The Association between Herpes Zoster and Increased Cancer Risk: A Nationwide Population-Based Matched Control Study
Abstract
:1. Introduction
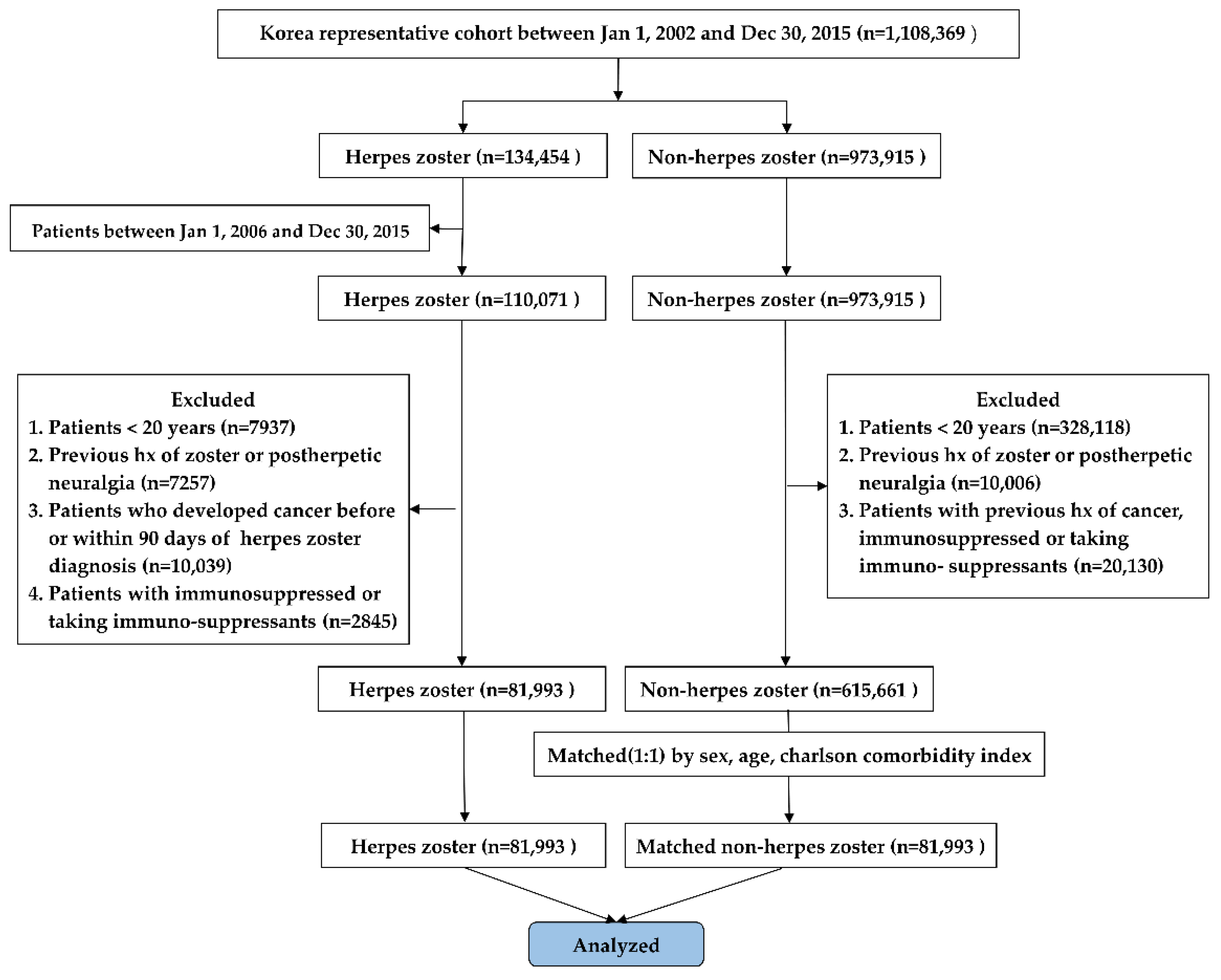
2. Materials and Methods
2.1. Study Design and Population
2.2. Identification of Herpes Zoster, Cancer, and Confounding Factors
2.3. Primary and Secondary Outcomes
2.4. Statistical Analysis
3. Results
3.1. Primary Outcomes
3.2. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jih, J.S.; Chen, Y.J.; Lin, M.W.; Chen, Y.C.; Chen, T.J.; Huang, Y.L.; Chen, C.C.; Lee, D.D.; Chang, Y.T.; Wang, W.J.; et al. Epidemiological features and costs of herpes zoster in Taiwan: A national study 2000 to 2006. Acta Derm. Venereol. 2009, 89, 612–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.L.; Hall, A.J. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect. Dis. 2004, 4, 26–33. [Google Scholar] [CrossRef]
- Nagel, M.A.; Gilden, D. Update on varicella zoster virus vasculopathy. Curr. Infect. Dis. Rep. 2014, 16, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donahue, J.G.; Choo, P.W.; Manson, J.E.; Platt, R. The incidence of herpes zoster. Arch. Intern. Med. 1995, 155, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.J.; Kim, S.H.; Lee, E.H.; Choi, J.H. Disseminated herpes zoster in an immunocompetent elderly patient. Korean J. Pain 2013, 26, 195–198. [Google Scholar] [CrossRef]
- McDonald, J.R.; Zeringue, A.L.; Caplan, L.; Ranganathan, P.; Xian, H.; Burroughs, T.E.; Fraser, V.J.; Cunningham, F.; Eisen, S.A. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin. Infect. Dis. 2009, 48, 1364–1371. [Google Scholar] [CrossRef]
- Engels, E.A.; Rosenberg, P.S.; Biggar, R.J. Zoster incidence in human immunodeficiency virus-infected hemophiliacs and homosexual men, 1984-1997. District of Columbia Gay Cohort Study. Multicenter Hemophilia Cohort Study. J. Infect. Dis. 1999, 180, 1784–1789. [Google Scholar] [CrossRef] [Green Version]
- Shiels, M.S.; Copeland, G.; Goodman, M.T.; Harrell, J.; Lynch, C.F.; Pawlish, K.; Pfeiffer, R.M.; Engels, E.A. Cancer stage at diagnosis in patients infected with the human immunodeficiency virus and transplant recipients. Cancer 2015, 121, 2063–2071. [Google Scholar] [CrossRef] [Green Version]
