WNT8B as an Independent Prognostic Marker for Nasopharyngeal Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Specimens
2.2. Tissues Microarray Construction
2.3. Immunohistochemistry
2.4. IHC Scoring
2.5. Statistical Analysis
3. Results
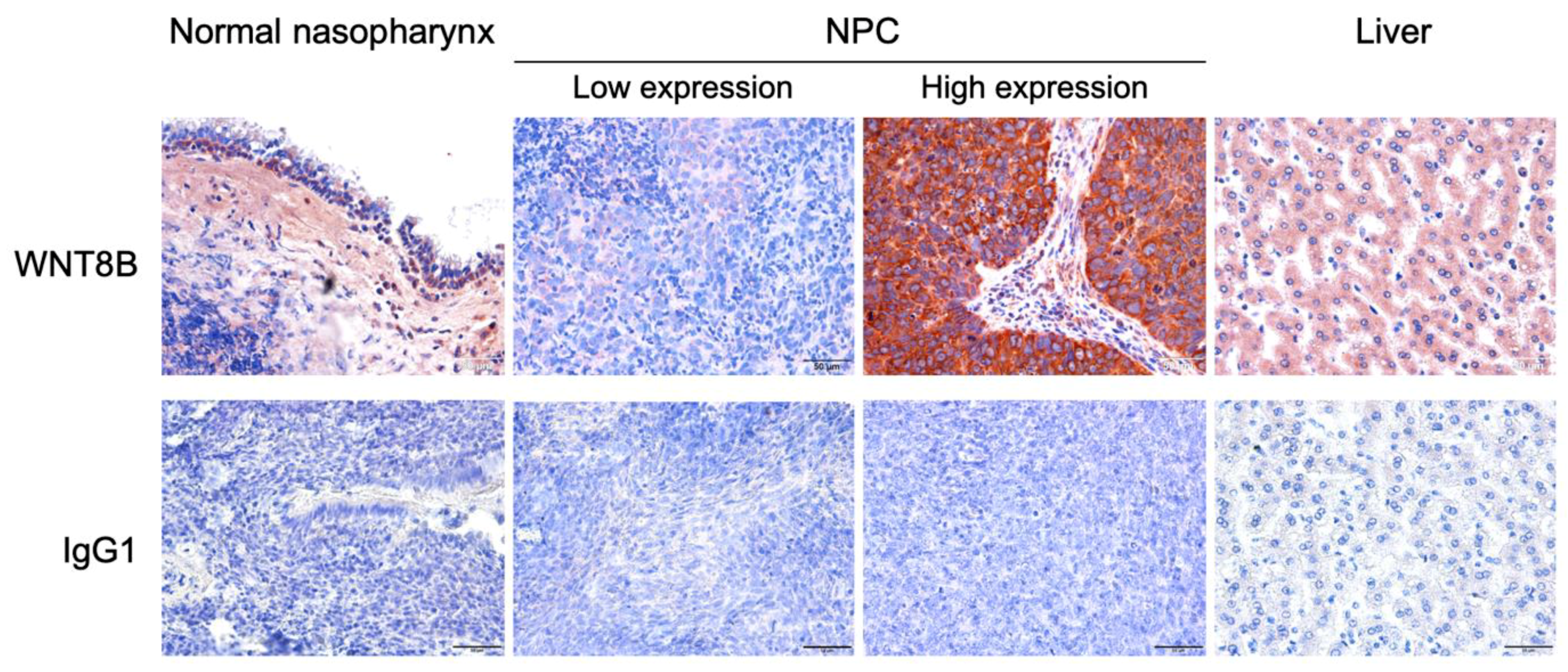
3.1. WNT8B Expression in NPC and Normal Nasopharyngeal Tissues
3.2. Correlation of WNT8B and Clinicopathological Features
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Mahdavifar, N.; Ghoncheh, M.; Mohammadian-Hafshejani, A.; Khosravi, B.; Salehiniya, H. Epidemiology and inequality in the incidence and mortality of nasopharynx cancer in Asia. Osong Public Health Res. Perspect. 2016, 7, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Poirier, S.; Hubert, A.; De-Thé, G.; Ohshima, H.; Bourgade, M.C.; Bartsch, H. Occurrence of volatile nitrosamines in food samples collected in three high-risk areas for nasopharyngeal carcinoma. IARC Sci. Publ. 1987, 1987, 415–419. [Google Scholar]
- Polesel, J.; Franceschi, S.; Talamini, R.; Negri, E.; Barzan, L.; Montella, M.; Libra, M.; Vaccher, E.; Franchin, G.; La Vecchia, C.; et al. Tobacco smoking, alcohol drinking, and the risk of different histological types of nasopharyngeal cancer in a low-risk population. Oral Oncol. 2011, 47, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liebowitz, D.; Kieff, E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell 1985, 43, 831–840. [Google Scholar] [CrossRef]
- Chang, E.T.; Adami, H.-O. The Enigmatic Epidemiology of Nasopharyngeal Carcinoma. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1765–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardo, T.; Prattapong, P.; Ngernsombat, C.; Wisetyaningsih, N.; Iizasa, H.; Yoshiyama, H.; Janvilisri, T. Epstein-Barr Virus Mediated Signaling in Nasopharyngeal Carcinoma Carcinogenesis. Cancers 2020, 12, 2441. [Google Scholar] [CrossRef]
- Bedwinek, J.M.; Perez, C.A.; Keys, D.J. Analysis of failures after definitive irradiation for epidermoid carcinoma of the nasopharynx. Cancer 1980, 45, 2725–2729. [Google Scholar] [CrossRef]
- Paiar, F.; Di Cataldo, V.; Zei, G.; Pasquetti, E.M.; Cecchini, S.; Meattini, I.; Mangoni, M.; Agresti, B.; Iermano, C.; Bonomo, P.; et al. Role of chemotherapy in nasopharyngeal carcinoma. Oncol. Rev. 2012, 6, 1. [Google Scholar] [CrossRef] [Green Version]
- Baker, B.S. The maternal and zygotic control of development by cinnamon, a new mutant in Drosophila melanogaster. Dev. Biol. 1973, 33, 429–440. [Google Scholar] [CrossRef]
