Dissociation and Combustion of a Layer of Methane Hydrate Powder: Ways to Increase the Efficiency of Combustion and Degassing
Abstract
:1. Introduction
2. Experimental Methods
3. Modeling and Experimental Results
3.1. Dissociation of Gas Hydrates at Negative Temperatures
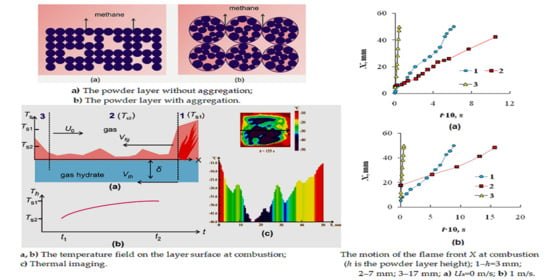
3.2. Combustion of Methane Hydrate at Dissociation of a Powder Layer
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Nomenclature
| A | the degree of transformation of methane hydrate (A = mH/m0) |
| a | the thermal diffusivity |
| C | the mass concentration |
| Cp | the heat capacity |
| E | the activation energy |
| h | the molar enthalpy |
| h | the powder layer height |
| Mr | the molecular weight of gas |
| m | mass |
| n | the molar concentrations of gas components |
| Por | the porosity in the powder layer |
| P | the gas pressure |
| Q | the heat of the methane hydrate dissociation |
| q | the heat flux density |
| Rg | the universal gas constant |
| r | the reaction rate |
| r | the radius of particle |
| t | time |
| tfull | the total dissociation time |
| T | temperature |
| J | gas flow |
| kR | the kinetic constant |
| kD | the permeability coefficient |
| k0 | the kinetic constant refers to methane hydrate at negative temperatures |
| U | the convective gas velocity |
| X | the motion of the flame front |
| z | the transverse coordinate |
| Greek symbols | |
| α | the convective heat transfer coefficient |
| δ | the ice crust layer |
| λ | the thermal conductivity |
| µ | the dynamic viscosity |
| ν | the kinematic viscosity |
| ρ | density |
| σp | the pore density |
| χ | the effective volumetric heat transfer |
| Subscripts | |
| a | air |
| 0 | the outer boundary |
| eq | equilibrium |
| eff | effective |
| f | the reaction front |
| g | gas |
| H | hudrate |
| I | ice |
| m | methane |
| s | the layer surface |
| S | solid |
| w | wall |
| w | water |
References
- Yin, Z.; Linga, P. Methane hydrates: A future clean energy resource. Chin. J. Chem. Eng. 2019, 27, 2026–2036. [Google Scholar] [CrossRef]
- Cui, Y.; Lu, C.; Wu, M.; Peng, Y.; Yao, Y.; Luo, W. Review of exploration and production technology of natural gas hydrate. Adv. Geo Energy Res. 2018, 2, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.-S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Lu, S.M. A global survey of gas hydrate development and reserves: Specifically in the marine field. Renew. Sustain. Energy Rev. 2015, 41, 884–900. [Google Scholar] [CrossRef]
- Sloan, E.D., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Istomin, V.A.; Yakushev, V.S. Gas Hydrates in Nature; Nedra: Moscow, Russia, 1992. [Google Scholar]
- Sum, A.K.; Koh, C.A.; Sloan, E.D. Developing a comprehensive understanding and model of hydrate in multiphase flow: From laboratory measurements to field applications. Energy Fuels 2012, 26, 4046–4052. [Google Scholar] [CrossRef]
- Xie, Y.; Li, G.; Liu, D.; Liu, N.; Qi, Y.; Liang, D.; Guo, K.; Fan, S. Experimental study on a small scale of gas hydrate cold storage apparatus. Appl. Energy 2010, 87, 3340–3346. [Google Scholar] [CrossRef]
- Javanmardi, J.; Nasrifar, K.; Najibi, S.H.; Moshfeghian, M. Economic evaluation of natural gas hydrate as an alternative for natural gas transportation. Appl. Therm. Eng. 2005, 25, 1708–1723. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.C.; Li, G.; Zhang, Y.; Li, B.; Chen, Z.Y. Experimental investigation into methane hydrate production during three-dimensional thermal stimulation with five-spot well system. Appl. Energy 2013, 110, 90–97. [Google Scholar] [CrossRef]
