Hydrogels Synthesized by Electron Beam Irradiation for Heavy Metal Adsorption
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Experimental Installation and Sample Preparation
2.3. Measurements
3. Results and Discussion
3.1. Swelling and Diffusion Experiments
3.1.1. The Measurement of Gel Content
3.1.2. Degree of Swelling
3.1.3. Equilibrium Water Content
3.1.4. Swelling Kinetics
3.1.5. Determination of Swelling Power
3.1.6. Network Studies
3.1.7. Spectral Characterization
3.1.8. SEM Analysis
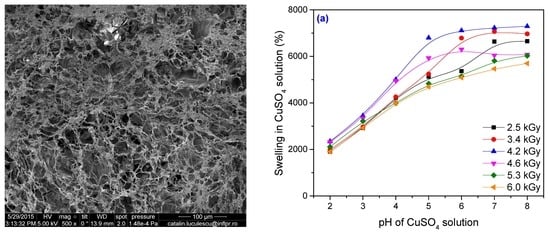
3.2. Metal Ion Uptake Studies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fernandez-Luqueno, F.; Lopez-Valdez, F.; Gamero-Melo, P.; Suarez, S.L.; Aguilera-Gonzalez, E.N.; Martinez, A.; del Socorro Garcia Guillermo, M.; Hernandez-Martinez, G.; Herrera-Mendoza, R.; Álvarez Garza, M.A.; et al. Heavy metal pollution in drinking water—A global risk for human health: A review. Afr. J. Environ. Sci. Technol. 2013, 7, 567–584. [Google Scholar]
- Aragay, G.; Pons, J.; Merkoci, A. Recent trends in macro-, micro- and nanomaterial-based tools and strategies for heavy-metal detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef] [PubMed]
- Wan Ngah, W.S.; Hanafiah, M.A.K.M. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S. P. Heavy metal pollution in China: Origin, pattern and control. Environ. Sci. Pollut. Res. 2003, 10, 192–195. [Google Scholar] [CrossRef]
- Ip, C.C.M.; Li, X.D.; Zhang, G.; Wong, C.S.C.; Zhang, W.L. Heavy metal and Pb isotopic compositions of aquatic organisms in the Pearl River Estuary, South China. Environ. Pollut. 2005, 138, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Peralta-Videa, J.R.; Lopez, M.L.; Narayan, M.; Saupe, G.; Gardea-Torresdey, J. The biochemistry of environmental heavy metal uptake by plants: Implications for the food chain. Int. J. Biochem. Cell Biol. 2009, 41, 1665–1677. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.P.; Fu, S.Y.; Liu, H.; Zhou, Y.M.; Li, X.Y. Hydrogel Beads Based on Carboxymethyl Cellulose for Removal Heavy Metal Ions. J. Appl. Polym. Sci. 2011, 119, 1204–1210. [Google Scholar] [CrossRef]
- Meunier, N.; Drogui, P.; Montane, C.; Hausler, R.; Mercier, G.; Blais, J.F. Comparison between electrocoagulation and chemical precipitation for metals removal from acidic soil leachate. J. Hazard. Mater. 2006, 137, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Charerntanyarak, L. Heavy metals removal by chemical coagulation and precipitation. Water Sci. Technol. 1999, 39, 135–138. [Google Scholar] [CrossRef]
- Bessbousse, H.; Rhlalou, T.; Verchere, J.F.; Lebrun, L. Removal of heavy metal ions from aqueous solutions by filtration with a novel complexing membrane containing poly(ethyleneimine) in a poly(vinyl alcohol) matrix. J. Membr. Sci. 2008, 307, 249–259. [Google Scholar] [CrossRef]
- Vijayalakshmi, A.; Arockiasam, D.L.; Nagendran, A.; Mohan, D. Separation of proteins and toxic heavy metal ions from aqueous solution by CA/PC blend ultrafiltration membranes. Sep. Purif. Technol. 2008, 62, 32–38. [Google Scholar] [CrossRef]