- Biechele, S.; Cockburn, K.; Lanner, F.; Cox, B.J.; Rossant, J. Porcn-dependent Wnt signaling is not required prior to mouse gastrulation. Development 2013, 140, 2961–2971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Zhu, H.; Gao, Z.; Li, J.; Zhuang, J.; Dong, Y.; Shen, B.; Li, M.; Zhou, H.; Guo, H.; et al. Wnt7a activates canonical Wnt signaling, promotes bladder cancer cell invasion, and is suppressed by miR-370-3p. J. Biol. Chem. 2018, 293, 6693–6706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Su, Q.; Liu, H.; Wang, D.; Zhang, W.; Lu, Z. Frizzled7 promotes epithelial-to-mesenchymal transition and stemness via activating canonical Wnt/beta-catenin pathway in gastric cancer. Int. J. Biol. Sci. 2018, 14, 280–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, D.J. Wnt Signaling Pathway in non-small cell lung cancer. J. Natl. Cancer Inst. 2014, 106, 356. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.-Y.; Zhou, Y.-H.; Zhang, W.-L.; Xiong, W.; Fan, S.-Q.; Li, X.-L.; Luo, X.-M.; Wu, M.-H.; Yang, Y.-X.; Huang, C.; et al. Gene expression profiling of nasopharyngeal carcinoma reveals the abnormally regulated Wnt signaling pathway. Hum. Pathol. 2007, 38, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Staal, F.J.T.; Famili, F.; Perez, L.G.; Pike-Overzet, K. Aberrant Wnt signaling in Leukemia. Cancers 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-L.; Guo, X.; Yuan, T.-Z.; Cao, S.-M.; Rao, H.-L.; Hou, J.-H.; Shao, Q.; Li, N.-W.; Hong, M.-H. Expression and clinical significance of Wnt-1 and beta-catenin in nasopharyngeal carcinoma. Ai Zheng Aizheng Chin. J. Cancer 2009, 28, 72–75. [Google Scholar]
- Tulalamba, W.; Janvilisri, T. Nasopharyngeal carcinoma signaling pathway: An update on molecular biomarkers. Int. J. Cell Biol. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janvilisri, T.; Suzuki, H.; Scaria, J.; Chen, J.-W.; Charoensawan, V. High-throughput screening for biomarker discovery. Dis. Mark. 2015, 2015, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Lako, M.; Strachan, T.; Curtis, A.R.; Lindsay, S. Isolation and characterization of WNT8B, a novel human Wnt gene that maps to 10q24. Genomics 1996, 35, 386–388. [Google Scholar] [CrossRef]
- Kelly, G.M.; Greenstein, P.; Erezyilmaz, D.F.; Moon, R.T. Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development 1995, 121, 1787–1799. [Google Scholar] [CrossRef]
- Liu, W. Focus on molecules: Wnt8b: A suppressor of early eye and retinal progenitor formation. Exp. Eye Res. 2012, 101, 113–114. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.Y.; Yao, L.; Sheng, Z.T.; Wan, P.Z.; Qiu, X.B.; Wang, J. Specific knockdown of WNT8b expression protects against phosphate-induced calcification in vascular smooth muscle cells by inhibiting the Wnt-beta-catenin signaling pathway. J. Cell. Physiol. 2019, 234, 3469–3477. [Google Scholar] [CrossRef]
- Saitoh, T.; Mine, T.; Katoh, M. Up-regulation of WNT8B mRNA in human gastric cancer. Int. J. Oncol. 2002, 20, 343–348. [Google Scholar] [CrossRef]