- Li, G.; Li, X.-S.; Yang, B.; Duan, L.-P.; Huang, N.-S.; Zhang, Y.; Tang, L.-G. The use of dual horizontal wells in gas production from hydrate accumulations. Appl. Energy 2013, 112, 1303–1310. [Google Scholar] [CrossRef]
- Zhong, D.; Englezos, P. Methane separation from coal mine methane gas by tetra-n-butyl ammonium bromide semiclathrate hydrate formation. Energy Fuels 2012, 26, 2098–2106. [Google Scholar] [CrossRef]
- Lee, Y.; Seo, Y.-J.; Ahn, T.; Lee, J.; Lee, J.X.; Kim, S.-J.; Seo, Y. CH4-Flue gas replacement occurring in sH hydrates and its significance for CH4 recovery and CO2 sequestration. Chem. Eng. J. 2017, 308, 50–58. [Google Scholar] [CrossRef]
- Hu, C.G.; Li, X.-S. Research progress of hydrate-based CO2 separation and capture from gas mixture. RSC Adv. 2014, 4, 18301–18316. [Google Scholar]
- Cai, L.; Pethica, B.A.; Debenedetti, P.G.; Sundaresan, S. Formation of cyclopentane methane binary clathrate hydrate in brine solutions. Chem. Eng. Sci. 2016, 141, 125–132. [Google Scholar] [CrossRef]
- Maslin, M.; Owen, M.; Betts, R.; Day, S.; Jones, T.D.; Ridgwell, A. Gas hydrates: Past and future geohazard? Philos. Trans. A. Math. Phys. Eng. Sci. 2010, 368, 2369–2393. [Google Scholar] [CrossRef] [PubMed]
- Hatzikiriakos, S.G.; Englezos, P. The relationship between global warming and methane gas hydrates in the earth. Chem. Eng. Sci. 1993, 48, 3963–3969. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Manakov, A.Y.; Morozov, V.S.; Nyashina, G.S.; Gaidukova, O.S.; Skiba, S.S.; Volkov, R.S.; Voytkov, I.S. The influence of key parameters on combustion of double gas hydrate. J. Nat. Gas Sci. Eng. 2020, 80, 103396. [Google Scholar] [CrossRef]
- Misyura, S.Y. Developing the environmentally friendly technologies of combustion of gas hydrates. Reducing harmful emissions during combustion. Environ. Pollut. 2020, 265, 114871. [Google Scholar] [CrossRef]
- Clarke, M.; Bishnoi, P.R. Determination of the activation energy and intrinsic rate constant of methane gas hydrate decomposition. Can. J. Chem. Eng. 2001, 79, 143–147. [Google Scholar] [CrossRef]
- Kim, H.C.; Bishnoi, P.R.; Heidemann, R.A.; Rizvi, S.S.H. Kinetics of methane hydrate decomposition. Chem. Eng. Sci. 1987, 42, 1645–1653. [Google Scholar] [CrossRef]
- Vysniauskas, A.; Bishnoi, P. A kinetic study of methane hydrate formation. Chem. Eng. Sci. 1983, 38, 1061–1072. [Google Scholar] [CrossRef]
- Misyura, S.Y. Comparing the dissociation kinetics of various gas hydrates during combustion: Assessment of key factors to improve combustion efficiency. Appl. Energy 2020, 270, 115042. [Google Scholar] [CrossRef]
- Misyura, S.Y. Non-stationary combustion of natural and artificial methane hydrate at heterogeneous dissociation. Energy 2019, 181, 589–602. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, X.; Guo, W.; Jia, R.; Li, B. Numerical simulation of the short- and long-term production behavior of the first offshore gas hydrate production test in the South China Sea. J. Pet. Sci. Eng. 2019, 181, 106196. [Google Scholar] [CrossRef]
- Yu, T.; Guan, G.; Abudula, A. Production performance and numerical investigation of the 2017 offshore methane hydrate production test in the Nankai Trough of Japan. Appl. Energy 2019, 251, 113338. [Google Scholar] [CrossRef]
- Khasanov, M.K.; Rafikova, G.R.; Musakaev, N.G. Mathematical model of carbon dioxide injection into a porous Reservoir saturated with methane and its gas hydrate. Energies 2020, 13, 440. [Google Scholar] [CrossRef] [Green Version]
