Purpose in Thermodynamics
Abstract
:1. Introduction
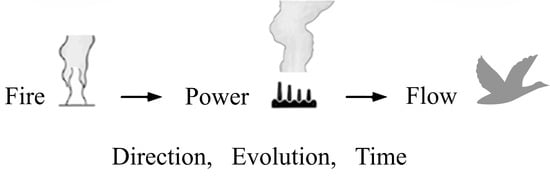
2. Fire
- (i)
- With fire we have access to power.
- (ii)
- With the freedom to change the shape we have access to even more power.
3. More Power
4. Methods
4.1. Exergy Analysis (EA)
4.2. Entropy Generation Minimization (EGM)
4.3. Cumulative Exergy Consumption (CEC)
4.4. Extended Exergy Analysis
4.5. Cost Minimization
4.6. Minimization of Environmental Impact
4.7. Pinch Analysis
4.8. Finite Time Thermodynamics (FTT)
- It generates power because it receives a heat current from its environment.
- Heat input comes from a fuel (a part of the environment) that is burned at a finite (expensive) rate; the fuel is hard to come by, it is eminently not a ‘reservoir’.
- Even in a heat engine driven by solar heating (on earth, or in outer space), the heating rate is finite because it is dictated by the size of the collector, which is finite and expensive (land area, materials, construction costs, mass launched in orbit).
- On earth, where people with their ‘heat engines’ and science live, there is only one heat reservoir: the ambient (atmosphere, hydrosphere, T0).
4.9. Constructal Law
5. Remarks
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| E | energy, J |
| EW | exergy, J |
| h° | specific enthalpy, J/kg, including kinetic and gravitational energy |
| mass flow rate, kg/s | |
| M | mass, kg |
| n | number of temperature reservoirs |
| P | pressure, Pa |
| heat transfer rate, W | |
| s | specific entropy, J/(kg K) |
| S | entropy, J/K |
| entropy generation rate, W/K | |
| t | time, s |
| T | temperature, K |
| V | volume, m3 |
| work transfer rate, W | |
| environment |
References
- Kakac, S. Evolution of the science of thermodynamics: The history. J. Therm. Sci. Technol. 2016, 36, 1–6. [Google Scholar]
- Bejan, A. Evolution in thermodynamics. Appl. Phys. Rev. 2017, 4, 011305. [Google Scholar] [CrossRef]
- Bejan, A. Thermodynamics today. Energy 2018, 160, 1208–1219. [Google Scholar] [CrossRef]
- Bejan, A. Thermodynamics of heating. Proc. R. Soc. Math. Phys. Eng. Sci 2019, 475, 20180820. [Google Scholar] [CrossRef]
- Schmidt-Nielsen, K. Scaling: Why Is Animal Size So Important? Cambridge University Press: Cambridge, UK, 1984. [Google Scholar]
- Weibel, E.R. Symmorphosis: On Form and Function in Shaping Life; Harvard University Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Hoppeler, H.; Weibel, E.R. Scaling functions to body size: Theories and facts. J. Exp. Biol. 2005, 208, 1573–1574. [Google Scholar] [CrossRef] [Green Version]
- Miguel, A.F. Constructal pattern formation in stony corals, bacterial colonies and plant roots under different hydrodynamics conditions. J. Theor. Biol. 2006, 242, 954–961. [Google Scholar] [CrossRef]
- Reis, A.H.; Miguel, A.F.; Aydin, M. Constructal theory of flow architecture of the lungs. Med. Phys. 2004, 31, 1135–1140. [Google Scholar] [CrossRef] [Green Version]
- Kasimova, R.G.; Obnosov, Y.V.; Baksht, F.B.; Kacimov, A.R. Optimal shape of an anthill dome: Bejan’s constructal law revisited. Ecol. Model. 2013, 250, 384–390. [Google Scholar] [CrossRef]
- Bejan, A.; Marden, J.H. Unifying constructal theory for scale effects in running, swimming and flying. J. Exp. Biol. 2006, 209, 238–248. [Google Scholar] [CrossRef] [Green Version]
- Weibel, E.R.; Webel, E.R.; Taylor, C.R.; Bolis, L. Principles of Animal Design: The Optimization and Symmorphosis Debate; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Turner, J.S. Purpose and Desire; HarperCollins: New York, NY, USA, 2017. [Google Scholar]
- Reis, A.H. Design in nature, and the laws of physics. Phys. Life Rev. 2011, 8, 255–256. [Google Scholar] [CrossRef]
- Wang, L. Universality of design and its evolution. Phys. Life Rev. 2011, 8, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Bejan, A.; Zane, J.P. Design in Nature: How the Constructal Law Governs Evolution in Biology, Physics, Technology, and Social Organization; Doubleday: New York, NY, USA, 2012. [Google Scholar]