- Saitoh, T.; Mine, T.; Katoh, M. Expression and regulation of WNT8A and WNT8B mRNAs in human tumor cell lines: Up-regulation of WNT8B mRNA by beta-estradiol in MCF-7 cells, and down-regulation of WNT8A and WNT8B mRNAs by retinoic acid in NT2 cells. Int. J. Oncol. 2002, 20, 999–1003. [Google Scholar] [CrossRef]
- Schmidt, L.H.; Biesterfeld, S.; Kuemmel, A.; Faldum, A.; Sebastian, M.; Taube, C.; Buhll, R.; Wiewrodt, R. Tissue microarrays are reliable tools for the clinicopathological characterization of lung cancer tissue. Anticancer Res. 2009, 29, 201–209. [Google Scholar] [PubMed]
- Ilyas, M.; Grabsch, H.; Ellis, I.O.; Womack, C.; Brown, R.; Berney, D.; Fennell, D.; Salto-Tellez, M.; Jenkins, M.; Landberg, G.; et al. Guidelines and considerations for conducting experiments using tissue microarrays. Histopathology 2013, 62, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tian, Z.; Qin, H.; Li, N.; Zhou, X.; Li, J.; Ni, B.; Ruan, Z. High expression of sphingosine kinase 1 is associated with poor prognosis in nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 2015, 460, 341–347. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, H.; Wu, S.; Lei, F.; Zhu, X.; Song, L.; Hong, M.; Guo, L. Prostate Tumor overexpressed 1 (PTOV1) is a novel prognostic marker for nasopharyngeal carcinoma progression and poor survival outcomes. PLoS ONE 2015, 10, e0136448. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhan, Y.; Zheng, H.; Wang, W.; Fan, S. Increased expression of heat shock protein (HSP) 10 and HSP70 correlates with poor prognosis of nasopharyngeal carcinoma. Cancer Manag. Res. 2019, 11, 8219–8227. [Google Scholar] [CrossRef] [Green Version]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 145, 146589. [Google Scholar] [CrossRef] [Green Version]
- Nusse, R. Wnt signaling and stem cell control. Cell Res. 2008, 18, 523–527. [Google Scholar] [CrossRef] [Green Version]
- Van Camp, J.K.; Beckers, S.; Zegers, D.; Van Hul, W. Wnt signaling and the control of human stem cell fate. Stem Cell Rev. Rep. 2014, 10, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signaling in cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Paluszczak, J. The significance of the dysregulation of canonical Wnt signaling in head and neck squamous cell carcinomas. Cells 2020, 9, 723. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Min, L.; Ren, C.; Xu, X.; Yang, J.; Sun, X.; Wang, T.; Wang, F.; Sun, C.; Zhang, X. miRNA-148a serves as a prognostic factor and suppresses migration and invasion through Wnt1 in non-small cell lung cancer. PLoS ONE 2017, 12, e0171751. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Zhan, P.; Qian, H.; Wang, X.; Katoh, M.; Phan, K.; Chung, J.-H.; Lv, T.; Song, Y. Prognostic value of wingless-type proteins in non-small cell lung cancer patients: A meta-analysis. Transl. Lung Cancer Res. 2016, 5, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Sun, Z.; Su, L.; Wang, F.; Jiang, Y.; Yu, D.; Zhang, F.; Sun, Z.; Liang, W. miRNA-185 serves as a prognostic factor and suppresses migration and invasion through Wnt1 in colon cancer. Eur. J. Pharmacol. 2018, 825, 75–84. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Zhu, Y.; Zhu, J.; Lai, Q. miR200b3p inhibits proliferation and induces apoptosis in colorectal cancer by targeting Wnt1. Mol. Med. Rep. 2018, 18, 2571–2580. [Google Scholar] [PubMed] [Green Version]
