Exploring the Self-Assembly Capabilities of ABA-Type SBS, SIS, and Their Analogous Hydrogenated Copolymers onto Different Nanostructures Using Atomic Force Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thin-Film Preparation
2.3. Morphological Characterization
3. Results
3.1. Effect of Block Nature and Solution Concentration
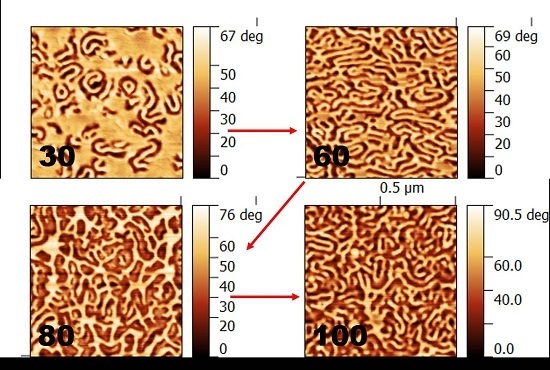
3.2. Effect of Annealing Temperature
3.3. Effect of Solvent Nature
3.4. Detailed Analysis of the Effect of Parameters on Morphology and Topography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rodríguez-Hernández, J.; Chécot, F.; Gnanou, Y.; Lecommandoux, S. Toward ‘smart’ nano-objects by self-assembly of block copolymers in solution. Prog. Polym. Sci. 2005, 30, 691–724. [Google Scholar] [CrossRef]
- Darling, S.B. Directing the self-assembly of block copolymers. Prog. Polym. Sci. 2007, 32, 1152–1204. [Google Scholar] [CrossRef]
- Kim, J.K.; Yang, S.Y.; Lee, Y.; Kim, Y. Functional nanomaterials based on block copolymer self-assembly. Prog. Polym. Sci. 2010, 35, 1325–1349. [Google Scholar] [CrossRef]
- Kimishima, K.; Jinnai, H.; Hashimoto, T. Control of Self-Assembled Structures in Binary Mixtures of A–B Diblock Copolymer and A–C Diblock Copolymer by Changing the Interaction between B and C Block Chains. Macromolecules 1999, 32, 2585–2596. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Bates, F.S. Synthesis and Characterization of Model Polyalkane–Poly(ethylene oxide) Block Copolymers. Macromolecules 1996, 29, 6994–7002. [Google Scholar] [CrossRef]
- Escobar, V.A.; Herrera, R.; Petit, A.; Pla, F. Selective hydrogenation of butadiene–styrene copolymers using a Ziegler–Natta type catalyst: 1. Kinetic study. Eur. Polym. J. 2000, 36, 1817–1834. [Google Scholar] [CrossRef]
- Peng, J.; Kim, D.H.; Knoll, W.; Xuan, Y.; Li, B.; Han, Y. Morphologies in solvent-annealed thin films of symmetric diblock copolymer. J. Chem. Phys. 2006, 125, 064702. [Google Scholar] [CrossRef] [PubMed]
- Politakos, N.; Ntoukas, E.; Avgeropoulos, A.; Krikorian, V.; Pate, B.D.; Thomas, E.L.; Hill, R.M. Strongly segregated cubic microdomain morphology consistent with the double gyroid phase in high molecular weight diblock copolymers of polystyrene and poly(dimethylsiloxane). J. Polym. Sci. Part B Polym. Phys. 2009, 47, 2419–2427. [Google Scholar] [CrossRef]
- Davidock, D.A.; Hillmyer, M.A.; Lodge, T.P. Mapping Large Regions of Diblock Copolymer Phase Space by Selective Chemical Modification. Macromolecules 2004, 37, 397–407. [Google Scholar] [CrossRef]
- Xue, F.; Li, H.; An, L.; Jiang, S. Constructional details of polystyrene-block-poly(4-vinylpyridine) ordered thin film morphology. J. Colloid Interface Sci. 2013, 399, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Politakos, N.; Weinman, C.J.; Paik, M.Y.; Sundaram, H.S.; Ober, C.L.; Avgeropoulos, A. Synthesis, molecular, and morphological characterization of initial and modified diblock copolymers with organic acid chloride derivatives. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4292–4305. [Google Scholar] [CrossRef]
- Politakos, N.; Kortaberria, G.; Zalakain, I.; Avgeropoulos, A.; Mondragon, I. Modified Diblock Copolymer Bearing Fluoro Groups and Evaluation of its Hydrophobic Properties. Macromol. Symp. 2012, 321–322, 53–58. [Google Scholar] [CrossRef]
- Samaddar, P.; Deep, A.; Kim, K.-H. An engineering insight into block copolymer self-assembly: Contemporary application from biomedical research to nanotechnology. Chem. Eng. J. 2018, 342, 71–89. [Google Scholar] [CrossRef]