- Bejan, A. The Physics of Life: The Evolution of Everything; St. Martin’s Press: New York, NY, USA, 2016. [Google Scholar]
- Bejan, A. Why humans build fires shaped the same way. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bejan, A.; Perin, S. Constructal Theory of Egyptian Pyramids and Flow Fossils in General. In Advanced Engineering Thermodynamics, 3rd ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Bejan, A. Freedom and Evolution; Springer Nature: New York, NY, USA, 2020. [Google Scholar]
- Bejan, A. Advanced Engineering Thermodynamics, 4th ed.; John Wiley & Sons Inc: Hoboken, NJ, USA, 2016. [Google Scholar]
- Cambel, A. (Ed.) Second law analysis of energy devices and processes. Energy 1980, 5, 665–1012. [Google Scholar]
- Bejan, A.; Tsatsaronis, G. Thermal Design and Optimization; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Rant, Z. Exergie ein neues wort für technische arbeitsfähigkeit. Forsch Im Ingenieurwesen 1956, 22, 36. [Google Scholar]
- Tsatsaronis, G.; Pisa, J. Exergoeconomic evaluation and optimization of energy systems—Application to the CGAM problem. Energy 1994, 19, 287–321. [Google Scholar] [CrossRef]
- Szargut, J. Application of exergy to the approximate economic optimization. Brennst Wärme Kraft 1971, 23, 516–519. [Google Scholar]
- Camberos, J.A.; Moorhouse, D.J. Exergy Analysis and Design Optimization for Aerospace Vehicles and Systems; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 2011; Volume 238. [Google Scholar]
- Kotas, T.J. The Exergy Method of Thermal Plant. Analysis, 2nd ed.; Krieger Publishing Company: Malabar, FL, USA, 1995. [Google Scholar]
- Szargut, J.; Morris, D.R.; Steward, F.R. Exergy Analysis of Thermal, Chemical, and Metallurgical Processes; Hemisphere: New York, NY, USA, 1988. [Google Scholar]
- Valero, A.; Lozano, M.A.; Serra, L.; Torres, C. Application of the exergetic cost theory to the CGAM problem. Energy 1994, 19, 365–381. [Google Scholar] [CrossRef]
- Melli, R.; Paoletti, B.; Sciubba, E. Design and functional optimization of thermo-mechanical plants via an interactive expert system. In Computer-Aided Energy System Analysis; ASME: New York, NY, USA, 1990. [Google Scholar]
- Kenney, W.F. Energy Conservation in the Process. Industries; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Tsatsaronis, G. Strengths and Limitations of Exergy Analysis. In Thermodynamic Optimization of Complex Energy Systems; Bejan, A., Mamut, E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 93–100. [Google Scholar] [CrossRef]
- Tsatsaronis, G. Combination of Exergetic and Economic Analysis in Energy-Conversion Processes. In Energy Economics and Management in Industry: Proceedings of the European Congress, Algarve, Portugal, 2–5 April 1984; Pergamon Press: Oxford, UK, 1985; pp. 151–157. [Google Scholar]
- Tsatsaronis, G.; Park, M.-H. On avoidable and unavoidable exergy destructions and investment costs in thermal systems. Energy Convers. Manag. 2002, 43, 1259–1270. [Google Scholar] [CrossRef]
- Kelly, S.; Tsatsaronis, G.; Morosuk, T. Advanced exergetic analysis: Approaches for splitting the exergy destruction into endogenous and exogenous parts. Energy 2009, 34, 384–391. [Google Scholar] [CrossRef]
- Morosuk, T.; Tsatsaronis, G. Advanced Exergetic Analysis is a Modern Tool for Evaluation and Optimization of Refrigeration Systems. In Handbook of Research on Advances and Applications in Refrigeration Systems and Technologies; Gaspar, P.D., da Silva, P.D., Eds.; IGI Global: Hershey, PA, USA, 2015; pp. 5–105. [Google Scholar] [CrossRef]
- Tsatsaronis, G. Exergoeconomics and Exergoenvironmental Analysis. In Thermodynamics and the Destruction of Resources; Bakshi, B.R., Gutowski, T., Sekulic, D., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 377–401. [Google Scholar] [CrossRef]
