Lithium-Ion Battery Operation, Degradation, and Aging Mechanism in Electric Vehicles: An Overview
Abstract
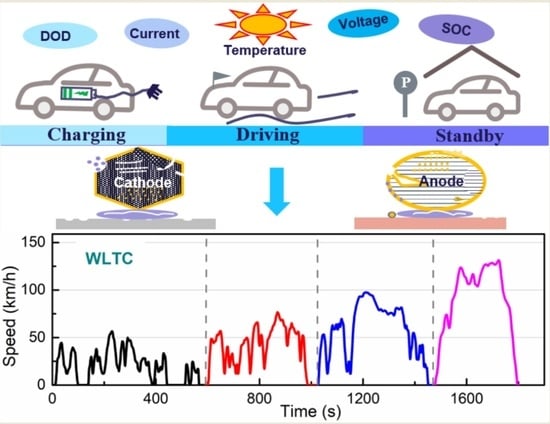
:1. Introduction
2. LiBs in EV Applications
2.1. Electric Vehicle Applications
2.2. Lithium-Ion Chemistries in EV Applications
3. Battery Operation in EV Application
3.1. EV Battery Charging
3.2. EV Driving Operation
3.2.1. Real-Life Scenarios
3.2.2. Driving Cycle Profiles
3.3. EV Standby Operation
4. Aging in EV Application
4.1. Aging in Charging
4.1.1. Impact of Charging Voltage
4.1.2. Impact of the Charging Current
4.1.3. Impact of Charging Temperature
4.2. Aging in Driving
4.3. Aging in Standby
4.4. Aging in Whole Application
5. Aging on Lithium-Ion Batteries
5.1. Aging at the Cathode
5.2. Aging at the Electrolyte
5.3. Aging at the Anode
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, J.; Bae, C.; Denlinger, A.; Miller, T. Electric Vehicles Batteries: Requirements and Challenges. Joule 2020, 4, 511–515. [Google Scholar] [CrossRef]
- Albertsen, L.; Richter, J.L.; Peck, P.; Dalhammar, C.; Plepys, A. Circular business models for electric vehicle lithium-ion batteries: An analysis of current practices of vehicle manufacturers and policies in the EU. Resour. Conserv. Recycl. 2021, 172, 105658. [Google Scholar] [CrossRef]
- Pellow, M.A.; Ambrose, H.; Mulvaney, D.; Betita, R.; Shaw, S. Research gaps in environmental life cycle assessments of lithium ion batteries for grid-scale stationary energy storage systems: End-of-life options and other issues. Sustain. Mater. Technol. 2020, 23, e00120. [Google Scholar] [CrossRef]
- El Ghossein, N.; Sari, A.; Venet, P.; Genies, S.; Azaïs, P. Post-Mortem Analysis of Lithium-Ion Capacitors after Accelerated Aging Tests. J. Energy Storage 2021, 33, 102039. [Google Scholar] [CrossRef]
- Han, X.; Lu, L.; Zheng, Y.; Feng, X.; Li, Z.; Li, J.; Ouyang, M. A review on the key issues of the lithium ion battery degradation among the whole life cycle. eTransportation 2019, 1, 100005. [Google Scholar] [CrossRef]
- Tian, H.; Qin, P.; Li, K.; Zhao, Z. A review of the state of health for lithium-ion batteries: Research status and suggestions. J. Clean. Prod. 2020, 261, 120813. [Google Scholar] [CrossRef]
- Mocera, F.; Soma, A.; Clerici, D. Study of aging mechanisms in lithium-ion batteries for working vehicle applications. In Proceedings of the 2020 Fifteenth International Conference on Ecological Vehicles and Renewable Energies, Monte-Carlo, Monaco, 10–12 September 2020. [Google Scholar]
- Woody, M.; Arbabzadeh, M.; Lewis, G.M.; Keoleian, G.A.; Stefanopoulou, A. Strategies to limit degradation and maximize Li-ion battery service lifetime-Critical review and guidance for stakeholders. J. Energy Storage 2020, 28, 101231. [Google Scholar] [CrossRef]