- Jiang, H.; Li, Q.; He, C.; Li, F.; Sheng, H.; Shen, X.; Zhang, X.; Zhu, S.; Chen, H.; Chen, X.; et al. Activation of the Wnt pathway through Wnt2 promotes metastasis in pancreatic cancer. Am. J. Cancer Res. 2014, 4, 537–544. [Google Scholar]
- Zhang, Z.; Wang, J.; Dong, X. Wnt2 contributes to the progression of gastric cancer by promoting cell migration and invasion. Oncol. Lett. 2018, 16, 2857–2864. [Google Scholar] [CrossRef]
- Aizawa, T.; Karasawa, H.; Funayama, R.; Shirota, M.; Suzuki, T.; Maeda, S.; Suzuki, H.; Yamamura, A.; Naitoh, T.; Nakayama, K.; et al. Cancer-associated fibroblasts secrete Wnt2 to promote cancer progression in colorectal cancer. Cancer Med. 2019, 8, 6370–6382. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Huang, Y.; Cao, X.; Xu, J.; Zhang, L.; Wang, J.; Huang, L.; Huang, S.; Yuan, L.; Jia, W.; et al. WNT2 promotes cervical carcinoma metastasis and induction of epithelial-mesenchymal transition. PLoS ONE 2016, 11, e0160414. [Google Scholar] [CrossRef]
- Yang, L.; Hu, B.; Zhang, Y.; Qiang, S.; Cai, J.; Huang, W. Suppression of the nuclear transporter-KPNbeta1 expression inhibits tumor proliferation in hepatocellular carcinoma. Med. Oncol. 2015, 32, 128. [Google Scholar] [CrossRef]
- Pan, L.-H.; Yao, M.; Cai, Y.; Gu, J.-J.; Yang, X.-L.; Wang, L.; Yao, D.-F. Oncogenic Wnt3a expression as an estimable prognostic marker for hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 3829–3836. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; He, Y.; Duan, J.; Yang, Y.; Zhong, C.; Zhang, J.; Liao, W.; Huang, X.; Zhu, R.; Li, M. Expression of Wnt3a in hepatocellular carcinoma and its effects on cell cycle and metastasis. Int. J. Oncol. 2017, 51, 1135–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, W.; Yao, M.; Fang, M.; Pan, L.; Wang, L.; Yang, J.; Dong, Z.; Yao, D. Oncogenic Wnt3a: A candidate specific marker and novel molecular target for hepatocellular carcinoma. J. Cancer 2019, 10, 5862–5873. [Google Scholar] [CrossRef]