- Bejan, A. Entropy Generation through Heat and Fluid Flow; Wiley: New York, NY, USA, 1982. [Google Scholar]
- Bejan, A. Entropy Generation Minimization: The Method of Thermodynamic Optimization of Finite-Size Systems and Finite-Time Processes; CRC Press: Boca Raton, FL, USA; New York, NY, USA, 1995. [Google Scholar]
- Naterer, G.F.; Camberos, J.A. Entropy-Based Design and Analysis of Fluids Engineering Systems; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Giangaspero, G.; Sciubba, E. Application of the entropy generation minimization method to a solar heat exchanger: A pseudo-optimization design process based on the analysis of the local entropy generation maps. Energy 2013, 58, 52–65. [Google Scholar] [CrossRef]
- Haseli, Y.; Dincer, I.; Naterer, G.F. Entropy generation of vapor condensation in the presence of a non-condensable gas in a shell and tube condenser. Int. J. Heat Mass. Transf. 2008, 51, 1596–1602. [Google Scholar] [CrossRef]
- Jankowski, T.A. Minimizing entropy generation in internal flows by adjusting the shape of the cross-section. Int. J. Heat Mass. Transf. 2009, 52, 3439–3445. [Google Scholar] [CrossRef]
- Maheshkumar, P.; Muraleedharan, C. Minimization of entropy generation in flat heat pipe. Int. J. Heat Mass. Transf. 2011, 54, 645–648. [Google Scholar] [CrossRef]
- Haseli, Y. Efficiency improvement of thermal power plants through specific entropy generation. Energy Conv. Manag. 2018, 159, 109–120. [Google Scholar] [CrossRef]
- Hung, Y.-M. A comparative study of viscous dissipation effect on entropy generation in single-phase liquid flow in microchannels. Int. J. Therm. Sci. 2009, 48, 1026–1035. [Google Scholar] [CrossRef]
- Mirzazadeh, M.; Shafaei, A.; Rashidi, F. Entropy analysis for non-linear viscoelastic fluid in concentric rotating cylinders. Int. J. Therm. Sci. 2008, 47, 1701–1711. [Google Scholar] [CrossRef]
- Bejan, A. Letter to the editor on “Temperature-heat diagram analysis method for heat recovery physical adsorption refrigeration cycle—Taking multi stage cycle as an example” by Xu, S.Z.; et al., vol. 74, 2017, pp. 254–268. Int. J. Refrig. 2018, 90, 277–279. [Google Scholar] [CrossRef]
- Sciacovelli, A.; Verda, V.; Sciubba, E. Entropy generation analysis as a design tool—A review. Renew. Sustain. Energy Rev. 2015, 43, 1167–1181. [Google Scholar] [CrossRef]
- Bejan, A. The Concept of Irreversibility in Heat Exchanger Design: Counterflow Heat Exchangers for Gas-to-Gas Applications. J. Heat Transf. 1977, 99, 374–380. [Google Scholar] [CrossRef]
- Bejan, A. A Study of Entropy Generation in Fundamental Convective Heat Transfer. J. Heat Transf. 1979, 101, 718–725. [Google Scholar] [CrossRef]
- Benedetti, P.; Sciubba, E. Numerical calculation of the local rate of entropy generation in the flow around a heated finned-tube. ASME HTD 1993, 30, 81–91. [Google Scholar]
- Lior, N.; Rudy, G.J. Second-law analysis of an ideal Otto cycle. Energy Convers. Manag. 1988, 28, 327–334. [Google Scholar] [CrossRef]
- Arpaci, V.S.; Selamet, A. Entropy Production in Flames. Combust. Flame 1988, 73, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Bidi, M.; Nobari, M.R.H.; Avval, M.S. A numerical evaluation of combustion in porous media by EGM (Entropy Generation Minimization). Energy 2010, 35, 3483–3500. [Google Scholar] [CrossRef]
- Bejan, A.; Lorente, S.; Martins, L.; Meyer, J.P. The constructal size of a heat exchanger. J. Appl. Phys. 2017, 122, 064902. [Google Scholar] [CrossRef]
- Szargut, J. Analysis of cumulative exergy consumption. Int. J. Energy Res. 1987, 11, 541–547. [Google Scholar] [CrossRef]
- Morris, D.R. Exergy analysis and cumulative exergy consumption of complex chemical processes: The industrial chlor-alkali processes. Chem. Eng. Sci. 1991, 46, 459–465. [Google Scholar] [CrossRef]