- Balakrishnan, P.G.; Ramesh, R.; Prem Kumar, T. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Broussely, M.; Biensan, P.; Bonhomme, F.; Blanchard, P.; Herreyre, S.; Nechev, K.; Staniewicz, R.J. Main aging mechanisms in Li ion batteries. J. Power Sources 2005, 146, 90–96. [Google Scholar] [CrossRef]
- Barré, A.; Deguilhem, B.; Grolleau, S.; Gérard, M.; Suard, F.; Riu, D. A review on lithium-ion battery ageing mechanisms and estimations for automotive applications. J. Power Sources 2013, 241, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Birkl, C.R.; Roberts, M.R.; McTurk, E.; Bruce, P.G.; Howey, D.A. Degradation diagnostics for lithium ion cells. J. Power Sources 2017, 341, 373–386. [Google Scholar] [CrossRef]
- Palacín, M.R. Understanding ageing in Li-ion batteries: A chemical issue. Chem. Soc. Rev. 2018, 47, 4924–4933. [Google Scholar] [CrossRef]
- Xiong, R.; Pan, Y.; Shen, W.; Li, H.; Sun, F. Lithium-ion battery aging mechanisms and diagnosis method for automotive applications: Recent advances and perspectives. Renew. Sustain. Energy Rev. 2020, 131, 110048. [Google Scholar] [CrossRef]
- Teichert, P.; Eshetu, G.G.; Jahnke, H.; Figgemeier, E. Degradation and aging routes of ni-rich cathode based li-ion batteries. Batteries 2020, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Alipour, M.; Ziebert, C.; Conte, F.V.; Kizilel, R. A review on temperature-dependent electrochemical properties, aging, and performance of lithium-ion cells. Batteries 2020, 6, 35. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, C.; Jiang, J.; Zhang, W.; Zhang, L.; Wang, Y. Review on state-of-health of lithium-ion batteries: Characterizations, estimations and applications. J. Clean. Prod. 2021, 314, 128015. [Google Scholar] [CrossRef]
- Balali, Y.; Stegen, S. Review of energy storage systems for vehicles based on technology, environmental impacts, and costs. Renew. Sustain. Energy Rev. 2021, 135, 110185. [Google Scholar] [CrossRef]
- Tesla Other Europe. Powerwall. Available online: https://www.tesla.com/en_eu/powerwall (accessed on 18 July 2021).
- Gerssen-Gondelach, S.J.; Faaij, A.P.C. Performance of batteries for electric vehicles on short and longer term. J. Power Sources 2012, 212, 111–129. [Google Scholar] [CrossRef]
- Ajanovic, A.; Haas, R. Electric vehicles: Solution or new problem? Environ. Dev. Sustain. 2018, 20, 7–22. [Google Scholar] [CrossRef]
- Ahmadian, A.; Mohammadi-Ivatloo, B.; Elkamel, A. A Review on Plug-in Electric Vehicles: Introduction, Current Status, and Load Modeling Techniques. J. Mod. Power Syst. Clean Energy 2020, 8, 412–425. [Google Scholar] [CrossRef]
- EVD Compare Hybrid and Electric Vehicles-EV Database UK 2019. Available online: https://ev-database.uk/ (accessed on 18 July 2021).
- Bureau of Transportation Statistics. Hybrid-Electric, Plug-in Hybrid-Electric and Electric Vehicle Sales. Available online: https://www.bts.gov/content/gasoline-hybrid-and-electric-vehicle-sales (accessed on 18 July 2021).