- Coghill, A.E.; Shiels, M.S.; Suneja, G.; Engels, E.A. Elevated Cancer-Specific Mortality among HIV-Infected Patients in the United States. J. Clin. Oncol. 2015, 33, 2376–2383. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Mine, H.; Akazawa, K.; Maehara, Y.; Sugimachi, K. Gastrointestinal cancer and herpes zoster in adults. Hepatogastroenterology 2003, 50, 1043–1046. [Google Scholar]
- Wyburn-Mason, R. Malignant change arising in tissues affected by herpes. Br. Med. J. 1955, 2, 1106–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.P.; Liu, C.J.; Hu, Y.W.; Chen, T.J.; Lin, Y.T.; Fung, C.P. Risk of cancer among patients with herpes zoster infection: A population-based study. Cmaj 2012, 184, E804–E809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, H.T.; Olsen, J.H.; Jepsen, P.; Johnsen, S.P.; Schønheyder, H.C.; Mellemkjaer, L. The risk and prognosis of cancer after hospitalisation for herpes zoster: A population-based follow-up study. Br. J. Cancer 2004, 91, 1275–1279. [Google Scholar] [CrossRef] [Green Version]
- Smyth, M.J.; Dunn, G.P.; Schreiber, R.D. Cancer immunosurveillance and immunoediting: The roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 2006, 90, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.A.; Mor, A.; Schønheyder, H.C.; Sørensen, H.T.; Dekkers, O.M.; Cronin-Fenton, D. Herpes zoster as a marker of occult cancer: A systematic review and meta-analysis. J. Infect. 2017, 74, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Ragozzino, M.W.; Melton, L.J., III; Kurland, L.T.; Chu, C.P.; Perry, H.O. Risk of cancer after herpes zoster: A population-based study. N. Engl. J. Med. 1982, 307, 393–397. [Google Scholar] [CrossRef]
- Mahale, P.; Yanik, E.L.; Engels, E.A. Herpes Zoster and Risk of Cancer in the Elderly U.S. Population. Cancer Epidemiol. Prev. Biomark. 2016, 25, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.C.; Yang, Y.H.; Hsiao, H.H.; Yang, W.C.; Liu, T.C.; Chang, C.S.; Yang, M.Y.; Lin, P.M.; Hsu, J.F.; Chang, P.Y.; et al. Herpes zoster is associated with an increased risk of subsequent lymphoid malignancies—a nationwide population-based matched-control study in Taiwan. BMC Cancer 2012, 12, 503. [Google Scholar] [CrossRef]
