar]
- Soto, K.F.; Carrasco, A.; Powell, T.G.; Garza, K.M.; Murr, L.E. Comparative in vitro cytotoxicity assessment of some manufactured nanoparticulate materials characterized by transmission electron microscopy. J. Nanopart. Res. 2005, 7, 145–169. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; Kwon, I.H.; Jeong, J.; Han, B.S.; Yu, I.J. Twenty-eight day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef]
- AshaRani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar]
- Rahman, M.F.; Wang, J.; Patterson, T.A.; Saini, U.T.; Robinson, B.L.; Newport, G.D.; Murdock, R.C.; Schlager, J.J.; Hussain, S.M.; Ali, S.F. Expression of genes related to oxidative stress in mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009, 187, 15–21. [Google Scholar]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Brandich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. 2008, 112, 13608–13619. [Google Scholar]
- Vinardell, M.P. In vitro cytotoxicity of nanoparticles in mammalian germ-line stem cell. Toxicol. Sci. 2005, 88, 285–286. [Google Scholar] [CrossRef]
- Kim, S.; Choi, E.J.; Choi, J.; Chung, K.; Park, K.; Yi, J. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol. In Vitro 2009, 23, 1076–1084. [Google Scholar]
- Zanette, C.; Pelin, M.; Crosera, M.; Adami, G.; Bovenzi, M.; Larese, F.F.; Florio, C. Silver nanoparticles exert a long-lasting antiproliferative effect on human keratinocyte HaCaT cell line Toxicol. In Vitro 2011, 25, 1053–1060. [Google Scholar]
- Mamta, K.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef]
- Oberdörster, E. Manufactured nanomaterials (fullerenes, C60) induce oxidative stress in the brain of juvenile Largemouth Bass. Environ. HealthPerspect. 2004, 112, 1058–1062. [Google Scholar]
- Ahmed, M.; Posgai, R.; Gorey, T.J.; Nielsen, M.; Hussain, S.M.; Rowe, J.J. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol. Appl. Pharmacol. 2010, 242, 263–269. [Google Scholar] [CrossRef]
- USEPA, Nanotechnology White Paper; Science Policy Council, USEPA: Washington, DC, USA, 2007.
- Lu, C.M.; Zhang, C.Y.; Wen, J.Q.; Wu, G.R.; Tao, M.X. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2002, 21, 168–172. [Google Scholar]
- Hong, F.S.; Yang, F.; Liu, C.; Gao, Q.; Wan, Z.G.; Gu, F.G.; Wu, C.; Ma, Z.N.; Zhou, J.; Yang, P. Influences of nano-TiO2 on the chloroplast aging of spinach under light. Biol. Trace Elem. Res. 2005, 104, 249–260. [Google Scholar]
- Hong, F.S.; Zhou, J.; Liu, C.; Yang, F.; Wu, C.; Zheng, L.; Yang, P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem.Res. 2005, 105, 269–279. [Google Scholar]
- Yang, L.; Watts, D.J. Particle surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005, 158, 122–132. [Google Scholar]
- Zheng, L.; Hong, F.S.; Lu, S.P.; Liu, C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005, 104, 83–91. [Google Scholar]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar]
- Tripathy, A.; Chandrasekran, N.; Raichur, A.M.; Mukherjee, A. Antibacterial applications of silver nanoparticles synthesized by aqueous extract of Azadirachta indica (Neem) leaves. J. Biomed. Nanotechnol. 2008, 4, 1–6. [Google Scholar]
- Luoma, S.N. Silver Anotechnologies and the Environment: Old Problems or New Challenges? The Project of Emerging Nanotechnologies: Washington, DC, USA, 2008. [Google Scholar]
- Grant, W.F. Chromosome aberration assays in Allium. A report of the US Environmental Protection Agency gene-Tox Program. Mutat. Res. 1982, 99, 273–291. [Google Scholar]