- Sciubba, E. Beyond thermoeconomics? The concept of Extended Exergy Accounting and its application to the analysis and design of thermal systems. Exergy Int. J. 2001, 1, 68–84. [Google Scholar] [CrossRef]
- Stoll, H.G. Least-Cost Electric Utility Planning; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Gundepsen, T.; Naess, L. The synthesis of cost optimal heat exchanger networks: An industrial review of the state of the art. Comput Chem. Eng. 1988, 12, 503–530. [Google Scholar] [CrossRef]
- Tsatsaronis, G. Thermoeconomic analysis and optimization of energy systems. Prog. Energy Combust. Sci. 1993, 19, 227–257. [Google Scholar] [CrossRef]
- Mishra, M.; Das, P.K. Thermoeconomic design-optimisation of crossflow plate-fin heat exchanger using Genetic Algorithm. Int. J. Exergy 2009, 6, 837–852. [Google Scholar] [CrossRef]
- Rocco, M.V.; Colombo, E.; Sciubba, E. Advances in exergy analysis: A novel assessment of the Extended Exergy Accounting method. Appl. Energy 2014, 113, 1405–1420. [Google Scholar] [CrossRef]
- Ahadi-Oskui, T.; Alperin, H.; Nowak, I.; Cziesla, F.; Tsatsaronis, G. A relaxation-based heuristic for the design of cost-effective energy conversion systems. Energy 2006, 31, 1346–1357. [Google Scholar] [CrossRef] [Green Version]
- Ahadi-Oskui, T.; Vigerske, S.; Nowak, I.; Tsatsaronis, G. Optimizing the design of complex energy conversion systems by Branch and Cut. Comput Chem. Eng. 2010, 34, 1226–1236. [Google Scholar] [CrossRef] [Green Version]
- Jüdes, M.; Vigerske, S.; Tsatsaronis, G. Optimization of the Design and Partial-Load Operation of Power Plants Using Mixed-Integer Nonlinear Programming. In Optimization in the Energy Industry; Kallrath, J., Pardalos, P.M., Rebennack, S., Scheidt, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 193–220. [Google Scholar] [CrossRef] [Green Version]
- Jüdes, M.; Tsatsaronis, G. Improving Mathematical Optimization Techniques with the Aid of Exergy-Based Variables. Int. J. Thermodyn. 2009, 12, 75–82. [Google Scholar]
- Bausa, J.; Tsatsaronis, G. Dynamic Optimization of Startup and Load-Increasing Processes in Power Plants—Part I: Method. J. Eng. Gas. Turbines Power 2001, 123, 246–250. [Google Scholar] [CrossRef]
- Tsatsaronis, G.; Moran, M.J. Exergy-aided cost minimization. Energy Convers. Manag. 1997, 38, 1535–1542. [Google Scholar] [CrossRef]
- Tsatsaronis, G.; Winhold, M. Exergoeconomic analysis and evaluation of energy-conversion plants—I. A new general methodology. Energy 1985, 10, 69–80. [Google Scholar] [CrossRef]
- Tsatsaronis, G.; Lin, L.; Tawfik, T.; Gallaspy, D.T. Exergoeconomic Evaluation of a KRW-Based IGCC Power Plant. J. Eng. Gas. Turbines Power 1994, 116, 300–306. [Google Scholar] [CrossRef]
- Hamdy, S.; Morosuk, T.; Tsatsaronis, G. Exergoeconomic optimization of an adiabatic cryogenics-based energy storage system. Energy 2019, 183, 812–824. [Google Scholar] [CrossRef]
- Tsatsaronis, G. Design Optimization Using Exergoeconomics. In Thermodynamic Optimization of Complex Energy Systems; Bejan, A., Mamut, E., Eds.; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1999; pp. 101–115. [Google Scholar]
- Tsatsaronis, G.; Morosuk, T. Understanding and improving energy conversion systems with the aid of exergy-based methods. Int. J. Exergy 2012, 11, 518–542. [Google Scholar] [CrossRef]
- Tsatsaronis, G.; Winhold, M. Thermoeconomic Analysis of Power Plants; EPRI AP-3651, RP 2029-8. Final Report; Electric Power Research Institute: Palo Alto, CA, USA, 1984. [Google Scholar]
- Lazzaretto, A.; Tsatsaronis, G. SPECO: A systematic and general methodology for calculating efficiencies and costs in thermal systems. Energy 2006, 31, 1257–1289. [Google Scholar] [CrossRef]
- Tsatsaronis, G. Definitions and nomenclature in exergy analysis and exergoeconomics. Energy 2007, 32, 249–253. [Google Scholar] [CrossRef]
- The Exergo Ecology Portal 2018. Available online: http://www.exergoecology.com (accessed on 7 November 2019).