- Ho, J.D.; Xirasagar, S.; Lin, H.C. Increased risk of a cancer diagnosis after herpes zoster ophthalmicus: A nationwide population-based study. Ophthalmology 2011, 118, 1076–1081. [Google Scholar] [CrossRef]
- Fueyo, M.A.; Lookingbill, D.P. Herpes zoster and occult malignancy. J. Am. Acad. Dermatol. 1984, 11, 480–482. [Google Scholar] [CrossRef]
- Doody, M.M.; Linet, M.S.; Glass, A.G.; Friedman, G.D.; Pottern, L.M.; Boice, J.D., Jr.; Fraumeni, J.F., Jr. Leukemia, lymphoma, and multiple myeloma following selected medical conditions. Cancer Causes Control 1992, 3, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Chen, B.K.; Yang, C.Y. Herpes zoster and subsequent risk of cancer: A population-based study. J. Epidemiol. 2013, 23, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Buntinx, F.; Wachana, R.; Bartholomeeusen, S.; Sweldens, K.; Geys, H. Is herpes zoster a marker for occult or subsequent malignancy? Br. J. Gen. Pract. 2005, 55, 102–107. [Google Scholar]
- Anderson, L.A.; Landgren, O.; Engels, E.A. Common community acquired infections and subsequent risk of chronic lymphocytic leukaemia. Br. J. Haematol. 2009, 147, 444–449. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef]
- Khang, Y.H.; Bahk, J.; Yi, N.; Yun, S.C. Age- and cause-specific contributions to income difference in life expectancy at birth: Findings from nationally representative data on one million South Koreans. Eur. J. Public Health 2016, 26, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.J.; Bang, S.M.; Oh, D. Incidence of venous thromboembolism in Korea: From the Health Insurance Review and Assessment Service database. J. Thromb. Haemost. 2011, 9, 85–91. [Google Scholar] [CrossRef]
- Klabunde, C.N.; Legler, J.M.; Warren, J.L.; Baldwin, L.M.; Schrag, D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann. Epidemiol. 2007, 17, 584–590. [Google Scholar] [CrossRef]
- Lin, C.S.; Lin, Y.C.; Lao, H.C.; Chen, C.C. Interventional Treatments for Postherpetic Neuralgia: A Systematic Review. Pain Physician 2019, 22, 209–228. [Google Scholar] [CrossRef]
- Zaha, M.; Hayashi, I.; Odashiro, M.; Mizoguchi, H.; Fujiwara, M.; Kato, H.; Kawamura, J. Herpes zoster and malignancy. Masui 1993, 42, 1343–1346. [Google Scholar] [PubMed]