- Li, Y.; Guo, J.; Chen, Y.; Deng, S.; Zhu, J.; Cao, G.; Lei, T.; Zhang, J. Phase transition regulation and Cd-O/Cd-F compounds multi-effect. Ionics 2020, 26, 1681–1693. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, Y.; Wang, J.; Lin, J.; Wang, C.; Chen, Y. Lanthanum and cerium Co-doped LiFePO4: Morphology, electrochemical performance and kinetic study from −30–+50 °C. Electrochim. Acta 2019, 322, 134686. [Google Scholar] [CrossRef]
- Sui, X.; Świerczyński, M.; Teodorescu, R.; Stroe, D.I. The degradation behavior of lifepo4/c batteries during long-term calendar aging. Energies 2021, 14, 1732. [Google Scholar] [CrossRef]
- Zhu, J.; Cao, G.; Li, Y.; Wang, S.; Deng, S.; Guo, J.; Chen, Y.; Lei, T.; Zhang, J.; Chang, S. Nd2O3 encapsulation-assisted surface passivation of Ni-rich LiNi0.8Co0.1Mn0.1O2 active material and its electrochemical performance. Electrochim. Acta 2019, 325, 134889. [Google Scholar] [CrossRef]
- Wu, F.; Maier, J.; Yu, Y. Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lee, S.; Manthiram, A. High-Nickel NMA: A Cobalt-Free Alternative to NMC and NCA Cathodes for Lithium-Ion Batteries. Adv. Mater. 2020, 32, 2002718. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, F.; Liu, B.; Zhou, H.; Zhang, Q.; Kong, J.; Wang, Q. Cathode Material for High Energy Density Li-Ion Batteries. Int. J. Electrochem. 2018, 2018, 6930386. [Google Scholar] [CrossRef] [Green Version]
- Noh, H.; Youn, S.; Seung, C.; Sun, Y. Comparison of the structural and electrochemical properties cathode material for lithium-ion batteries. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, Z.; Dai, A.; Tang, L.; Wei, H.; Li, Y.; He, Z.; Lu, J. Boosting Cell Performance of LiNi0.8Co0.15Al0.05O2 via Surface Structure Design. Small 2019, 15, 1904854. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Liu, W.; Peng, Z.; Dua, K.; Cao, Y. Synthesis and electrochemical properties of LiNi0.8Co0.15Al0.05O2 prepared from the precursor Ni0.8Co0.15Al0.05OOH. J. Power Sources 2012, 198, 258–263. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Liu, S.; Deng, S.; Chen, Y.; Zhu, J.; Zhang, J.; Guo, J.; Chang, S. Superior Electrochemical and Kinetics Performance of LiNi0.8Co0.15Al0.05O2 Cathode by Neodymium Synergistic Modifying for Lithium Ion Batteries. J. Electrochem. Soc. 2020, 167, 090509. [Google Scholar] [CrossRef]
- He, Y.F.; Chu, D.Y.; Zhuo, Z. Cycle Stability of Dual-Phase Lithium Titanate (LTO)/TiO2 Nanowires as Lithium Battery Anode. J. Multidiscip. Appl. Nat. Sci. 2021, 1, 54–61. [Google Scholar] [CrossRef]
- Keil, P.; Jossen, A. Charging protocols for lithium-ion batteries and their impact on cycle life-An experimental study with different 18650 high-power cells. J. Energy Storage 2016, 6, 125–141. [Google Scholar] [CrossRef]
- Tomaszewska, A.; Chu, Z.; Feng, X.; O’Kane, S.; Liu, X.; Chen, J.; Ji, C.; Endler, E.; Li, R.; Liu, L.; et al. Lithium-ion battery fast charging: A review. eTransportation 2019, 1, 100011. [Google Scholar] [CrossRef]
- Serhan, H.A.; Ahmed, E.M. Effect of the different charging techniques on battery life-time: Review. In Proceedings of the 2018 International Conference on Innovative Trends in Computer Engineering, ITCE, Aswan, Egypt, 19–21 February 2018; pp. 421–426. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Acharya, A.B.; Sui, X.; Meng, J.; Teodorescu, R.; Stroe, D.I. A review of pulsed current technique for lithium-ion batteries. Energies 2020, 13, 2458. [Google Scholar] [CrossRef]