- Tsatsaronis, G.; Morosuk, T. Understanding the Formation of Costs and Environmental Impacts Using Exergy-Based Methods. In Energy Security and Development; Reddy, B.S., Ulgiati, S., Eds.; Springer: New Delhi, India, 2015; pp. 271–291. [Google Scholar] [CrossRef]
- Tsatsaronis, G. Recent developments in exergy analysis and exergoeconomics. Int. J. Exergy 2008, 5, 489–499. [Google Scholar] [CrossRef]
- Morosuk, T.; Tsatsaronis, G. Advanced Exergy Analysis for Chemically Reacting Systems—Application to a Simple Open Gas-Turbine System. Int. J. Thermodyn. 2009, 12, 105–111. [Google Scholar]
- Morosuk, T.; Tsatsaronis, G. Advanced exergetic evaluation of refrigeration machines using different working fluids. Energy 2009, 34, 2248–2258. [Google Scholar] [CrossRef]
- Petrakopoulou, F.; Boyano, A.; Cabrera, M.; Tsatsaronis, G. Exergoeconomic and exergoenvironmental analyses of a combined cycle power plant with chemical looping technology. Int. J. Greenh. Gas. Control. 2011, 5, 475–482. [Google Scholar] [CrossRef]
- Petrakopoulou, F.; Tsatsaronis, G.; Morosuk, T. Exergoeconomic Analysis of an Advanced Zero Emission Plant. J. Eng. Gas. Turbines Power 2011, 133, 113001. [Google Scholar] [CrossRef]
- Petrakopoulou, F.; Tsatsaronis, G.; Morosuk, T.; Carassai, A. Conventional and advanced exergetic analyses applied to a combined cycle power plant. Energy 2012, 41, 146–152. [Google Scholar] [CrossRef]
- Petrakopoulou, F.; Tsatsaronis, G.; Morosuk, T. Evaluation of a power plant with chemical looping combustion using an advanced exergoeconomic analysis. Sustain. Energy Technol. Assess. 2013, 3, 9–16. [Google Scholar] [CrossRef]
- Penkuhn, M.; Tsatsaronis, G. A decomposition method for the evaluation of component interactions in energy conversion systems for application to advanced exergy-based analyses. Energy 2017, 133, 388–403. [Google Scholar] [CrossRef]
- Valero, A.; Valero, A.; Stanek, W. Assessing the exergy degradation of the natural capital: From Szargut’s updated reference environment to the new thermoecological-cost methodology. Energy 2018, 163, 1140–1149. [Google Scholar] [CrossRef]
- Frangopoulos, C.A. Introduction to environomics. In Symposium onThermodynamics of Energy Systems; Reistad, G.M., Ed.; ASME: Atlanta, GA, USA, 1991; pp. 49–54. [Google Scholar]
- Frangopoulos, C.A.; von Spakovsky, M.R. A global environomic approach for energy systems analysis and optimization. In Proceedings of the Energy Systems and Ecology: Proceedings of the International Conference (ENSEC 93), Cracow, Poland, 5–9 July 1993; Szargut, J., Ed.; Advanced Energy Systems Division, American Society of Mechanical Engineers: Atlanta, GA, USA, 1993; pp. 123–132. [Google Scholar]
- Meyer, L.; Tsatsaronis, G.; Buchgeister, J.; Schebek, L. Exergoenvironmental analysis for evaluation of the environmental impact of energy conversion systems. Energy 2009, 34, 75–89. [Google Scholar] [CrossRef]
- Boyano, A.; Blanco-Marigorta, A.M.; Morosuk, T.; Tsatsaronis, G. Exergoenvironmental analysis of a steam methane reforming process for hydrogen production. Energy 2011, 36, 2202–2214. [Google Scholar] [CrossRef]
- Petrakopoulou, F.; Tsatsaronis, G.; Morosuk, T.; Paitazoglou, C. Environmental evaluation of a power plant using conventional and advanced exergy-based methods. Energy 2012, 45, 23–30. [Google Scholar] [CrossRef]
- Morosuk, T.; Tsatsaronis, G.; Koroneos, C. Environmental impact reduction using exergy-based methods. J. Clean Prod. 2016, 118, 118–123. [Google Scholar] [CrossRef]