- Muller-Buschbaum, P. Preface to Forum on Block Copolymers for Nanotechnology Applications. ACS Appl. Mater. Interfaces 2017, 9, 31213–31214. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gopinadhan, M.; Osuji, C. Directed self-assembly of block copolymers: A tutorial review of strategies for enabling nanotechnology with soft matter. Soft Matter 2014, 10, 3867–3889. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Maiti, C.; Rajesh, Y.; Dey, K.; Pal, I.; Parekh, A.; Patra, R.; Dhara, D.; Dutta, P.; Mandal, M. Gold nanorod embedded reduction responsive block copolymer micelle-triggered drug delivery combined with photothermal ablation for targeted cancer therapy. Biochim. Biophys. Acta 2017, 1861, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Muljajew, I.; Weber, C.; Nischang, I.; Schubert, U. PMMA-g-OEtOx Graft Copolymers: Influence of Grafting Degree and Side Chain Length on the Conformation in Aqueous Solution. Materials 2018, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, S.; Liu, H.; Yang, C.; Kang, Y.; Wang, M. Unimolecular micelles of amphiphilic cyclodextrin-core star-like block copolymers for anticancer drug delivery. Chem. Commun. 2015, 51, 15768–15771. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.-K.; Chen, R.; Zhu, H.-Y.; Li, Y.; Liu, Y.; Hao, J.-Y. Carboxylic Terminated Thermo-Responsive Copolymer Hydrogel and Improvement in Peptide Release Profile. Materials 2018, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Kataoka, K. Polyion complex micelle formation from double-hydrophilic block copolymers composed of charged and non-charged segments in aqueous media. Polym. J. 2018, 50, 95–100. [Google Scholar] [CrossRef]
- Wang, J.; Rahman, M.; Abetz, C.; Rangou, S.; Zhang, Z.; Abetz, V. Novel Post-Treatment Approaches to Tailor the Pore Size of PS-b-PHEMA Isoporous Membranes. Macromol. Rapid Commun. 2018, 1800435. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Ferebee, R.; Lee, B.; Mitra, I.; Lynd, N.A.; Hayat, J.; Stein, G.E.; Bockstaller, M.R.; Tang, C. Symmetric Poly(ethylene oxide-b-styrene-b-isoprene) Triblock Copolymers: Synthesis, Characterization, and Self-Assembly in Bulk and Thin Film. Macromolecules 2014, 47, 6373–6381. [Google Scholar] [CrossRef]
- Krishnan, M.R.; Lu, K.-Y.; Chiu, W.-Y.; Chen, I.-C.; Lin, J.-W.; Lo, T.-Y.; Georgopanos, P.; Avgeropoulos, A.; Lee, M.-C.; Ho, R.-M. Directed Self-Assembly of Star-Block Copolymers by Topographic Nanopatterns through Nucleation and Growth Mechanism. Small 2018, 14, 1704005. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-C.; Wang, T.-C.; Ho, R.-M.; Georgopanos, P.; Avgeropoulos, A.; Thomas, E.L. Robust Block Copolymer Mask for Nanopatterning Polymer Films. ACS Nano 2010, 4, 2088–2094. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-C.; Ho, R.-M.; Georgopanos, P.; Avgeropoulos, A.; Thomas, E.L. Silicon oxy carbide nanorings from polystyrene-b-polydimethylsiloxane diblock copolymer thin films. Soft Matter 2010, 6, 3582–3587. [Google Scholar] [CrossRef]
- Shi, X.; Xu, Z.; Huang, C.; Wang, Y.; Cui, Z. Selective Swelling of Electrospun Block Copolymers: From Perforated Nanofibers to High Flux and Responsive Ultrafiltration Membranes. Macromolecules 2018, 51, 2283–2292. [Google Scholar] [CrossRef]
- Cetintas, M.; de Grooth, J.; Hofman, A.H.; van der Kooij, H.M.; Loos, K.; de Vos, W.M.; Kamperman, M. Free-standing thermo-responsive nanoporous membranes from high molecular weight PS-PNIPAM block copolymers synthesized via RAFT polymerization. Polym. Chem. 2017, 8, 2235–2243. [Google Scholar] [CrossRef]