- La Vecchia, C.; Negri, E.; Franceschi, S. Medical history and the risk of non-Hodgkin’s lymphomas. Cancer Epidemiol. Prev. Biomark. 1992, 1, 533–536. [Google Scholar]
- Gramenzi, A.; Buttino, I.; D’Avanzo, B.; Negri, E.; Franceschi, S.; La Vecchia, C. Medical history and the risk of multiple myeloma. Br. J. Cancer 1991, 63, 769–772. [Google Scholar] [CrossRef] [Green Version]
- Cuzick, J.; De Stavola, B. Multiple myeloma—A case-control study. Br. J. Cancer 1988, 57, 516–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, H.H.; Wendt, V.; Winzer, M. Cutaneous pseudolymphoma at the site of prior herpes zoster eruption. Arch. Dermatol. Res. 1987, 279. [Google Scholar] [CrossRef] [PubMed]
- Hudson, C.P.; Hanno, R.; Callen, J.P. Cutaneous angiosarcoma in a site of healed herpes zoster. Int. J. Dermatol. 1984, 23, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Weller, T.H. Varicella and herpes zoster. Changing concepts of the natural history, control, and importance of a not-so-benign virus. N. Engl. J. Med. 1983, 309, 1434–1440. [Google Scholar] [CrossRef]
- Arvin, A. Aging, immunity, and the varicella-zoster virus. N. Engl. J. Med. 2005, 352, 2266–2267. [Google Scholar] [CrossRef] [PubMed]
- Konjević, G.; Jurisić, V.; Banićevic, B.; Spuzić, I. The difference in NK-cell activity between patients with non-Hodgkin’s lymphomas and Hodgkin’s disease. Br. J. Haematol. 1999, 104, 144–151. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.; Han, K.; Yoo, S.A.; Lee, J.H. Herpes Zoster and Subsequent Cancer Risk: A Nationwide Population-Based Cohort Study in Korea. Dermatology 2020, 1–6. [Google Scholar] [CrossRef]
- Kim, Y.; Park, J.; Nam, B.H.; Ki, M. Stomach cancer incidence rates among Americans, Asian Americans and Native Asians from 1988 to 2011. Epidemiol. Health 2015, 37, e2015006. [Google Scholar] [CrossRef] [PubMed]
- Pourhoseingholi, M.A.; Vahedi, M.; Baghestani, A.R. Burden of gastrointestinal cancer in Asia; an overview. Gastroenterol. Hepatol. Bed Bench 2015, 8, 19–27. [Google Scholar] [PubMed]

| Study Population | HZ | Non-HZ |
|---|---|---|
| Total | 81,993 | 81,993 |
| Sex (male) | 33,003 (40.3%) | 33,003 (40.3%) |