- Lara, Y.; Petrakopoulou, F.; Morosuk, T.; Boyano, A.; Tsatsaronis, G. An exergy-based study on the relationship between costs and environmental impacts in power plants. Energy 2017, 138, 920–928. [Google Scholar] [CrossRef] [Green Version]
- Morosuk, T.; Tsatsaronis, G. Advanced exergy-based methods used to understand and improve energy-conversion systems. Energy 2019, 169, 238–246. [Google Scholar] [CrossRef]
- Petrakopoulou, F.; Tsatsaronis, G.; Morosuk, T. Advanced Exergoenvironmental Analysis of a Near-Zero Emission Power Plant with Chemical Looping Combustion. Environ. Sci. Technol. 2012, 46, 3001–3007. [Google Scholar] [CrossRef]
- Petrakopoulou, F.; Tsatsaronis, G.; Morosuk, T. Assessment of a Power Plant With CO2 Capture Using an Advanced Exergoenvironmental Analysis. J. Energy Resour. Technol. 2014, 136, 022001. [Google Scholar] [CrossRef]
- Linnhoff, B.; Alanis, F.J. Integration of a New Process Into an Existing Site: A Case Study in the Application of Pinch Technology. J. Eng. Gas. Turbines Power 1991, 113, 159–168. [Google Scholar] [CrossRef]
- Smith, R. Chemical Process. Design; McGraw-Hill: New York, NY, USA, 1995. [Google Scholar]
- Goedkoop, M.; Spriensma, R. The Eco-Indicator 99: A Damage Oriented Method for Life Cycle Impact Assessment; Methodology Report; PRé Consultants B.V.: Amersfoort, The Netherlands, 2001. [Google Scholar]
- Linnhoff, B. A User Guide on Process. Integration for the Efficient Use of Energy; Institution of Chemical Engineers: Warks, UK, 1982. [Google Scholar]
- Andresen, B.; Salamon, P.; Berry, R.S. Thermodynamics in finite time: Extremals for imperfect heat engines. J. Chem. Phys. 1977, 66, 1571–1577. [Google Scholar] [CrossRef]
- Chambadal, P. Les Centrales Nucléaires; Colin: Paris, France, 1957. [Google Scholar]
- Novikov, I.I. The efficiency of atomic power stations. J. Nucl. Energy 1958, 7, 125–128. [Google Scholar] [CrossRef]
- El-Wakil, M.M. Nuclear Power Engineering; McGraw-Hill: New York, NY, USA, 1962. [Google Scholar]
- El-Wakil, M.M. Nuclear Energy Conversion; International Textbook Co.: Scranton, PA, USA, 1971. [Google Scholar]
- Bejan, A.; Smith, J.L. Thermodynamic optimization of mechanical supports for cryogenic apparatus. Cryogenics 1974, 14, 158–163. [Google Scholar] [CrossRef]
- Curzon, F.L.; Ahlborn, B. Efficiency of a Carnot engine at maximum power output. Am. J. Phys. 1975, 43, 22–24. [Google Scholar] [CrossRef]
- Bejan, A. Engineering Advances on Finite-Time Thermodynamics. Am. J. Phys. 1994, 62, 11–12. [Google Scholar] [CrossRef]
- Gyftopoulos, E.P. Infinite time (reversible) versus finite time (irreversible) thermodynamics: A misconceived distinction. Energy 1999, 24, 1035–1039. [Google Scholar] [CrossRef]
- Gyftopoulos, E.P. On the Curzon–Ahlborn efficiency and its lack of connection to power producing processes. Energy Convers. Manag. 2002, 43, 609–615. [Google Scholar] [CrossRef]
- Moran, M.J. On second-law analysis and the failed promise of finite-time thermodynamics. Energy 1998, 23, 517–519. [Google Scholar] [CrossRef]
- Basak, T. The law of life: The bridge between physics and biology. Phys. Life Rev. 2011, 8, 249–252. [Google Scholar] [CrossRef]
- Chen, L. Progress in study on constructal theory and its applications. Sci. China Technol. Sci. 2012, 55, 802–820. [Google Scholar] [CrossRef]
- Miguel, A.F. Natural flow systems: Acquiring their constructal morphology. Int. J. Des. Nat. Ecodynamics 2010, 5, 230–241. [Google Scholar] [CrossRef]