- Jing, Q.; Li, G.; Chen, X.; Liu, C.; Lu, S.; Zheng, H.; Ma, H.; Qin, Y.; Zhang, D.; Zhang, S.; et al. Wnt3a promotes radioresistance via autophagy in squamous cell carcinoma of the head and neck. J. Cell. Mol. Med. 2019, 23, 4711–4722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Li, G.; Chen, X.; Jing, Q.; Liu, C.; Lu, S.; Huang, N.; Wang, Y.; Tan, P.; Chen, J.; et al. Wnt3a protein overexpression predicts worse overall survival in laryngeal squamous cell carcinoma. J. Cancer 2019, 10, 4633–4638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benhaj, K.; Akcali, K.C.; Ozturk, M. Redundant expression of canonical Wnt ligands in human breast cancer cell lines. Oncol. Rep. 2006, 15, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Siar, C.H.; Nagatsuka, H.; Han, P.P.; Buery, R.R.; Tsujigiwa, H.; Nakano, K.; Ng, K.H.; Kawakami, T. Differential expression of canonical and non-canonical Wnt ligands in ameloblastoma. J. Oral Pathol. Med. 2011, 41, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wu, D.; Cheng, H.; Chen, L.; Zhang, W.; Zou, L.; Gao, Q.; Zhao, Z.; Chen, Q.; Zeng, W.; et al. Wnt8B, transcriptionally regulated by ZNF191, promotes cell proliferation of hepatocellular carcinoma via Wnt signaling. Cancer Sci. 2021, 112, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Li, A.-C.; Xiao, W.; Shen, G.-Z.; Wang, L.; Xu, A.-A.; Cao, Y.-Q.; Huang, S.-M.; Lin, C.-G.; Han, F.; Deng, X.-W.; et al. Distant metastasis risk and patterns of nasopharyngeal carcinoma in the era of IMRT: Long-term results and benefits of chemotherapy. Oncotarget 2015, 6, 24511–24521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmeister, W.; Key, B. Frizzled-3a and Wnt-8b genetically interact during forebrain commissural formation in embryonic zebrafish. Brain Res. 2013, 1506, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Jiang, Y.; Zheng, J.; Xie, G.; Li, J.; Shi, L. Aberrant expression of beta-catenin and E-cadherin is correlated with poor prognosis of nasopharyngeal cancer. Hum. Pathol. 2013, 44, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.-Y.; Zheng, Z.-H.; Lu, H.-J.; Yan, J.; Zheng, G.-H.; Zheng, Y.-L.; Wu, D.-M.; Lu, J. Roles of β-catenin, TCF-4, and survivin in nasopharyngeal carcinoma: Correlation with clinicopathological features and prognostic significance. Cancer Cell Int. 2019, 19, 48. [Google Scholar] [CrossRef] [Green Version]
- Pang, Q.; Hu, W.; Zhang, X.; Pang, M. Wnt/β-catenin signaling pathway-related proteins (DKK-3, β-Catenin, and c-MYC) are involved in prognosis of nasopharyngeal carcinoma. Cancer Biother. Radiopharm. 2019, 34, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, M.; Wu, X.; Yang, C.; Zhang, Y.; Xu, Z. Overexpression of N-cadherin and beta-catenin correlates with poor prognosis in patients with nasopharyngeal carcinoma. Oncol. Lett. 2017, 13, 1725–1730. [Google Scholar] [CrossRef] [Green Version]