| Age | 51.3 ± 15.7 | 51.3 ± 15.7 |
| 20~29 years | 8213 (10.0%) | 8213 (10.0%) |
| 30~39 years | 12,205 (14.9%) | 12,205 (14.9%) |
| 40~49 years | 15,896 (19.4%) | 15,896 (19.4%) |
| 50~59 years | 20,245 (24.7%) | 20,245 (24.7%) |
| 60~69 years | 13,960 (17.0%) | 13,960 (17.0%) |
| 70~79 years | 8630 (10.5%) | 8630 (10.5%) |
| ≥80 years | 2844 (3.5%) | 2844 (3.5%) |
| CCI | ||
| 0 | 37,556 (45.8%) | 37,556 (45.8%) |
| 1 | 22,258 (27.2%) | 22,258 (27.2%) |
| 2 | 11,335 (13.8%) | 11,335 (13.8%) |
| 3 | 5496 (6.7%) | 5496 (6.7%) |
| 4 | 2760 (3.4%) | 2760 (3.4%) |
| 5 | 2588 (3.2%) | 2588 (3.2%) |
| Variable | HZ | Non-HZ | HR | 95% CI | p |
|---|---|---|---|---|---|
| Total | 10.55 | 11.34 | 0.94 | 0.90–0.97 | 0.002 |
| Sex | |||||
| Male | 12.26 | 13.40 | 0.93 | 0.87–0.98 | 0.015 |
| Female | 9.42 | 9.98 | 0.94 | 0.89–1.00 | 0.052 |
| Follow-up interval | |||||
| 1 year | 8.69 | 10.20 | 0.85 | 0.77–0.94 | 0.002 |
| 3 years | 10.16 | 11.52 | 0.89 | 0.84–0.94 | <0.001 |
| 5 years | 10.43 | 11.43 | 0.92 | 0.88–0.96 | 0.001 |
| Follow Up Period | Cancer Type | Total | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | ||
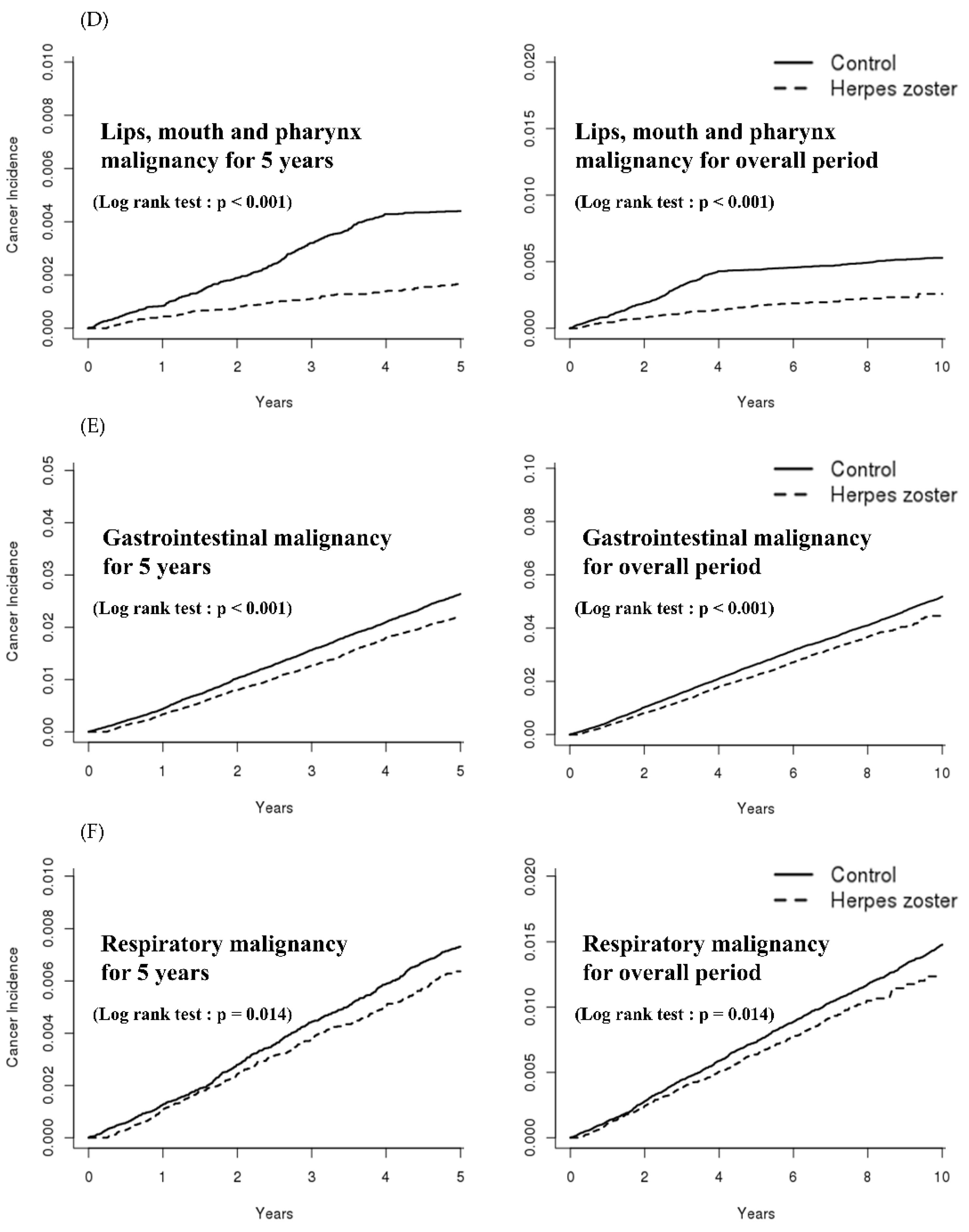
| Overall | Lips, mouth, and pharynx | 0.41 | 0.33–0.50 | <0.001 | 0.41 | 0.33–0.50 | <0.001 | 0.41 | 0.33–0.50 | <0.001 |
| Digestive system | 0.86 | 0.81–0.91 | <0.001 | 0.86 | 0.81–0.91 | <0.001 | 0.86 | 0.81–0.91 | <0.001 | |
| Respiratory system | 0.87 | 0.78–0.97 | 0.014 | 0.87 | 0.78–0.97 | 0.014 | 0.87 | 0.78–0.97 | 0.014 | |
| Bone and articular cartilage | 1.03 | 0.63–1.66 | 0.904 | 1.03 | 0.63–1.66 | 0.904 | 1.03 | 0.63–1.66 | 0.904 | |
| Melanoma and skin | 1.15 | 0.90–1.47 | 0.260 | 1.15 | 0.90–1.47 | 0.260 | 1.15 | 0.90–1.47 | 0.260 | |
| Mesothelial and soft tissue | 1.02 | 0.71–1.46 | 0.911 | 1.02 | 0.71–1.46 | 0.911 | 1.02 | 0.71–1.46 | 0.911 | |
| Urinary tract | 1.04 | 0.87–1.25 | 0.608 | 1.04 | 0.87–1.25 | 0.608 | 1.04 | 0.87–1.25 | 0.608 | |
| Eye, brain, and CNS system | 0.79 | 0.59–1.07 | 0.139 | 0.79 | 0.59–1.07 | 0.139 | 0.79 | 0.59–1.07 | 0.139 | |
| Thyroid gland and endocrine gland | 1.20 | 1.07–1.34 | 0.001 | 1.20 | 1.07–1.34 | 0.001 | 1.20 | 1.07–1.34 | 0.001 | |