- Attia, P.M.; Grover, A.; Jin, N.; Severson, K.A.; Markov, T.M.; Liao, Y.H.; Chen, M.H.; Cheong, B.; Perkins, N.; Yang, Z.; et al. Closed-loop optimization of fast-charging protocols for batteries with machine learning. Nature 2020, 578, 397–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Li, Y.; Meng, J.; Sui, X.; Teodorescu, R.; Stroe, D.I. The Effect of Pulsed Current on the Performance of Lithium-ion Batteries. In Proceedings of the ECCE 2020-IEEE Energy Conversion Congress and Exposition, Detroit, MI, USA, 11–15 October 2020; pp. 5633–5640. [Google Scholar] [CrossRef]
- Paul, T.; Mesbahi, T.; Durand, S.; Flieller, D.; Uhring, W. Study and influence of standardized driving cycles on the sizing of li-ion battery / supercapacitor hybrid energy storage. In Proceedings of the 2019 IEEE Vehicle Power and Propulsion Conference, VPPC 2019-Proceedings, Hanoi, Vietnam, 14–17 October 2019. [Google Scholar] [CrossRef]
- Sikha, G.; Ramadass, P.; Haran, B.S.; White, R.E.; Popov, B.N. Comparison of the capacity fade of Sony US 18650 cells charged with different protocols. J. Power Sources 2003, 122, 67–76. [Google Scholar] [CrossRef]
- Varga, B.O.; Sagoian, A.; Mariasiu, F. Prediction of electric vehicle range: A comprehensive review of current issues and challenges. Energies 2019, 12, 946. [Google Scholar] [CrossRef] [Green Version]
- Jafari, M.; Gauchia, A.; Zhao, S.; Zhang, K.; Gauchia, L. Electric Vehicle Battery Cycle Aging Evaluation in Real-World Daily Driving and Vehicle-to-Grid Services. IEEE Trans. Transp. Electrif. 2017, 4, 122–134. [Google Scholar] [CrossRef]
- Patel, S.S.; Asakit, J. Real Driving Particulate Emissions from a Gasoline PHEV. Master’s Thesis, Chalmers University of Technology, Goteborg, Sweden, 2020. [Google Scholar]
- Ben-marzouk, M.; Pelissier, S.; Clerc, G.; Member, S.; Sari, A. Generation of a real-life battery usage pattern for electrical vehicle application and aging comparison with the WLTC profile. IEEE Trans. Veh. Technol. 2021, 9545, 5618–5627. [Google Scholar] [CrossRef]
- Eddahech, A.; Briat, O.; Woirgard, E.; Vinassa, J.M. Remaining useful life prediction of lithium batteries in calendar ageing for automotive applications. Microelectron. Reliab. 2012, 52, 2438–2442. [Google Scholar] [CrossRef]
- Swierczynski, M.; Stroe, D.I.; Stan, A.I.; Teodorescu, R.; Kær, S.K. Lifetime Estimation of the Nanophosphate LiFePO4/C Battery Chemistry Used in Fully Electric Vehicles. IEEE Trans. Ind. Appl. 2015, 51, 3453–3461. [Google Scholar] [CrossRef]
- Liu, J.; Duan, Q.; Ma, M.; Zhao, C.; Sun, J.; Wang, Q. Aging mechanisms and thermal stability of aged commercial 18650 lithium ion battery induced by slight overcharging cycling. J. Power Sources 2020, 445, 227263. [Google Scholar] [CrossRef]
- Mathieu, R.; Briat, O.; Gyan, P.; Vinassa, J.M. Comparison of the impact of fast charging on the cycle life of three lithium-ion cells under several parameters of charge protocol and temperatures. Appl. Energy 2021, 283, 116344. [Google Scholar] [CrossRef]
- Zhu, J.; Dewi Darma, M.S.; Knapp, M.; Sørensen, D.R.; Heere, M.; Fang, Q.; Wang, X.; Dai, H.; Mereacre, L.; Senyshyn, A.; et al. Investigation of lithium-ion battery degradation mechanisms by combining differential voltage analysis and alternating current impedance. J. Power Sources 2020, 448, 28–30. [Google Scholar] [CrossRef]