- Khasanov, M.K.; Musakaev, N.G.; Stolpovsky, M.V.; Kildibaeva, S.R. Mathematical model of decomposition of methane hydrate during the injection of liquid carbon dioxide into a reservoir saturated with methane and its hydrate. Mathematics 2020, 8, 1482. [Google Scholar] [CrossRef]
- Selim, M.S.; Sloan, E.D. Heat and mass transfer during the dissociation of hydrates in porous media. AIChE J. 1989, 35, 1049–1052. [Google Scholar] [CrossRef]
- Kuhs, W.F.; Genov, G.; Staykova, D.K.; Hansen, T. Ice perfection and onset of anomalous preservation of gas hydrates. Phys. Chem. Chem. Phys. 2004, 6, 4917–4920. [Google Scholar] [CrossRef]
- Falenty, A.; Kuhs, W.F. Self-preservation of CO2 gas hydrates-surface microstructure and ice perfection. J. Phys. Chem. B 2009, 113, 5975–5988. [Google Scholar] [CrossRef]
- Nguyen, A.H.; Koc, M.A.; Shepherd, T.D.; Molinero, V. Structure of the ice-clathrate interface. J. Phys. Chem. C 2015, 119, 4104–4117. [Google Scholar] [CrossRef]
- Takeya, S.; Uchida, T.; Nagao, J.; Ohmura, R.; Shimada, W.; Kamata, Y.; Ebinuma, T.; Narita, H. Particle size effect of CH4 hydrate for self-preservation. Chem. Eng. Sci. 2005, 60, 1383–1387. [Google Scholar] [CrossRef]
- Misyura, S.Y. Dissociation of various gas hydrates (methane hydrate, double gas hydrates of methane-propane and methane-isopropanol) during combustion: Assessing the combustion efficiency. Energy 2020, 206, 118120. [Google Scholar] [CrossRef]
- Sato, H.; Sakamoto, H.; Ogino, S.; Mimachi, H.; Kinoshita, T.; Iwasaki, T.; Sano, K.; Ohgaki, K. Self-preservation of methane hydrate revealed immediately below the eutectic temperature of the mother electrolyte solution. Chem. Eng. Sci. 2013, 91, 86–89. [Google Scholar] [CrossRef]
- Takeya, S.; Ripmeester, J.A. Anomalous preservation of CH4 hydrate and its dependence on the morphology hexagonal of ice. ChemPhysChem 2010, 11, 70–73. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Rogers, R.E. Ultra-stability of gas hydrates at 1 atm and 268.2 K. Chem. Eng. Sci. 2008, 63, 2066–2074. [Google Scholar] [CrossRef]
- Shimada, W.; Takeya, S.; Kamata, Y.; Uchida, T.; Nagao, J.; Ebinuma, T.; Narita, H. Texture change of ice on anomalously preserved methane clathrate hydrate. J. Phys. Chem. B 2005, 109, 5802–5807. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Donskoy, I.G. Dissociation of natural and artificial gas hydrate. Chem. Eng. Sci. 2016, 148, 65–77. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Oxford, UK, 1975; pp. 89–103. [Google Scholar]
- Misyura, S.Y.; Donskoy, I.G. Dissociation kinetics of methane hydrate and CO2 hydrate for different granular composition. Fuel 2020, 262, 116614. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Donskoy, I.G. Ways to improve the efficiency of carbon dioxide utilization and gas hydrate storage at low temperatures. J. CO2 Util. 2019, 34, 313–324. [Google Scholar] [CrossRef]
- Vlasov, V.A. Diffusion model of gas hydrate dissociation into ice and gas: Simulation of the self-preservation effect. Int. J. Heat Mass Transf. 2016, 102, 631–636. [Google Scholar] [CrossRef]
- Misyura, S.Y. The influence of porosity and structural parameters on different kinds of gas hydrate dissociation. Sci. Rep. 2016, 6, 30324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassanpouryouzband, A.; Joonaki, E.; Farahani, M.V.; Takeya, S.; Ruppel, C.; Yang, J.; English, N.J.; Schicks, J.M.; Edlmann, K.; Mehrabian, H.; et al. Gas hydrates in sustainable chemistry. Chem. Soc. Rev. 2020, 49, 5225–5309. [Google Scholar] [CrossRef] [PubMed]
- Misyura, S.Y. Effect of heat transfer on the kinetics of methane hydrate dissociation. Chem. Phys. Lett. 2013, 583, 34–37. [Google Scholar] [CrossRef]