- Lecarpentier, Y.; Schussler, O.; Hebert, J.L.; Vallee, A. Multiple targets of the canonical WNT/beta-catenin signaling in cancers. Front. Oncol. 2019, 9, 1248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Wang, Z.; Chen, M.; Chen, Y.; Li, X.; Huang, D.; Fan, S.; Xiong, W.; Li, G.; et al. Upregulation of cyclin D1 can act as an independent prognostic marker for longer survival time in human nasopharyngeal carcinoma. J. Clin. Lab. Anal. 2020, 34, e23298. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, C.; Zeng, L.; Liu, G.; Jiang, W.; Zhang, X.; Chen, S.; Guo, J.; Jian, X.; Ouyang, J.; et al. High COX-2 expression in cancer-associated fibiroblasts contributes to poor survival and promotes migration and invasiveness in nasopharyngeal carcinoma. Mol. Carcinog. 2020, 59, 265–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.-J.; Lee, S.-W.; Lin, L.-C.; Chen, T.-J.; Chang, I.-W.; Hsu, H.-P.; Chang, K.-Y.; Huang, H.-Y.; Li, C.-F. Fibronectin overexpression is associated with latent membrane protein 1 expression and has independent prognostic value for nasopharyngeal carcinoma. Tumor Biol. 2013, 35, 1703–1712. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | No. of Patients | ||
|---|---|---|---|
| Age | |||
| Mean | 49.29 | years | |
| Median | 50 | years | |
| Range | 16–79 | years | |
| Gender | % | ||
| Male | 57 | 69.5 | |
| Female | 25 | 30.5 | |
| WHO classification | % | ||
| Type 1 | 2 | 2.4 | |
| Type 2 | 46 | 56.1 | |
| Type 3 | 34 | 41.5 | |
| AJCC staging | % | ||
| Stage I–II | 12 | 14.6 | |
| Stage III–IV | 68 | 82.9 | |
| N/A | 2 | 2.4 | |
| T stage | % | ||
| T1–T2 | 30 | 36.6 | |
| T3–T4 | 51 | 62.2 | |
| N/A | 1 | 1.2 | |
| Regional lymph node metastasis | % | ||
| No | 21 | 25.6 | |
| Yes (N1–N3) | 61 | 74.4 | |
| Systemic metastasis | % | ||
| No | 69 | 84.1 | |
| Yes (M1) | 12 | 14.6 | |
| N/A | 1 | 1.2 | |
| Recurrence | % | ||
| No | 58 | 70.7 | |
| Yes | 24 | 29.3 | |
| Total | 82 | 100.0 | |
| Prognostic Variables | No. of Patients | 5-Year Survival | Survival (Months) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | % | p Value | Univariate | Multivariate | |||||
| HR (95% CI) | p Value | HR (95% CI) | p-Value | ||||||
| Age (years) | 0.406 | 1.342 (0.858 to 2.099) | 0.197 | 1.326 (0.799 to 2.201) | 0.275 | ||||
| <50 | 44 | 19 | 43.2 | ||||||
| ≥50 | 38 | 13 | 34.2 | ||||||
| Gender | 0.905 | 1.006 (0.626 to 1.614) | 0.982 | 0.870 (0.525 to 1.440) | 0.587 | ||||
| Male | 57 | 22 | 38.6 | ||||||
| Female | 25 | 10 | 40.0 | ||||||
| WHO classification | 0.146 | 1.426 (0.926 to 2.196) | 0.107 | 1.202 (0.760 to 1.903) | 0.432 | ||||
| Type 1 | 2 | 1 | 50.0 | ||||||
| Type 2 | 46 | 22 | 47.8 | ||||||
| Type 3 | 34 | 9 | 26.5 | ||||||
| AJCC staging | 0.106 | 1.557 (0.820 to 2.956) | 0.176 | 1.376 (0.477 to 3.974) | 0.555 | ||||
| I–II | 12 | 7 | 58.3 | ||||||
| III–IV | 68 | 23 | 33.8 | ||||||
| N/A | 2 | - | - | ||||||
| T classification | 0.096 | 1.414 (0.893 to 2.238) | 0.140 | 1.043 (0.559 to1.943) | 0.895 | ||||
| T1–T2 | 30 | 15 | 50.0 | ||||||
| T3–T4 | 51 | 16 | 31.4 | ||||||
| N/A | 1 | - | - | ||||||
| Regional lymph node metastasis | 0.048 * | 1.451 (0.874 to 2.411) | 0.150 | 1.131 (0.549 to 2.329) | 0.738 | ||||