- Spingler, F.B.; Wittmann, W.; Sturm, J.; Rieger, B.; Jossen, A. Optimum fast charging of lithium-ion pouch cells based on local volume expansion criteria. J. Power Sources 2018, 393, 152–160. [Google Scholar] [CrossRef]
- You, H.; Dai, H.; Li, L. The Aging Law of Low Temperature Charging of Lithium-Ion Battery; SAE Technical Papers; Tongji University: Shanghai, China, 2019. [Google Scholar]
- Lindgren, J.; Lund, P.D. Effect of extreme temperatures on battery charging and performance of electric vehicles. J. Power Sources 2016, 328, 37–45. [Google Scholar] [CrossRef]
- Stroe, D.I.; Swierczynski, M.; Kær, S.K.; Laserna, E.M.; Zabala, E.S. Accelerated aging of lithium-ion batteries based on electric vehicle mission profile. In Proceedings of the 2017 IEEE Energy Conversion Congress and Exposition, ECCE 2017, Cincinnati, OH, USA, 1–5 October 2017; pp. 5631–5637. [Google Scholar] [CrossRef] [Green Version]
- Hosen, M.S.; Karimi, D.; Kalogiannis, T.; Pirooz, A.; Jaguemont, J.; Berecibar, M.; Van Mierlo, J. Electro-aging model development of nickel-manganese-cobalt lithium-ion technology validated with light and heavy-duty real-life profiles. J. Energy Storage 2020, 28, 101265. [Google Scholar] [CrossRef]
- Simolka, M.; Heger, J.F.; Kaess, H.; Biswas, I.; Friedrich, K.A. Influence of cycling profile, depth of discharge and temperature on commercial LFP/C cell ageing: Post-mortem material analysis of structure, morphology and chemical composition. J. Appl. Electrochem. 2020, 50, 1101–1117. [Google Scholar] [CrossRef]
- Ouyang, D.; Weng, J.; Chen, M.; Liu, J.; Wang, J. Experimental analysis on the degradation behavior of overdischarged lithium-ion battery combined with the effect of high-temperature environment. Int. J. Energy Res. 2020, 44, 229–241. [Google Scholar] [CrossRef]
- Lai, X.; Zheng, Y.; Zhou, L.; Gao, W. Electrical behavior of overdischarge-induced internal short circuit in lithium-ion cells. Electrochim. Acta 2018, 278, 245–254. [Google Scholar] [CrossRef]
- Ouyang, D.; Chen, M.; Liu, J.; Wei, R.; Weng, J.; Wang, J. Investigation of a commercial lithium-ion battery under overcharge/over-discharge failure conditions. RSC Adv. 2018, 8, 33414–33424. [Google Scholar] [CrossRef] [Green Version]
- De Hoog, J.; Timmermans, J.M.; Ioan-Stroe, D.; Swierczynski, M.; Jaguemont, J.; Goutam, S.; Omar, N.; Van Mierlo, J.; Van Den Bossche, P. Combined cycling and calendar capacity fade modeling of a Nickel-Manganese-Cobalt Oxide Cell with real-life profile validation. Appl. Energy 2017, 200, 47–61. [Google Scholar] [CrossRef]
- Bank, T.; Feldmann, J.; Klamor, S.; Bihn, S.; Sauer, D.U. Extensive aging analysis of high-power lithium titanate oxide batteries: Impact of the passive electrode effect. J. Power Sources 2020, 473, 228566. [Google Scholar] [CrossRef]
- Stroe, D.I.; Schaltz, E. Lithium-Ion Battery State-of-Health Estimation Using the Incremental Capacity Analysis Technique. IEEE Trans. Ind. Appl. 2020, 56, 678–685. [Google Scholar] [CrossRef]
- Ben-Marzouk, M.; Chaumond, A.; Redondo-Iglesias, E.; Montaru, M.; Pélissier, S. Experimental protocols and first results of calendar and/or cycling aging study of lithium-ion batteries-The MOBICUS project. World Electr. Veh. J. 2016, 8, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Ashwin, T.R.; Hu, X.; Lucu, M.; Widanage, W.D. An evaluation study of different modelling techniques for calendar ageing prediction of lithium-ion batteries. Renew. Sustain. Energy Rev. 2020, 131, 110017. [Google Scholar] [CrossRef]