| Unknown secondary and unspecified sites | 0.80 | 0.73–0.87 | <0.001 | 0.80 | 0.73–0.87 | <0.001 | 0.80 | 0.73–0.87 | <0.001 | |
| Lymphoid and hematopoietic system | 1.66 | 1.35–2.03 | <0.001 | 1.66 | 1.35–2.03 | <0.001 | 1.66 | 1.35–2.03 | <0.001 | |
| 1 year | Lips, mouth, and pharynx | 0.50 | 0.33–0.76 | 0.001 | 0.80 | 0.39–1.61 | 0.538 | 0.40 | 0.24–0.67 | 0.001 |
| Digestive system | 0.76 | 0.65–0.90 | 0.001 | 0.85 | 0.68–1.06 | 0.172 | 0.68 | 0.54–0.86 | 0.001 | |
| Respiratory system | 0.84 | 0.63–1.12 | 0.256 | 0.90 | 0.61–1.32 | 0.606 | 0.77 | 0.49–1.20 | 0.257 | |
| Bone and articular cartilage | 0.87 | 0.26–2.86 | 0.827 | 0.52 | 0.09–2.84 | 0.451 | 1.59 | 0.26–9.44 | 0.607 | |
| Melanoma and skin | 0.98 | 0.49–1.95 | 0.958 | 1.20 | 0.43–3.32 | 0.715 | 0.82 | 0.33–2.11 | 0.690 | |
| Mesothelial and soft tissue | 1.82 | 0.53–6.26 | 0.338 | 3.19 | 0.33–3.10 | 0.310 | 1.37 | 0.35–6.21 | 0.678 | |
| Urinary tract | 0.95 | 0.59–1.53 | 0.858 | 0.92 | 0.51–1.63 | 0.780 | 1.04 | 0.40–2.41 | 0.922 | |
| Eye, brain, and CNS system | 1.13 | 0.51–2.49 | 0.749 | 0.70 | 0.19–2.48 | 0.584 | 1.56 | 0.57–4.42 | 0.394 | |
| Thyroid gland and endocrine gland | 1.36 | 0.98–1.89 | 0.065 | 1.05 | 0.36–2.99 | 0.926 | 1.40 | 0.90–1.98 | 0.057 | |
| Unknown secondary and unspecified sites | 0.78 | 0.60–1.00 | 0.056 | 0.69 | 0.47–1.02 | 0.065 | 0.85 | 0.60–1.19 | 0.359 | |
| Lymphoid and hematopoietic system | 2.44 | 1.33–4.48 | 0.004 | 1.30 | 0.61–2.80 | 0.491 | 7.00 | 2.09–23.61 | 0.002 | |
| 3 years | Lips, mouth, and pharynx | 0.35 | 0.27–0.45 | <0.001 | 0.47 | 0.31–0.72 | 0.001 | 0.30 | 0.22–0.42 | <0.001 |
| Digestive system | 0.80 | 0.73–0.87 | <0.001 | 0.83 | 0.73–0.93 | 0.003 | 0.76 | 0.67–0.87 | <0.001 | |
| Respiratory system | 0.85 | 0.72–1.00 | 0.052 | 0.84 | 0.68–1.03 | 0.105 | 0.87 | 0.67–1.12 | 0.291 | |
| Bone and articular cartilage | 0.53 | 0.25–1.11 | 0.096 | 0.28 | 0.08–1.01 | 0.053 | 0.82 | 0.31–2.15 | 0.694 | |
| Melanoma and skin | 1.34 | 0.90–2.01 | 0.147 | 1.06 | 0.58–1.94 | 0.838 | 1.62 | 0.93–2.80 | 0.082 | |
| Mesothelial and soft tissue | 1.07 | 0.61–1.87 | 0.807 | 0.97 | 0.44–2.13 | 0.943 | 1.18 | 0.53–2.65 | 0.672 | |
| Urinary tract | 1.07 | 0.82–1.40 | 0.590 | 1.12 | 0.81–1.55 | 0.487 | 0.99 | 0.62–1.58 | 0.980 | |