- Alyuz, B.; Veli, S. Kinetics and equilibrium studies for the removal of nickel and zinc from aqueous solutions by ion exchange resins. J. Hazard. Mater. 2009, 167, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, A.; Hubicki, Z.; Podko′scielny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Basha, C.A.; Somasundaram, M.; Kannadasan, T.; Lee, C.W. Heavy metals removal from copper smelting effluent using electrochemical filter press cells. Chem. Eng. J. 2011, 171, 563–571. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Zhao, C.; Zheng, J. Hydrogels for Removal of Heavy Metals from Aqueous Solution. J. Environ. Anal. Toxicol. 2012. [Google Scholar] [CrossRef]
- Sprynskyy, M. Solid-liquid-solid extraction of heavy metals (Cr, Cu, Cd, Ni and Pb) in aqueous systems of zeolite-sewage sludge. J. Hazard. Mater. 2009, 161, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Eloussaief, M.; Kallel, N.; Yaacoubi, A.; Benzina, M. Mineralogical identification, spectroscopic characterization and potential environmental use of natural clay materials on chromate removal from aqueous solutions. Chem. Eng. J. 2011, 168, 1024–1031. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Coll. Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Eloussaief, M.; Benzina, M. Efficiency of natural and acid-activated calys in the removal of Pb(II) from aqueous solutions. J. Hazard. Mater. 2010, 178, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Eloussaief, M.; Sdiri, A.; Benzina, M. Modelling the adsorption of mercury onto natural and aluminium pillared clays. Environ. Sci. Pollut. Res. 2013, 20, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arabian J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Peng, X.W.; Zhong, L.X.; Ren, J.L.; Sun, R.C. Highly effective adsorption of heavy metal ions from aqueous solutions by macroporous xylan-rich hemicelluloses-based hydrogel. J. Agric. Food Chem. 2012, 60, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, A. Evaluation of ammonium removal using a chitosan-gpoly(acrylic acid)/rectorite hydrogel composite. J. Hazard. Mater. 2009, 171, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Yetimoglu, E.K.; Kahraman, M.V.; Ercan, O.; Akdemir, Z.S.; Apohan, N.K. N-vinylpyrrolidone/acrylic acid/2-acrylamido-2-methylpropane sulfonic acid based hydrogels: Synthesis, characterization and their application in the removal of heavy metals. React. Funct. Polym. 2007, 67, 451–460. [Google Scholar] [CrossRef]
- Gulrez, K.H.S.; Al-Assaf, S.; Phillips, O.G. Chapter 5: Hydrogels: Methods of Preparation, Characterisation and Applications. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; Capri, A., Ed.; InTech: Rijeka, Croatia, 2011; pp. 117–150. [Google Scholar]
- Hennink, W.E. van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug. Deliver. Rev. 2002, 54, 13–36. [Google Scholar] [CrossRef]
- Barbucci, R.; Leone, G.; Vecchiullo, A. Novel carboxymethylcellulose-based microporous hydrogels suitable for drug delivery. J. Biomat. Sci. Polym. E 2004, 15, 607–619. [Google Scholar] [CrossRef]
- Said, H.M.; Abd Alla, S.G.A.; El-Naggar, A.W.M. Synthesis and characterization of novel gels based on carboxymethyl cellulose/acrylic acid prepared by electron beam irradiation. React. Funct. Polym. 2004, 61, 397–404. [Google Scholar] [CrossRef]
- Fei, B.; Wach, R.A.; Mitomo, H.; Yoshii, F.; Kume, T. Hydrogel of biodegradable cellulose derivatives. I. Radiation-induced crosslinking of CMC. J. Appl. Polym. Sci. 2000, 78, 278–283. [Google Scholar] [CrossRef]