- Keil, P.; Jossen, A. Impact of Dynamic Driving Loads and Regenerative Braking on the Aging of Lithium-Ion Batteries in Electric Vehicles. J. Electrochem. Soc. 2017, 164, A3081–A3092. [Google Scholar] [CrossRef]
- Keil, P.; Schuster, S.F.; Wilhelm, J.; Travi, J.; Hauser, A.; Karl, R.C.; Jossen, A. Calendar Aging of Lithium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1872–A1880. [Google Scholar] [CrossRef]
- Zilberman, I.; Sturm, J.; Jossen, A. Reversible self-discharge and calendar aging of 18650 nickel-rich, silicon-graphite lithium-ion cells. J. Power Sources 2019, 425, 217–226. [Google Scholar] [CrossRef]
- Wang, D.; Coignard, J.; Zeng, T.; Zhang, C.; Saxena, S. Quantifying electric vehicle battery degradation from driving vs. vehicle-to-grid services. J. Power Sources 2016, 332, 193–203. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Deng, C.; Zhang, Y.; He, Y. State of charge estimation for lithium-ion battery with a temperature-compensated model. Energies 2017, 10, 1560. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Heng, S.; Wang, Y.; Qu, Q.; Zheng, H. Anchoring Interfacial Nickel Cations on Single-Crystal LiNi0.8Co0.1Mn0.1O2 Cathode Surface via Controllable Electron Transfer. ACS Energy Lett. 2020, 5, 2421–2433. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Chen, Y.; Deng, S.; Zhu, J.; Wang, S.; Zhang, J.; Chang, S.; Zhang, D.; Xi, X. Stable interface Co3O4 -coated LiNi0.5 Mn1.5 O4 for lithium-ion batteries. J. Alloys Compd. 2019, 811, 152031. [Google Scholar] [CrossRef]
- Zhou, R.; Huang, J.; Lai, S.; Li, J.; Wang, F.; Chen, Z.; Lin, W.; Li, C.; Wang, J.; Zhao, J. A bifunctional electrolyte additive for H2O/HF scavenging and enhanced graphite/LiNi0.5Co0.2Mn0.3O2 cell performance at a high voltage. Sustain. Energy Fuels 2018, 2, 1481–1490. [Google Scholar] [CrossRef]
- Hemmelmann, H.; Dinter, J.K.; Elm, M.T. Thin Film NCM Cathodes as Model Systems to Assess the Influence of Coating Layers on the Electrochemical Performance of Lithium Ion Batteries. Adv. Mater. Interfaces 2021, 8, 2002074. [Google Scholar] [CrossRef]
- Jie, Y.; Ren, X.; Cao, R.; Cai, W.; Jiao, S. Advanced Liquid Electrolytes for Rechargeable Li Metal Batteries. Adv. Funct. Mater. 2020, 30, 1910777. [Google Scholar] [CrossRef]
- Hekmatfar, M.; Hasa, I.; Eghbal, R.; Carvalho, D.V.; Moretti, A.; Passerini, S. Effect of Electrolyte Additives on the LiNi0.5Mn0.3Co0.2O2 Surface Film Formation with Lithium and Graphite Negative Electrodes. Adv. Mater. Interfaces 2020, 7, 1901500. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.Z.; Zhao, Q.; Liu, X.; Zheng, J.; Stalin, S.; Zhang, Q.; Archer, L.A. Rechargeable Lithium Metal Batteries with an In-Built Solid-State Polymer Electrolyte and a High Voltage/Loading Ni-Rich Layered Cathode. Adv. Mater. 2020, 32, 1905629. [Google Scholar] [CrossRef]
- Li, Y.; Wan, S.; Veith, G.M.; Unocic, R.R.; Paranthaman, M.P.; Dai, S.; Sun, X.G. A Novel Electrolyte Salt Additive for Lithium-Ion Batteries with Voltages Greater than 4.7 V. Adv. Energy Mater. 2017, 7, 1601397. [Google Scholar] [CrossRef]
- Heiskanen, S.K.; Kim, J.; Lucht, B.L. Generation and Evolution of the Solid Electrolyte Interphase of Lithium-Ion Batteries. Joule 2019, 3, 2322–2333. [Google Scholar] [CrossRef]
- Horsthemke, F.; Leißing, M.; Winkler, V.; Friesen, A.; Ibing, L.; Winter, M.; Nowak, S. Development of a lithium ion cell enabling in situ analyses of the electrolyte using gas chromatographic techniques. Electrochim. Acta 2020, 338, 135894. [Google Scholar] [CrossRef]