| Eye, brain, and CNS system | 0.89 | 0.57–1.39 | 0.614 | 0.73 | 0.37–1.46 | 0.384 | 1.03 | 0.56–1.86 | 0.920 | |
| Thyroid gland and endocrine gland | 1.58 | 1.32–1.90 | <0.001 | 1.79 | 1.02–3.12 | 0.041 | 1.56 | 1.29–1.88 | <0.001 | |
| Unknown secondary and unspecified sites | 0.74 | 0.65–0.84 | <0.001 | 0.70 | 0.58–0.85 | <0.001 | 0.77 | 0.64–0.92 | 0.006 | |
| Lymphoid and hematopoietic system | 2.61 | 1.79–3.81 | <0.001 | 2.14 | 1.28–3.57 | 0.004 | 3.31 | 1.88–5.82 | <0.001 | |
| 5 years | Lips, mouth, and pharynx | 0.37 | 0.30–0.46 | <0.001 | 0.55 | 0.38–0.80 | 0.002 | 0.30 | 0.23–0.40 | <0.001 |
| Digestive system | 0.83 | 0.77–0.89 | <0.001 | 0.84 | 0.76–0.92 | <0.001 | 0.82 | 0.74–0.91 | <0.001 | |
| Respiratory system | 0.86 | 0.76–0.98 | 0.032 | 0.82 | 0.70–0.98 | 0.029 | 0.93 | 0.75–1.14 | 0.496 | |
| Bone and articular cartilage | 0.86 | 0.49–1.51 | 0.604 | 0.68 | 0.31–1.50 | 0.346 | 1.13 | 0.49–2.60 | 0.768 | |
| Melanoma and skin | 1.28 | 0.93–1.76 | 0.127 | 1.16 | 0.70–1.93 | 0.548 | 1.36 | 0.90–2.06 | 0.136 | |
| Mesothelial and soft tissue | 0.99 | 0.64–1.55 | 0.992 | 1.15 | 0.61–2.15 | 0.652 | 0.85 | 0.45–1.61 | 0.634 | |
| Urinary tract | 1.04 | 0.84–1.29 | 0.679 | 1.11 | 0.85–1.45 | 0.432 | 0.93 | 0.64–1.35 | 0.719 | |
| Eye, brain, and CNS system | 0.86 | 0.61–1.23 | 0.428 | 0.80 | 0.46–1.40 | 0.449 | 0.91 | 0.57–1.43 | 0.693 | |
| Thyroid gland and endocrine gland | 1.36 | 1.19–1.56 | <0.001 | 1.32 | 0.90–1.93 | 0.153 | 1.37 | 1.18–1.59 | <0.001 | |
| Unknown secondary and unspecified sites | 0.72 | 0.65–0.80 | <0.001 | 0.73 | 0.63–0.84 | <0.001 | 0.71 | 0.62–0.82 | <0.001 | |
| Lymphoid and hematopoietic system | 2.00 | 1.52–2.63 | <0.001 | 1.85 | 1.24–2.77 | 0.002 | 2.14 | 1.47–3.11 | <0.001 | |
| Cancer Type | Overall | Follow-Up at 1 year | Follow-Up at 3 Years | Follow-Up at 5 Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | HR | 95% Cl | p | |
| Lips, mouth, and pharynx | 1.43 | 0.97–2.10 | 0.071 | 1.26 | 0.60–2.64 | 0.534 | 1.18 | 0.72–1.93 | 0.514 | 1.22 | 0.80–1.87 | 0.358 |
| Digestive system | 1.33 | 1.20–1.47 | <0.001 | 1.20 | 0.92–1.57 | 0.169 | 1.38 | 1.19–1.59 | <0.001 | 1.34 | 1.19–1.51 | <0.001 |