- Chen, X.R.; Li, X.S.; Chen, Z.Y.; Zhang, Y.; Yan, K.F.; Lv, Q.-N. Experimental investigation into the combustion characteristics of propane hydrates in porous media. Energies 2015, 8, 1242–1255. [Google Scholar] [CrossRef] [Green Version]
- Maruyama, Y.; Yokomori, T.; Ohmura, R.; Ueda, T. Flame spreading over combustible hydrate in a laminar boundary layer. In Proceedings of the 7th International Conference on Gas Hydrate, Edinburgh, UK, 17–21 July 2011. [Google Scholar]
- Maruyama, Y.; Fuse, M.J.; Yokomori, T.; Ohmura, R.; Watanabe, S.; Iwasaki, T.; Iwabuchi, W.; Ueda, T. Experimental investigation of flame spreading over pure methane hydrate in a laminar boundary layer. Proc. Combust. Inst. 2013, 34, 2131–2138. [Google Scholar] [CrossRef]
- Nakamura, Y.; Katsuki, R.; Yokomori, T.; Ohmura, R.; Takahashi, M.; Iwasaki, T.; Uchida, K.; Ueda, T. Combustion characteristics of methane hydrate in a laminar boundary layer. Energy Fuels 2009, 23, 1445–1449. [Google Scholar] [CrossRef]
- Chien, Y.-C.; Dunn-Rankin, D. Combustion characteristics of methane hydrate flames. Energies 2019, 12, 1939. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.H.; Padilla, R.E.; Dunn-Rankin, D.; Chen, G.B.; Chao, Y.C. Thermal structure of methane hydrate fueled flames. Proc. Combust. Inst. 2017, 36, 4391–4398. [Google Scholar] [CrossRef]
- Yoshioka, T.; Yamamoto, Y.; Yokomori, T.; Ohmura, R.; Ueda, T. Experimental study on combustion of a methane hydrate sphere. Exp. Fluids 2015, 56, 192. [Google Scholar] [CrossRef]
- Cui, G.; Wang, S.; Dong, Z.; Xing, X.; Shan, T.; Li, Z. Effects of the diameter and the initial center temperature on the combustion characteristics of methane hydrate spheres. Appl. Energy 2020, 257, 114058. [Google Scholar] [CrossRef]
- Bar-Kohany, T.; Sirignano, W.A. Transient combustion of a methane-hydrate sphere. Combust. Flame 2016, 163, 284–300. [Google Scholar] [CrossRef]
- Dagan, Y.; Bar-Kohany, T. Flame propagation through three-phase methane-hydrate particles. Combust. Flame 2018, 193, 25–35. [Google Scholar] [CrossRef]
- Cui, G.; Dong, Z.; Wang, S.; Xing, X.; Shan, T.; Li, Z. Effect of the water on the flame characteristics of methane hydrate combustion. Appl. Energy 2020, 259, 114205. [Google Scholar] [CrossRef]
- Aerov, M.E.; Todes, O.M.; Narinsky, D.A. Apparatuses with the Steady Grain Layer: Hydraulic and Thermal Fundamentals of Operation; Khimiya: Saint Petersburg, Russia, 1979. [Google Scholar]
- Glushko, V.P. Thermodynamic and Thermophysical Properties of Combustion Products; VINITY AS USSR: Moscow, Russia, 1971; Volume 5. [Google Scholar]
- Snegirev, A.Y. Perfectly stirred reactor model to evaluate extinction of diffusion flame. Combust. Flame 2015, 162, 3622–3631. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misyura, S.Y.; Donskoy, I.G. Dissociation and Combustion of a Layer of Methane Hydrate Powder: Ways to Increase the Efficiency of Combustion and Degassing. Energies 2021, 14, 4855. https://0-doi-org.brum.beds.ac.uk/10.3390/en14164855
Misyura SY, Donskoy IG. Dissociation and Combustion of a Layer of Methane Hydrate Powder: Ways to Increase the Efficiency of Combustion and Degassing. Energies. 2021; 14(16):4855. https://0-doi-org.brum.beds.ac.uk/10.3390/en14164855
Chicago/Turabian StyleMisyura, Sergey Y., and Igor G. Donskoy. 2021. "Dissociation and Combustion of a Layer of Methane Hydrate Powder: Ways to Increase the Efficiency of Combustion and Degassing" Energies 14, no. 16: 4855. https://0-doi-org.brum.beds.ac.uk/10.3390/en14164855