- Wang, C.; Yu, L.; Fan, W.; Liu, J.; Ouyang, L.; Yang, L.; Zhu, M. Lithium Difluorophosphate As a Promising Electrolyte Lithium Additive for High-Voltage Lithium-Ion Batteries. ACS Appl. Energy Mater. 2018, 1, 2647–2656. [Google Scholar] [CrossRef]
- Deng, K.; Zeng, Q.; Wang, D.; Liu, Z.; Wang, G.; Qiu, Z.; Zhang, Y.; Xiao, M.; Meng, Y. Nonflammable organic electrolytes for high-safety lithium-ion batteries. Energy Storage Mater. 2020, 32, 425–447. [Google Scholar] [CrossRef]
- Schultz, C.; Vedder, S.; Streipert, B.; Winter, M.; Nowak, S. Quantitative investigation of the decomposition of organic lithium ion battery electrolytes with LC-MS/MS. RSC Adv. 2017, 7, 27853–27862. [Google Scholar] [CrossRef] [Green Version]
- Rinkel, B.L.D.; Hall, D.S.; Temprano, I.; Grey, C.P. Electrolyte oxidation pathways in lithium-ion batteries. J. Am. Chem. Soc. 2020, 142, 15058–15074. [Google Scholar] [CrossRef]
- Gerelt-Od, B.; Kim, J.; Shin, E.; Kang, H.; Kim, N.; Jo, C.; Son, H.; Yoon, S. In situ Raman investigation of resting thermal effects on gas emission in charged commercial 18650 lithium ion batteries. J. Ind. Eng. Chem. 2021, 96, 339–344. [Google Scholar] [CrossRef]
- Fedorov, R.G.; Maletti, S.; Heubner, C.; Michaelis, A.; Ein-Eli, Y. Molecular Engineering Approaches to Fabricate Artificial Solid-Electrolyte Interphases on Anodes for Li-Ion Batteries: A Critical Review. Adv. Energy Mater. 2021, 2101173, 2101173. [Google Scholar] [CrossRef]
- An, S.J.; Li, J.; Daniel, C.; Mohanty, D.; Nagpure, S.; Wood, D.L. The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon 2016, 105, 52–76. [Google Scholar] [CrossRef] [Green Version]
- Agubra, V.; Fergus, J. Lithium ion battery anode aging mechanisms. Materials 2013, 6, 1310–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Cheng, X.B.; Tian, Y.; Chen, X.; Zhang, X.Q.; Li, W.J.; Huang, J.Q.; Zhang, Q. Dual-Layered Film Protected Lithium Metal Anode to Enable Dendrite-Free Lithium Deposition. Adv. Mater. 2018, 30, 1707629. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Wang, S.J.; Wang, H.; Xu, B.; Hu, C.; Jin, Y.; Liu, J.B.; Yan, H. The suppression of lithium dendrite growth in lithium sulfur batteries: A review. J. Energy Storage 2017, 13, 387–400. [Google Scholar] [CrossRef]
- Persson, K.; Sethuraman, V.A.; Hardwick, L.J.; Hinuma, Y.; Meng, Y.S.; Van Der Ven, A.; Srinivasan, V.; Kostecki, R.; Ceder, G. Lithium diffusion in graphitic carbon. J. Phys. Chem. Lett. 2010, 1, 1176–1180. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xing, L.; Zhang, L.; Yu, L.; Fan, W.; Xu, M.; Li, W. Insight into self-discharge of layered lithium-rich oxide cathode in carbonate-based electrolytes with and without additive. J. Power Sources 2016, 324, 17–25. [Google Scholar] [CrossRef]
- Liao, X.; Huang, Q.; Mai, S.; Wang, X.; Xu, M.; Xing, L.; Liao, Y.; Li, W. Understanding self-discharge mechanism of layered nickel cobalt manganese oxide at high potential. J. Power Sources 2015, 286, 551–556. [Google Scholar] [CrossRef]















| References | Topic | Chemistries | ||
|---|---|---|---|---|
| Operation | Degradation | Aging Mechanism | ||
| Han et al., 2019 [5] | √ | √ | LMO, LCO, LFP, NMC | |
| Tian et al., 2020 [6] | √ | LMO, LFP, NMC | ||
| Mocera et al., 2020 [7] | √ | LFP | ||
| Woody et al., 2020 [8] | √ | √ | LCO, LMO, LFP, NCA, NMC | |
| Vetter et al., 2005 [9] | √ | LCO, LMO, NMC | ||
| Broussely et al., 2005 [10] | √ | LCO, NMC | ||