- Liu, P.F.; Zhai, M.L.; Li, J.Q.; Peng, J.; Wu, J.L. Radiation preparation and swelling behavior of sodium carboxymethyl cellulose hydrogels. Radiat. Phys. Chem. 2002, 63, 525–528. [Google Scholar] [CrossRef]
- Wang, M.; Xu, L.; Ju, X.; Peng, J.; Zhai, M.L.; Li, J.Q.; Wei, G.S. Enhanced radiation crosslinking of carboxymethylated chitosan in the presence of acids or polyfunctional monomers. Polym. Degrad. Stabil. 2008, 93, 1807–1813. [Google Scholar] [CrossRef]
- Ismail, O.; Kipcak, A.S.; Piskin, P. Modeling of absorption kinetics of poly(acrylamide) hydrogels crosslinked by EGDMA and PEGDMAs. Res. Chem. Intermediat. 2013, 39, 907–919. [Google Scholar] [CrossRef]
- Hassan, S.; Yasin, T. Synthesis of radiation crosslinked poly(acrylic acid) in the presence of phenyltriethoxysilane. Radiat. Phys. Chem. 2014, 97, 292–297. [Google Scholar] [CrossRef]
- Karadag, E.; Saraydin, D. Swelling studies of super water retainer acrylamide/crotonic acid hydrogels crosslinked by trimethylolpropane triacrylate and 1,4-butanediol dimethacrylate. Polym. Bull. 2002, 48, 299–307. [Google Scholar] [CrossRef]
- Craciun, G.; Manaila, E.; Stelescu, M.D. Electron Beam Synthesis and Characterization of Acrylamide/Acrylic Acid Hydrogels Using Trimethylolpropane Trimethacrylate as Cross-Linker. J. Chem. 2016, 2016, 1470965. [Google Scholar] [CrossRef]
- Fiti, M. Ionizing Radiation Chemical Dosimetry. In Dozimetria Chimica a Radiatiilor Ionizante; Editura Academiei Republicii Socialiste Romania: Bucuresti, Romania, 1973; pp. 24–70. [Google Scholar]
- Cleland, M.R. Industrial Applications of Electron Accelerators—Ion Beam Applications. In Proceedings of the CERN Accelerator School/Small Accelerator Course, Zeegse, The Netherlands, 24 May–2 June 2005. [Google Scholar]
- Marandi, G.B.; Mahdavinia, G.R.; Ghafary, S. Collagen-g-poly(Sodium Acrylate-co-Acrylamide)/sodium montmorillonite superabsorbent nanocomposites: Synthesis and swelling behavior. J. Polym. Res. 2011, 18, 1487–1499. [Google Scholar] [CrossRef]
- Karadag, E.; Saraydin, D.; Sahiner, N.; Güven, O. Radiation induced acrylamide/citric acid hydrogelas and their swelling behaviors. J. Macromol. Sci. Part A 2001, 38, 1105–1121. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Kurdtabar, M. Collagen-based highly porous hydrogel without any porogen: Synthesis and characteristics. Eur. Polym. J. 2007, 43, 877–889. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M.; Bianco-Peled, H. A quantitative analysis of alginate swelling. Carbohydr. Polym. 2010, 79, 1020–1027. [Google Scholar] [CrossRef]
- Karadag, E.; Uzum, O.B.; Saraydin, D. Swelling equilibria and dye adsorption studies of chemically crosslinked superabsorbent acrylamide/maleic acid hydrogels. Eur. Polym. J. 2002, 38, 2133–2141. [Google Scholar] [CrossRef]
- Jabbari, E.; Nozari, S. Swelling behaviour of acrylic acid hydrogels prepared by c-radiation crosslinking of polyacrylic acid in aqueous solution. Eur. Polym. J. 2000, 36, 2685–2692. [Google Scholar] [CrossRef]
- Ostrowska-Czubenko, J.; Gierszewska-Drużyńska, M. Mechanism of water diffusion into noncrosslinked and ionically crosslinked chitosan membranes. In Progress on Chemistry and Application of Chitin and its Derivatives; Jaworska, M.M., Ed.; Polish Chitin Society: Łódź, Poland, 2012; Volume 17, pp. 59–66. [Google Scholar]