- Ndaya, D.; Bosire, R.; Mahajan, L.; Huh, S.; Kasi, R. Synthesis of ordered, functional, robust nanoporous membranes from liquid crystalline brush-like triblock copolymers. Polym. Chem. 2018, 9, 1404–1411. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Noda, D.; Takahashi, T.; Mori, H. Design of stimuli-responsive nanoparticles with optoelectronic cores by post-assembly cross-linking and self-assembly of functionalized block copolymers. Polymer 2016, 86, 56–68. [Google Scholar] [CrossRef]
- Strover, L.T. Tuning the optoelectronic properties of P3EHT block copolymers by surface modification. Int. J. Nanotechnol. 2017, 14, 540–557. [Google Scholar] [CrossRef]
- Kocak, G.; Solmaz, G.; Dikmen, Z.; Butun, V. Preparation of Cross-Linked Micelles from Glycidyl Methacrylate Based Block Copolymers and Their Usages as Nanoreactors in the Preparation of Gold Nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 514–526. [Google Scholar] [CrossRef]
- Boucher-Jacobs, C.; Rabnawaz, M.; Katz, J.S.; Even, R.; Guironnet, D. Encapsulation of catalyst in block copolymer micelles for the polymerization of ethylene in aqueous medium. Nat. Commun. 2018, 9, 841. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Wan, W.-M.; Zhou, Y.; Sun, X.-L.; Bonder, E.M.; Jakle, F. Borinic acid block copolymers: New building blocks for supramolecular assembly and sensory applications. Polym. Chem. 2015, 6, 4650–4656. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, S.; Ji, X.; An, L.; Jiang, B. Study of the time evolution of the surface morphology of thin asymmetric diblock copolymer films under solvent vapor. Polymer 2005, 46, 6513–6521. [Google Scholar] [CrossRef]
- Xia, S.; Metwalli, E.; Opel, M.; Staniec, P.A.; Herzig, E.M.; Muller-Buschbaum, P. Printed Thin Magnetic Films Based on Diblock Copolymer and Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 2982–2991. [Google Scholar] [CrossRef] [PubMed]
- Tomita, E.; Kanehashi, S.; Ogino, K. Fabrication of Completely Polymer-Based Solar Cells with p- and n-Type Semiconducting Block Copolymers with Electrically Inert Polystyrene. Materials 2018, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, Y.; Chen, J.; Chance, W.M.; Hayat, J.; Gai, Z.; Tang, C. Nanostructured Metal/Carbon Composites from Heterobimetallic Block Copolymers with Controlled Magnetic Properties. Chem. Mater. 2014, 26, 3185–3190. [Google Scholar] [CrossRef]
- Ube, T.; Kosaka, T.; Okazaki, H.; Nakae, K.; Ikeda, T. A block copolymer of crosslinkable polythiophene and removable poly(ethylene oxide) for preparing heterostructures of organic semiconductors. J. Mater. Chem. C 2017, 5, 1414–1419. [Google Scholar] [CrossRef]
- Elacqua, E.; Weck, M. Fabrication of Supramolecular Semiconductor Block Copolymers by Ring-Opening Metathesis Polymerization. Chem. Eur. J. 2015, 21, 7151–7158. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yu, J.; Xue, L.; Zhang, C.; Zha, Y.; Gu, Y. Investigation of Molecular Structure and Thermal Properties of Thermo-Oxidative Aged SBS in Blends and Their Relations. Materials 2017, 10, 768. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Wu, S.; Pang, L.; Javilla, B. The Utilization of Multiple-Walled Carbon Nanotubes in Polymer Modified Bitumen. Materials 2017, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Hyun Lee, D.; Cho, H.; Yoo, S.; Park, S. Ordering evolution of block copolymer thin films upon solvent-annealing process. J. Colloid Interface Sci. 2012, 383, 118–123. [Google Scholar] [CrossRef]