| No | 21 | 12 | 57.1 | ||||||
| Yes (N1–N3) | 61 | 20 | 32.8 | ||||||
| Systemic metastasis | 0.003 * | 6.207 (2.977 to 12.939) | 0.000 * | 5.852 (2.614 to 13.103) | 0.000 * | ||||
| No | 69 | 31 | 44.9 | ||||||
| Yes | 12 | 0 | 0.0 | ||||||
| N/A | 1 | - | - | ||||||
| Recurrence | 0.856 | 0.819 (0.493 to 1.360) | 0.441 | 0.832 (0.495 to 1.397) | 0.486 | ||||
| No | 58 | 23 | 39.7 | ||||||
| Yes | 24 | 9 | 37.5 | ||||||
| WNT8B | 0.092 | 1.616 (0.951 to 2.744) | 0.076 | 1.744 (1.005 to 3.025) | 0.048 * | ||||
| Low expression | 20 | 11 | 55.0 | ||||||
| High expression | 62 | 21 | 33.9 | ||||||
| Characteristics | Case | No. of Patients (%) | p-Value | ||
|---|---|---|---|---|---|
| Low WNT8B | High WNT8B | ||||
| No. of patients | 82 | 20/82 (24.4) | 62/82 (75.6) | ||
| Age | 0.513 | ||||
| <50 | 44 | 12/20 (60.0) | 32/62 (51.6) | ||
| ≥50 | 38 | 8/20 (40.0) | 30/62 (48.4) | ||
| Gender | 0.241 | ||||
| Male | 57 | 16/20 (80.0) | 41/62 (66.1) | ||
| Female | 25 | 4/20 (20.0) | 21/62 (33.9) | ||
| WHO classification | 0.694 | ||||
| Type 1 | 2 | 0/20 (0.0) | 2/62 (3.2) | ||
| Type 2 | 46 | 11/20 (55.0) | 35/62 (56.5) | ||
| Type 3 | 34 | 9/20 (45.0) | 25/62 (40.3) | ||
| AJCC staging | 0.532 | ||||
| I–II | 12 | 2/20 (10.0) | 10/62 (16.1) | ||
| III–IV | 68 | 17/20 (85.0) | 51/62 (82.3) | ||
| N/A | 2 | 1/20 (5.0) | 1/62 (1.6) | ||
| T stage | 0.395 | ||||
| T1–T2 | 30 | 9/20 (45.0) | 21/62 (33.9) | ||
| T3–T4 | 51 | 11/20 (55.0) | 40/62 (64.5) | ||
| N/A | 1 | 0/20 (0.0) | 1/62 (1.6) | ||
| Regional lymph node metastasis | 0.509 | ||||
| No | 21 | 4/20 (20.0) | 17/62 (27.4) | ||
| Yes (N1–N3) | 61 | 16/20 (80.0) | 45/62 (72.6) | ||
| Systemic metastasis | 0.485 | ||||
| No | 69 | 18/20 (90.0) | 51/62 (82.2) | ||
| Yes | 12 | 2/20 (10.0) | 10/62 (16.1) | ||
| N/A | 1 | 0/20 (0.0) | 1/62 (1.6) | ||
| Recurrence | 0.517 | ||||
| No | 58 | 13/20 (65.0) | 45/62 (72.6) | ||
| Yes | 24 | 7/20 (35.0) | 17/62 (27.4) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngernsombat, C.; Prattapong, P.; Larbcharoensub, N.; Khotthong, K.; Janvilisri, T. WNT8B as an Independent Prognostic Marker for Nasopharyngeal Carcinoma. Curr. Oncol. 2021, 28, 2529-2539. https://0-doi-org.brum.beds.ac.uk/10.3390/curroncol28040230
Ngernsombat C, Prattapong P, Larbcharoensub N, Khotthong K, Janvilisri T. WNT8B as an Independent Prognostic Marker for Nasopharyngeal Carcinoma. Current Oncology. 2021; 28(4):2529-2539. https://0-doi-org.brum.beds.ac.uk/10.3390/curroncol28040230
Chicago/Turabian StyleNgernsombat, Chawalit, Pongphol Prattapong, Noppadol Larbcharoensub, Krittika Khotthong, and Tavan Janvilisri. 2021. "WNT8B as an Independent Prognostic Marker for Nasopharyngeal Carcinoma" Current Oncology 28, no. 4: 2529-2539. https://0-doi-org.brum.beds.ac.uk/10.3390/curroncol28040230