- Khare, A.R.; Peppas, N.A. Swelling/deswelling of anionic copolymer gels. Biomaterials 1995, 16, 559–567. [Google Scholar] [CrossRef]
- Lin, W.C.; Yu, D.G.; Yang, M.C. pH-sensitive polyelectrolyte complex gel microspheres composed of chitosan/sodium tripolyphosphate/dextran sulfate: Swelling kinetics and drug delivery properties. Coll. Surf. B 2005, 44, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.R.; Devi, K.P.; Buri, P. Cellulose matrices for zero-order release of soluble drugs. Drug Dev. Ind. Pharm. 1988, 14, 2299–2320. [Google Scholar]
- Munday, D.L.; Cox, P.J. Compressed xanthan and karaya gum matrices: Hydration, erosion and drug release mechanisms. Int. J. Pharm. 2000, 203, 179–192. [Google Scholar] [CrossRef]
- Hill, D.J.T.; Lim, M.C.H.; Whittaker, A.K. Water diffusion in hydroxyethyl methacrylate (HEMA)-based hydrogels formed by γ-radiolysis. Polym. Int. 1999, 48, 1046–1052. [Google Scholar] [CrossRef]
- Krongauz, V.V. Diffusion in polymers dependence on crosslink density. J. Therm. Anal. Calor. 2010, 102, 435–445. [Google Scholar] [CrossRef]
- Demeter, M.; Virgolici, M.; Vancea, C.; Scarisoreanu, A.; Kaya, M.G.A.; Meltzer, V. Network structure studies on gamma-irradiated collagen-PVP superabsorbent hydrogels. Radiat. Phys. Chem. 2017, 131, 51–59. [Google Scholar] [CrossRef]
- Yiamsawas, D.; Kangwansupamonkon, W.; Chailapakul, O.; Kiatkamjornwong, S. Synthesis and swelling properties of poly[acrylamide-co-(crotonic acid)] superabsorbents. React. Funct. Polym. 2007, 67, 865–882. [Google Scholar] [CrossRef]
- Ding, Z.Y.; Aklonis, J.J.; Salovey, R. Model filled polymers. VI. Determination of the crosslink density of polymeric beads by swelling. J. Polym. Sci. B 1991, 29, 1035–1038. [Google Scholar] [CrossRef]
- Karadag, E.; Saraydin, D.; Güven, O. Influence of some crosslinkers on the swelling of acrylamide-crotonic acid hydrogels. Turk. J. Chem. 1997, 21, 151–161. [Google Scholar]
- Thakur, A.; Wanchoo, R.K.; Singh, P. Structural Parameters and Swelling Behavior of pH Sensitive Poly(acrylamide-co-acrylic acid) Hydrogels. Chem. Biochem. Eng. Q 2011, 25, 181–194. [Google Scholar]
- Nesrinne, S.; Djamel, A. Synthesis, characterization and rheological behavior of pH sensitive poly(acrylamide-co-acrylic acid) hydrogels. Arabian J. Chem. 2017, 10, 539–547. [Google Scholar] [CrossRef]
- Murugan, R.; Mohan, S.; Bigotto, A. FTIR and Polarized Raman Spectra of Acrylamide and Polyacrylamide. J. Korean Phys. Soc. 1998, 32, 505–512. [Google Scholar]
- Geethanjali, R.; Sabirneeza, A.A.F.; Subhashini, S. Water-Soluble and Biodegradable Pectin-Grafted Polyacrylamide and Pectin-Grafted Polyacrylic Acid: Electrochemical Investigation of Corrosion-Inhibition Behaviour on Mild Steel in 3.5% NaCl Media. Indian J. Mater. Sci. 2014, 2014, 356075. [Google Scholar]
- Li, Y.H.; Chen, X.L.; Liu, Y.M.; Lu, J.Q.; Zhao, Y.S. Synthesis and characterization of poly(aspartic acid) composite hydrogels with inorganic MCM-41 cross-linker. Iran. Polym. J. 2014, 23, 907–916. [Google Scholar] [CrossRef]
- Xie, Y.T.; Wang, A.Q. Preparation and Swelling Behaviourof Chitosan-g-poly(acrylic acid)/Muscovite Superabsorbent Composites. Iran. Polym. J. 2010, 19, 131–141. [Google Scholar]