- Ma, T.H.; Xu, Z.; Xu, C.; McConnell, H.; Rabago, E.V.; Arreola, G.A.; Zhang, H. The improved Allium/Vicia root tip micronucleus assay for clastogenicity of environmental pollutants. Mutat. Res. 1995, 334, 185–195. [Google Scholar]
- Ma, T.H. Vicia cytogenetic tests for environmental mutagens. A report of the US Environmental Protection Agency Gene-Tox Program. Mutat Res. 1982, 99, 259–271. [Google Scholar]
- Kanaya, N.; Gill, B.S.; Grover, I.S.; Murin, A.; Osiecka, R.; Sandhu, S.S.; Andersson, H.C. Vicia faba chromosomal aberration assay. Mutat. Res. 1994, 310, 231–247. [Google Scholar] [CrossRef]
- Smaka-Kincl, V.; Stegnar, P.; Lovka, M.; Toman, J.M. The evaluation of waste, surface and ground water quality using the Allium test procedure. Mutat. Res. 1996, 368, 171–179. [Google Scholar] [CrossRef]
- Webster, P.L.; Davidson, D. Changes in the duration of mitotic cycle induced by colchicine and indole-3-yl acetic acid in V. faba roots. J. Exp. Bot. 1969, 20, 671–685. [Google Scholar] [CrossRef]
- Macleod, R.D. Some effects of 2,4,5-trichlorophenoxy acetic acid on the mitotic cycle of lateral root apical meristems of V. faba. Chromosoma 1969, 27, 327–337. [Google Scholar] [CrossRef]
- Kumari, M.; Khan, S.S.; Pakrashi, S.; Mukherjee, A.; Chandrasekaran, N. Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. J. Hazard Mater. 2011, 90, 613–621. [Google Scholar]
- Topaktas, M.; Renciizogullar, E. Chromosomal aberrations in cultured human lymphocytes treated with Marshal and its effective ingredient Carbosulfan. Mutat. Res. 1993, 319, 103–111. [Google Scholar]
- Kihlman, B.A.; Anderson, H.C. Root-Tips of Vicia faba for the Study of the Induction of Chromosomal Aberrations and Sister-Chromatid Exchanges. In Handbook of Mutagenecity Test Procedures; Kilbey, B.J., Legator, M.S., Nichols, W., Ramel, C., Eds.; Elsevier: Amsterdam, The Netherland, 1984; pp. 531–554. [Google Scholar]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Mistra, C.; Sempf, J.M. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar]
- Chen, M.; Von Mickecz, A. Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticles. Exp. Cell Res. 2005, 305, 51–62. [Google Scholar]
- Racuciu, M.; Creanga, D.E. TMA-OH coated magnetic nanoparticles internalized in vegetal tissue. Rom. J. Phys. 2007, 52, 367–374. [Google Scholar]
- Panda, K.K.; Achary, M.M.; Krishnaveni, R.; Padhi, B.K.; Sarangi, S.N.; Sahu, S.N.; Panda, B.B. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. In Vitro 2011, 25, 1097–1105. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Patlolla, A.K.; Berry, A.; May, L.; Tchounwou, P.B. Genotoxicity of Silver Nanoparticles in Vicia faba: A Pilot Study on the Environmental Monitoring of Nanoparticles. Int. J. Environ. Res. Public Health 2012, 9, 1649-1662. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph9051649
Patlolla AK, Berry A, May L, Tchounwou PB. Genotoxicity of Silver Nanoparticles in Vicia faba: A Pilot Study on the Environmental Monitoring of Nanoparticles. International Journal of Environmental Research and Public Health. 2012; 9(5):1649-1662. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph9051649
Chicago/Turabian StylePatlolla, Anita K., Ashley Berry, LaBethani May, and Paul B. Tchounwou. 2012. "Genotoxicity of Silver Nanoparticles in Vicia faba: A Pilot Study on the Environmental Monitoring of Nanoparticles" International Journal of Environmental Research and Public Health 9, no. 5: 1649-1662. https://0-doi-org.brum.beds.ac.uk/10.3390/ijerph9051649