| Respiratory system | 1.65 | 1.37–1.99 | <0.001 | 1.67 | 1.07–2.62 | 0.023 | 1.71 | 1.33–2.21 | <0.001 | 1.62 | 1.31–2.00 | <0.001 |
| Bone and articular cartilage | 1.54 | 0.72–3.30 | 0.262 | 0.76 | 0.09–6.77 | 0.803 | 0.75 | 0.16–3.53 | 0.716 | 1.21 | 0.47–3.12 | 0.691 |
| Melanoma and skin | 1.50 | 0.96–2.19 | 0.079 | 1.37 | 0.48–3.95 | 0.556 | 1.13 | 0.61–2.09 | 0.693 | 1.11 | 0.67–1.86 | 0.681 |
| Mesothelial and soft tissue | 0.83 | 0.41–1.67 | 0.603 | 1.21 | 0.24–6.24 | 0.819 | 0.45 | 0.13–1.51 | 0.195 | 0.67 | 0.28–1.62 | 0.371 |
| Urinary tract | 1.18 | 0.86–1.61 | 0.303 | 0.81 | 0.35–1.88 | 0.628 | 1.35 | 0.89–2.05 | 0.160 | 1.27 | 0.89–1.81 | 0.182 |
| Eye, brain, and CNS system | 1.27 | 0.74–2.21 | 0.388 | 0.55 | 0.12–2.48 | 0.436 | 1.12 | 0.52–2.41 | 0.768 | 1.17 | 0.63–2.17 | 0.618 |
| Thyroid gland and endocrine gland | 1.05 | 0.86–1.28 | 0.638 | 0.85 | 0.50–1.43 | 0.542 | 0.96 | 0.73–1.25 | 0.739 | 1.06 | 0.85–1.32 | 0.594 |
| Unknown secondary and unspecified sites | 1.41 | 1.21–1.63 | <0.001 | 1.15 | 0.75–1.76 | 0.516 | 1.36 | 1.10–1.70 | 0.005 | 1.41 | 1.19–1.69 | <0.001 |
| Lymphoid and hematopoietic system | 1.74 | 1.27–2.39 | <0.001 | 1.78 | 0.90–3.54 | 0.097 | 1.34 | 0.85–2.12 | 0.198 | 1.46 | 1.01–2.13 | 0.043 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, J.-H.; Cho, H.-S.; Kim, Y.-D.; Mun, J.; Kim, S.-B.; Lee, J.-H.; Leem, J.-G. The Association between Herpes Zoster and Increased Cancer Risk: A Nationwide Population-Based Matched Control Study. Curr. Oncol. 2021, 28, 2720-2730. https://0-doi-org.brum.beds.ac.uk/10.3390/curroncol28040237
Sim J-H, Cho H-S, Kim Y-D, Mun J, Kim S-B, Lee J-H, Leem J-G. The Association between Herpes Zoster and Increased Cancer Risk: A Nationwide Population-Based Matched Control Study. Current Oncology. 2021; 28(4):2720-2730. https://0-doi-org.brum.beds.ac.uk/10.3390/curroncol28040237
Chicago/Turabian StyleSim, Ji-Hoon, Hyun-Seok Cho, Young-Do Kim, Juhan Mun, Sung-Bae Kim, Jong-Hyuk Lee, and Jeong-Gil Leem. 2021. "The Association between Herpes Zoster and Increased Cancer Risk: A Nationwide Population-Based Matched Control Study" Current Oncology 28, no. 4: 2720-2730. https://0-doi-org.brum.beds.ac.uk/10.3390/curroncol28040237