- Miguel, A.F. The physics principle of the generation of flow configuration. Phys. Life Rev. 2011, 8, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.H. Constructal theory: From engineering to physics, and how flow systems develop shape and structure. Appl. Mech. Rev. 2006, 59, 269–282. [Google Scholar] [CrossRef]
- Reis, A.H. Use and validity of principles of extremum of entropy production in the study of complex systems. Ann. Phys. 2014, 346, 22–27. [Google Scholar] [CrossRef]
- Queiros-Condé, D.; Feidt, M. Constructal Theory and Multi-Scale Geometries: Theory and Application in Energetics, Chemical Engineering and Materials; Les Presses de l’ENSTA: Paris, France, 2010. [Google Scholar]
- Rocha, L. Convection in Channels and Porous Media: Analysis, Optimization, and Constructal Design; VDM Verlag: Saarbrücken, Germany, 2009. [Google Scholar]
- Lorenzini, G.; Moretti, S.; Conti, A. Fin Shape Thermal Optimization Using Bejan’s Constructal Theory; Morgan & Claypool: San Rafael, CA, USA, 2011; Volume 6. [Google Scholar]
- Miguel, A.F. Dendritic structures for fluid flow: Laminar, turbulent and constructal design. J. Fluids Struct. 2010, 26, 330–335. [Google Scholar] [CrossRef]
- Kacimov, A.R.; Klammler, H.; Il’yinskii, N.; Hatfield, K. Constructal design of permeable reactive barriers: Groundwater-hydraulics criteria. J. Eng. Math. 2011, 71, 319–338. [Google Scholar] [CrossRef]
- Reis, A.H.; Gama, C. Sand size versus beachface slope—An explanation based on the Constructal Law. Geomorphology 2010, 114, 276–283. [Google Scholar] [CrossRef]
- Ventikos, Y. The importance of the constructal framework in understanding and eventually replicating structure in tissue. Phys. Life Rev. 2011, 8, 241–242. [Google Scholar] [CrossRef]
- Bejan, A. Street network theory of organization in nature. J. Adv. Transp. 1996, 30, 85–107. [Google Scholar] [CrossRef]
- Bejan, A. Constructal-theory network of conducting paths for cooling a heat generating volume. Int. J. Heat Mass. Transf. 1997, 40, 799–816. [Google Scholar] [CrossRef]
- Bejan, A. Constructal tree network for fluid flow between a finite-size volume and one source or sink. Rev. Générale Therm. 1997, 36, 592–604. [Google Scholar] [CrossRef]
- Errera, M.R.; Bejan, A. Deterministic Tree Networks for River Drainage Basins. Fractals 1998, 6, 245–261. [Google Scholar] [CrossRef]
- Kim, S.; Lorente, S.; Bejan, A.; Miller, W.; Morse, J. The emergence of vascular design in three dimensions. J. Appl. Phys. 2008, 103, 123511. [Google Scholar] [CrossRef]
- Bejan, A.; Lorente, S. Letter to editor “X.-B. Liu, Q. Chen, M. Wang, N. Pan and Z.-Y. Guo, Multi-dimensional effect on optimal network structure for fluid distribution, Chemical Engineering and Processing 49 (2010) 1038–1043.”. Chem. Eng. Process. Process. Intensif. 2012, 56, 34. [Google Scholar] [CrossRef]
- Bejan, A. Comment on “Study on the consistency between field synergy principle and entransy dissipation extremum principle. ” Int. J. Heat Mass. Transf. 2018, 120, 1187–1188. [Google Scholar] [CrossRef]
- Bejan, A. Comment on “Application of Entransy Analysis in Self-Heat Recuperation Technology.”. Ind. Eng. Chem. Res. 2014, 53, 18352–18353. [Google Scholar] [CrossRef]
- Bejan, A. Heatlines (1983) versus synergy (1998). Int. J. Heat Mass. Transf. 2015, 81, 654–658. [Google Scholar] [CrossRef]
- Bejan, A. “Entransy,” and Its Lack of Content in Physics. J. Heat Transf. 2014, 136, 055501. [Google Scholar] [CrossRef]