- Wu, Y.X.; Zhou, J.H.; Ye, C.C.; Sun, H.S.; Zhao, R.J. Optimized Synthesis of Lignosulphonate-g-poly(acrylic acid-co-acrylamide) Superabsorbent Hydrogel Based on the Taguchi Method. Iran. Polym. J. 2010, 19, 511–520. [Google Scholar]
- Kong, W.Q.; Ren, J.L.; Wang, S.Y.; Chen, Q.F. Removal of heavy metals from aqueous solutions using acrylic—Modified sugarcane bagasse—Based adsorbents: Equilibrium and kinetic studies. Bioresources 2014, 9, 3184–3196. [Google Scholar] [CrossRef]
- Ibrahim, A.G.; Hai, F.A.; Wahab, H.A.; Mahmoud, H. Synthesis, Characterization, Swelling Studies and Dye Removal of Chemically Crosslinked Acrylic Acid/Acrylamide/N, N-Dimethyl Acrylamide Hydrogels. Am. J. Appl. Chem. 2016, 4, 221–234. [Google Scholar] [CrossRef]
- Li, W.; Zhao, H.; Teasdale, P.R.; John, R.; Zhang, S. Synthesis and characterisation of a polyacrylamide-polyacrylic acid copolymer hydrogel for environmental analysis of Cu and Cd. React. Funct. Polym. 2002, 52, 31–41. [Google Scholar] [CrossRef]
- Atia, A.A.; Donia, A.M.; Hussin, R.A.; Rashad, R.T. Swelling and metal ion uptake characteristics of kaolinite containing poly [(acrylic acid)-co-acrylamide] hydrogels. Desalination Water Treat. 2009, 3, 73–82. [Google Scholar] [CrossRef]
- Shouman, M.A.; Fathy, N.A.; Khedr, S.A.; Attia, A.A. Comparative Biosorption Studies of Hexavalent Chromium Ion onto Raw and Modified Palm Branches. Adv. Phys. Chem. 2013, 2013, 159712. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y. Fixed bed studies for the sorption of chromium(VI) onto tea factory waste. Chem. Eng. Sci. 2006, 61, 4363–4372. [Google Scholar] [CrossRef]
- Dambies, L.; Guimon, C.; Yiacoumi, S.; Guibal, E. Characterization of metal ion interactions with chitosan by X-ray photoelectron spectroscopy. Coll. Surf. A 2001, 77, 203–214. [Google Scholar] [CrossRef]
- Rashid, J.; Barakat, M.A.; Alghamdi, M.A. Adsorption of Chromium (VI) from Wastewater by Anion Exchange Resin. J. Adv. Catal. Sci. Technol. 2014, 1, 26–34. [Google Scholar]
- Kumar, P.A.; Chakraborty, S.; Ray, M. Removal and recovery of chromium from wastewater using short chain polyaniline synthesized on jute fiber. Chem. Eng. J. 2008, 141, 130–140. [Google Scholar] [CrossRef]














| Materials | Chemical Characteristics | Chemical Structure |
|---|---|---|
| Acrylamide, AMD | molecular weight: 72.06 g/mol; density 1.051 g/cm3; solubility in water: 2.04 kg·L−1 at 25 °C |  |
| Acrylic acid, AA | molecular weight: 71.08 g/mol; density 1.13 g/cm3; soluble in water; |  |
| Potassium persulfate, PP (used as initiator) | molecular weight: 270.322 g/mol; density 2.477 g/cm3; solubility in water: 1.75 g/100 mL at 0 °C; | K2S2O8 |
| Trimethylolpropane trimethacrylate, TMPT (used as cross-linker) | molecular weight: 338.4 g/mol; boiling point: >200 °C; density 1.06 g/cm3; 75 ± 3% active ingredient; |  |
| Samples Codes | Amount of Chemicals (mol/L) | Irradiation Dose (kGy) | |||
|---|---|---|---|---|---|
| AMD | AA | PP | TMPT | ||
| Hyd1.1 | 5 | 0.5 | 3.70 × 10−3 | 2.95 × 10−3 | 2.5 |
| Hyd1.2 | 3.4 | ||||
| Hyd1.3 | 4.2 | ||||
| Hyd1.4 | 4.6 | ||||
| Hyd1.5 | 5.3 | ||||
| Hyd1.6 | 6.0 | ||||
| Hyd2.1 | 5 | 0.5 | 7.40 × 10−3 | 5.90 × 10−3 | 2.5 |