- Hyun Kim, S.; Misner, M.J.; Xu, T.; Kimura, M.; Russell, T.P. Highly oriented and ordered arrays from block copolymers via solvent evaporation. Adv. Mater. 2004, 16, 226–231. [Google Scholar]
- Hong, X.; Wang, B.; Wang, H.; Wang, Y. Mechanisms of ordering in block copolymer sub-monolayer films upon selective solvent annealing. Polymer 2013, 54, 6036–6044. [Google Scholar] [CrossRef]
- Sinturel, C.; Vayer, M.; Morris, M.; Hillmyer, M.A. Solvent Vapor Annealing of Block Polymer Thin Films. Macromolecules 2013, 46, 5399–5415. [Google Scholar] [CrossRef]
- Kim, G.; Libera, M. Morphological Development in Solvent-Cast Polystyrene−Polybutadiene−Polystyrene (SBS) Triblock Copolymer Thin Films. Macromolecules 1998, 31, 2569–2577. [Google Scholar] [CrossRef]
- Guo, R.; Huang, H.; Du, B.; He, T. Solvent-Induced Morphology of the Binary Mixture of Diblock Copolymer in Thin Film: The Block Length and Composition Dependence of Morphology. J. Phys. Chem. B 2009, 113, 2712–2724. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Huang, H.; Chen, Y.; Gong, Y.; Du, B.; He, T. Effect of the Nature of Annealing Solvent on the Morphology of Diblock Copolymer Blend Thin Films. Macromolecules 2008, 41, 890–900. [Google Scholar] [CrossRef]
- Albalak, R.J.; Capel, M.S.; Thomas, E.L. Solvent swelling of roll-cast triblock copolymer films. Polymer 1998, 39, 1647–1656. [Google Scholar] [CrossRef]
- Albert, J.N.L.; Young, W.-S.; Lewis, R.L.; Bogart, T.D.; Smith, J.R.; Epps, T.H. Systematic Study on the Effect of Solvent Removal Rate on the Morphology of Solvent Vapor Annealed ABA Triblock Copolymer Thin Films. ACS Nano 2012, 6, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.M. Hansen Solubility Parameters a User’s Handbook, 2nd ed.; Hansen, C.M., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2007; ISBN 9780849372483. [Google Scholar]
- Lindvig, T.; Michelesen, M.L.; Kontogeorgis, G.M. A Flory–Huggins model based on the Hansen solubility parameters. Fluid Phase Equilib. 2002, 203, 247–260. [Google Scholar] [CrossRef]
- Miller-Chou, B.A.; Koenig, J.L. A review of polymer dissolution. Prog. Polym. Sci. 2003, 28, 1223–1270. [Google Scholar] [CrossRef] [Green Version]
- Cortizo, M.S.; Larsen, D.O.; Bianchetto, H.; Alessandrini, J.L. Effect of the thermal degradation of SBS copolymers during the ageing of modified asphalts. Polym. Degrad. Stab. 2004, 86, 275–282. [Google Scholar] [CrossRef]
- Georgopanos, P.; Handge, U.A.; Abetz, C.; Abetz, V. Influence of block sequence and molecular weight on morphological, rheological and dielectric properties of weakly and strongly segregated styrene-isoprene triblock copolymers. Polymer 2016, 104, 279–295. [Google Scholar] [CrossRef] [Green Version]
- Jian, X.; Hay, A.S. Catalytic epoxidation of styrene–butadiene triblock copolymer with hydrogen peroxide. J. Polym. Sci. Part A Polym. Chem. 1991, 29, 1183–1189. [Google Scholar] [CrossRef]
- Kane, L.; Norman, D.A.; White, S.A.; Matsen, M.W.; Satkowski, M.M.; Smith, S.D.; Spontak, R.J. Molecular, Nanostructural and Mechanical Characteristics of Lamellar Triblock Copolymer Blends: Effects of Molecular Weight and Constraint. Macromol. Rapid Commun. 2001, 22, 281–296. [Google Scholar] [CrossRef]
- Breiner, U.; Krappe, U.; Abetz, V.; Stadler, R. Cylindrical morphologies in asymmetric ABC triblock copolymers. Macromol. Chem. Phys. 1997, 198, 1051–1083. [Google Scholar] [CrossRef]