- Bejan, A. Constructal Law: Optimization as Design Evolution. J. Heat Transf. 2015, 137, 061003. [Google Scholar] [CrossRef] [Green Version]
- Manuel, M.C.E.; Lin, P.T. Design explorations of heat conductive pathways. Int. J. Heat Mass. Transf. 2017, 104, 835–851. [Google Scholar] [CrossRef]
- Asfaw, B.; James, P. Constructal theory, adaptive motion, and their theoretical application to low-speed turbine design. J. Energy Eng. 2009, 135, 112–118. [Google Scholar] [CrossRef]
- Mehrgoo, M.; Amidpour, M. Constructal design of humidification–dehumidification desalination unit architecture. Desalination 2011, 271, 62–71. [Google Scholar] [CrossRef]
- Ojeda, J.; Mendez, F. Application of Bejan’s constructal theory to a solar collector system. Part I: The fundamentals to define the first construction. Int. J. Thermodyn. 2010, 13, 135–141. [Google Scholar] [CrossRef]
- Bejan, A. Convection Heat Transfer, 1st ed.; Wiley: New York, NY, USA, 1984. [Google Scholar]
- Bejan, A. Graphic Techniques for Teaching Engineering Thermodynamics. Mech Eng. News 1977, 14, 26–28. [Google Scholar]
- Bejan, A. Heat tubes: Conduction and convection. Int. J. Heat Mass. Transf. 2019, 137, 1258–1262. [Google Scholar] [CrossRef]
- Grassmann, P. Zur allgemeinen Definition des Wirkungsgrades. Chem. Ing. Tech. 1950, 22, 77–80. [Google Scholar] [CrossRef]
- Bejan, A. Letter to the editor of renewable and sustainable energy reviews. Renew. Sustain. Energy Rev. 2016, 53, 1636–1637. [Google Scholar] [CrossRef]
- Bejan, A. Plagiarism is not a Victimless Crime. Am. Soc. Eng. Educ. Prism 2019, 28, 52. [Google Scholar]
- Lorenzini, G.; Biserni, C. The Constructal law: From design in nature to social dynamics and wealth as physics. Phys. Life Rev. 2011, 8, 259–260. [Google Scholar] [CrossRef]
- Saslow, W.M. An economic analogy to thermodynamics. Am. J. Phys. 1999, 67, 1239–1247. [Google Scholar] [CrossRef]
- Kalason, P. Epistémologie Constructale du Lien Cultuel: Les Rites: Manipulation ou Médiation? L’Harmattan: Paris, France, 2007. [Google Scholar]
- Temple, H. Théorie Générale de la Nation: L’architecture du Monde; L’Harmattan: Paris, France, 2014. [Google Scholar]
- Smerlak, M. Thermodynamics of inequalities: From precariousness to economic stratification. Phys. Stat. Mech. Its Appl. 2016, 441, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Karpiarz, M.; Fronczak, P.; Fronczak, A. International trade network: Fractal properties and globalization puzzle. Phys. Rev. Lett. 2014, 113, 248701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisbuch, G.; Battiston, S. From production networks to geographical economics. J. Econ. Behav. Organ. 2007, 64, 448–469. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y. Maximum Profit Configurations of Commercial Engines. Entropy 2011, 13, 1137–1151. [Google Scholar] [CrossRef]
- Frederick, W.C. Natural Corporate Management: From the Big Bang to Wall Street; Greenleaf Publishing: Sheffield, UK, 2012. [Google Scholar]
- Mirowski, P. More Heat than Light: Economics as Social Physics, Physics as Nature’s Economics; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejan, A.; Tsatsaronis, G. Purpose in Thermodynamics. Energies 2021, 14, 408. https://0-doi-org.brum.beds.ac.uk/10.3390/en14020408
Bejan A, Tsatsaronis G. Purpose in Thermodynamics. Energies. 2021; 14(2):408. https://0-doi-org.brum.beds.ac.uk/10.3390/en14020408
Chicago/Turabian StyleBejan, Adrian, and George Tsatsaronis. 2021. "Purpose in Thermodynamics" Energies 14, no. 2: 408. https://0-doi-org.brum.beds.ac.uk/10.3390/en14020408