| Barre et al., 2013 [11] | √ | √ | LCO | |
| Birkl et al., 2017 [12] | √ | LCO | ||
| Palacin et al., 2018 [13] | √ | LMO, LCO, NMC, NCA | ||
| Xiong et al., 2020 [14] | √ | √ | LFP, NCA, NMC | |
| Teichert et al., 2020 [15] | √ | NMC | ||
| Alipour et al., 2020 [16] | √ | √ | LCO, LFP, NMC, NCA | |
| Chen et al., 2021 [17] | √ | √ | LCO | |
| Yang et al., 2021 [18] | √ | √ | NMC, NCA | |
| Type | Manufacturer | Battery-Only Range (km) | Battery Capacity (kWh) |
|---|---|---|---|
| HEV | Toyota Prius IV | / | 1.3–1.6 |
| BMW 225xe | |||
| Audi A3 e-tron | |||
| Toyota Prius III | |||
| PHEV | Chevy Volt | 64 | 18.4 |
| Toyota Prius XW30 | 21 | 5.2 | |
| Jaguar I-Pace | 470 | 90.0 | |
| BEV | Tesla Model S | 663 | 100.0 |
| BMW i3 | 246 | 42.2 | |
| Nissan Leaf | 364 | 62.0 | |
| Renault Zoe | 395 | 52 |
| Electrode | VAverage (v) | VMax (v) | Specific Capacity (mAh g−1/mAh cm−3) | Gravimetric Energy (wh kg−1) | |
|---|---|---|---|---|---|
| Cathode | LiNi0.8Co0.15Al0.05O2 | 3.7 | 4.6 | 220/979 | 758 |
| LiNi1/3Co1/3Mn1/3O2 | 3.6 | 4.7 | 160/712 | 576 | |
| LiNi0.5Co0.2Mn0.3O2 | 3.6 | 4.7 | 170/757 | 612 | |
| LiNi0.6Co0.2Mn0.2O2 | 3.6 | 4.7 | 180/810 | 648 | |
| LiNi0.7Co0.15Mn0.15O2 | 3.6 | 4.7 | 190/855 | 684 | |
| LiNi0.8Co0.1Mn0.1O2 | 3.6 | 4.7 | 200/930 | 720 | |
| Anode | graphite | 0.6 | 3 | 372/735 | 190 |
| LTO | 1.55 | 2.5 | 175/607 | 263.5 | |
| Battery Type | Chemistry | Temperature °C | SOC (%) | Main Conclusion | Sources |
|---|---|---|---|---|---|
| SIMCAL (2009–2012) | NMC | 0, 25, 45 and 60 | 0, 30, 65, 80 and 100 | Higher SOC leads to higher capacity loss; temperature over 30 °C begin to accelerate the aging | [67] |
| Panasonic NCR18650BD | LiC6/NCA | 10, 25 and 45 | 20, 50 and 90 | Accelerate aging obviously occurs in 45 °C or 90% SOC case | [68] |
| Panasonic NCR18650PD | G/NCA | 10, 25 and 40 | 30, 45, 60, 75 and 90 | Higher temperature leads to higher capacity fade from 10 °C to 40 °C | [69] |
| Panasonic NCR18650PD | G/NCA | 25, 40 and 50 | 5, 10, 20, 30, 40, 45, 50, 55, 60, 65, 70, 80, 90, 95 and 100 | Accelerate aging with the increasing of SOC or storage temperature | [70] |
| SanyoUR18650E | G/NMC | ||||
| EIG NMC pouch cell | G/NMC | 10, 25 and 45 | 10, 40 and 80 | Higher SOC accelerates capacity fade, which is prominent at a higher temperature than at moderate temperature | [59] |
| Pouch cell | LTO/NMC | 40, 60 and 80 | 5, 20, 55, 70, 90 and 95 | There is no obvious relationship between temperature and capacity fade for LTO/NMC, while the internal resistance increased significantly. | [65] |
| EIG NMC pouch cell | G/NMC | 25, 35 and 45 | 20, 35, 50, 65, 80 and 100 | Accelerate aging with the increasing of SOC or storage temperature | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Li, Y.; Pedersen, K.; Stroe, D.-I. Lithium-Ion Battery Operation, Degradation, and Aging Mechanism in Electric Vehicles: An Overview. Energies 2021, 14, 5220. https://0-doi-org.brum.beds.ac.uk/10.3390/en14175220
Guo J, Li Y, Pedersen K, Stroe D-I. Lithium-Ion Battery Operation, Degradation, and Aging Mechanism in Electric Vehicles: An Overview. Energies. 2021; 14(17):5220. https://0-doi-org.brum.beds.ac.uk/10.3390/en14175220
Chicago/Turabian StyleGuo, Jia, Yaqi Li, Kjeld Pedersen, and Daniel-Ioan Stroe. 2021. "Lithium-Ion Battery Operation, Degradation, and Aging Mechanism in Electric Vehicles: An Overview" Energies 14, no. 17: 5220. https://0-doi-org.brum.beds.ac.uk/10.3390/en14175220