| Hyd2.2 | 3.4 | ||||
| Hyd2.3 | 4.2 | ||||
| Hyd2.4 | 4.6 | ||||
| Hyd2.5 | 5.3 | ||||
| Hyd2.6 | 6.0 | ||||
| PP (mol/L) | TMPT (mol/L) | Absorbed Dose (kGy) | |||||
|---|---|---|---|---|---|---|---|
| 2.5 | 3.4 | 4.2 | 4.6 | 5.3 | 6.0 | ||
| k1,S × 103 | |||||||
| 3.70 × 10−3 | 2.95 × 10−3 | 5.42 | 3.81 | 4.55 | 3.12 | 3.48 | 3.98 |
| 7.40 × 10−3 | 5.90 × 10−3 | 3.11 | 4.19 | 3.58 | 3.55 | 3.67 | 3.78 |
| k2,S × 107 | |||||||
| 3.70 × 10−3 | 2.95 × 10−3 | 7.69 | 3.39 | 2.54 | 2.25 | 2.72 | 3.81 |
| 7.40 × 10−3 | 5.90 × 10−3 | 2.88 | 3.31 | 3.26 | 3.45 | 3.76 | 2.73 |
| PP (mol/L) | TMPT (mol/L) | Absorbed Dose (kGy) | |||||
|---|---|---|---|---|---|---|---|
| 2.5 | 3.4 | 4.2 | 4.6 | 5.3 | 6.0 | ||
| r0 × 102 | |||||||
| 3.70 × 10−3 | 2.95 × 10−3 | 3.13 | 3.83 | 4.62 | 5.46 | 4.71 | 3.89 |
| 7.40 × 10−3 | 5.90 × 10−3 | 3.14 | 2.17 | 2.16 | 2.45 | 2.37 | 3.78 |
| Smax. | |||||||
| 3.70 × 10−3 | 2.95 × 10−3 | 6440 | 8768 | 9215 | 9015 | 8820 | 8207 |
| 7.40 × 10−3 | 5.90 × 10−3 | 10,519 | 11,801 | 11,929 | 10,888 | 10,603 | 9843 |
| PP (mol/L) | TMPT (mol/L) | Absorbed Dose (kGy) | |||||
|---|---|---|---|---|---|---|---|
| 2.5 | 3.4 | 4.2 | 4.6 | 5.3 | 6.0 | ||
| n | |||||||
| 3.70 × 10−3 | 2.95 × 10−3 | 0.94 | 0.93 | 1.15 | 1.02 | 0.90 | 0.98 |
| 7.40 × 10−3 | 5.90 × 10−3 | 0.81 | 0.79 | 0.79 | 0.82 | 0.87 | 0.97 |
| k | |||||||
| 3.70 × 10−3 | 2.95 × 10−3 | 0.20 | 0.21 | 0.06 | 0.11 | 0.23 | 0.12 |
| 7.40 × 10−3 | 5.90 × 10−3 | 0.51 | 0.72 | 0.75 | 0.57 | 0.43 | 0.18 |
| D × 103 | |||||||
| 3.70 × 10−3 | 2.95 × 10−3 | 0.31 | 0.49 | 0.77 | 0.59 | 0.73 | 0.96 |
| 7.40 × 10−3 | 5.90 × 10−3 | 1.46 | 2.62 | 2.69 | 2.46 | 2.72 | 2.31 |
| PP (mol/L) | TMPT (mol/L) | Absorbed Dose (kGy) | |||||
|---|---|---|---|---|---|---|---|
| 2.5 | 3.4 | 4.2 | 4.6 | 5.3 | 6.0 | ||
| Mc × 10−3 | |||||||
| 3.70 × 10−3 | 2.95 × 10−3 | 179 | 184 | 190 | 176 | 179 | 164 |
| 7.40 × 10−3 | 5.90 × 10−3 | 625 | 668 | 684 | 582 | 559 | 458 |
| q × 104 | |||||||
| 3.70 × 10−3 | 2.95 × 10−3 | 4.013 | 3.916 | 3.783 | 4.089 | 4.001 | 4.391 |
| 7.40 × 10−3 | 5.90 × 10−3 | 1.160 | 1.086 | 1.061 | 1.246 | 1.297 | 1.583 |
| ξ/nm | |||||||
| 3.70 × 10−3 | 2.95 × 10−3 | 107 | 138 | 142 | 135 | 137 | 128 |
| 7.40 × 10−3 | 5.90 × 10−3 | 258 | 293 | 298 | 266 | 258 | 226 |
| P(%) | |||||||
| 3.70 × 10−3 | 2.95 × 10−3 | 98.73 | 98.74 | 98.77 | 98.71 | 98.73 | 98.66 |
| 7.40 × 10−3 | 5.90 × 10−3 | 99.05 | 99.09 | 99.10 | 99.01 | 98.99 | 98.87 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manaila, E.; Craciun, G.; Ighigeanu, D.; Cimpeanu, C.; Barna, C.; Fugaru, V. Hydrogels Synthesized by Electron Beam Irradiation for Heavy Metal Adsorption. Materials 2017, 10, 540. https://0-doi-org.brum.beds.ac.uk/10.3390/ma10050540
Manaila E, Craciun G, Ighigeanu D, Cimpeanu C, Barna C, Fugaru V. Hydrogels Synthesized by Electron Beam Irradiation for Heavy Metal Adsorption. Materials. 2017; 10(5):540. https://0-doi-org.brum.beds.ac.uk/10.3390/ma10050540
Chicago/Turabian StyleManaila, Elena, Gabriela Craciun, Daniel Ighigeanu, Catalina Cimpeanu, Catalina Barna, and Viorel Fugaru. 2017. "Hydrogels Synthesized by Electron Beam Irradiation for Heavy Metal Adsorption" Materials 10, no. 5: 540. https://0-doi-org.brum.beds.ac.uk/10.3390/ma10050540