- Laurer, J.H.; Hajduk, D.A.; Fung, J.C.; Sedat, J.W.; Smith, S.D.; Gruner, S.M.; Agard, D.A.; Spontak, R.J. Microstructural Analysis of a Cubic Bicontinuous Morphology in a Neat SIS Triblock Copolymer. Macromolecules 1997, 30, 3938–3941. [Google Scholar] [CrossRef]
- Kaewsaiha, P.; Matsumoto, K.; Matsuoka, H. Synthesis and Nanostructure of Strong Polyelectrolyte Brushes in Amphiphilic Diblock Copolymer Monolayers on a Water Surface. Langmuir 2004, 20, 6754–6761. [Google Scholar] [CrossRef] [PubMed]
- Zalakain, I.; Politakos, N.; Fernandez, R.; Etxeberria, H.; Ramos, J.A.; Corcuera, M.A.; Mondragon, I.; Eceiza, A. Morphology response by solvent and vapour annealing using polystyrene/poly(methyl methacrylate) brushes. Thin Solid Films 2013, 539, 201–206. [Google Scholar] [CrossRef]
- Politakos, N.; Kortaberria, G.; Zalakain, I.; Mondragon, I.; Avgeropoulos, A. Enhancing the hydrophobic properties of various commercial polymers through mixtures and coatings with a fluorinated diblock copolymer in low concentrations. Eur. Polym. J. 2013, 49, 1841–1851. [Google Scholar] [CrossRef]
- Zalakain, I.; Politakos, N.; Ramos, J.A.; Saralegi, A.; Etxeberria, H.; Mondragon, I.; Corcuera, M.A.; Eceiza, A. Chemical and morphological characterization of sulfonated polystyrene brushes in different environments. Eur. Polym. J. 2013, 49, 2120–2127. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 044482877X, 9780444828774. [Google Scholar]
- Fortunati, E.; Puglia, D.; Iannoni, A.; Terenzi, A.; Kenny, J.M.; Torre, L. Processing Conditions, Thermal and Mechanical Responses of Stretchable Poly (Lactic Acid)/Poly (Butylene Succinate) Films. Materials 2017, 10, 809. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Lionetto, F.; Maffezzoli, A. Processing and Characterization of Amorphous Polyethylene Terephthalate Fibers for the Alignment of Carbon Nanofillers in Thermosetting Resins. Polym. Compos. 2015, 36, 1096–1103. [Google Scholar] [CrossRef]
- Zalakain, I.; Ramos, J.A.; Fernandez, R.; Etxeberria, H.; Mondragon, I. Silicon and carbon substrates induced arrangement changes in poly(styrene-b-isoprene-b-styrene) block copolymer thin films. J. Appl. Polym. Sci. 2012, 125, 1552–1558. [Google Scholar] [CrossRef]
- Niu, S.; Saraf, R.F. Stability of Order in Solvent-Annealed Block Copolymer Thin Films. Macromolecules 2003, 36, 2428–2440. [Google Scholar] [CrossRef] [Green Version]
- Radzilowski, L.H.; Carvalho, B.L.; Thomas, E.L. Structure of minimum thickness and terraced free-standing films of block copolymers. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 3081–3093. [Google Scholar] [CrossRef]
- Mansky, P.; Russell, T.P.; Hawker, C.J.; Mays, J.; Cook, D.C.; Satija, S.K. Interfacial Segregation in Disordered Block Copolymers: Effect of Tunable Surface Potentials. Phys. Rev. Lett. 1997, 79, 237–240. [Google Scholar] [CrossRef]
- Karim, A.; Slawecki, T.M.; Kumar, S.K.; Douglas, J.F.; Satija, S.K.; Han, C.C.; Russell, T.P.; Liu, Y.; Overney, R.; Sokolov, J.; et al. Phase Separation-Induced Surface Patterns in Thin Polymer Blend Films. Macromolecules 1998, 31, 857–862. [Google Scholar] [CrossRef]
- Yeol, D.; Kyusoon, R.; Drockenmuller, S.E.; Hawker, C.J.; Russell, T.P. A Generalized Approach to the Modification of Solid Surfaces. Science 2005, 308, 236–239. [Google Scholar] [CrossRef]
- Kellogg, G.J.; Walton, D.G.; Mayes, A.M.; Lambooy, P.; Russell, T.P.; Gallagher, P.D.; Satija, S.K. Observed Surface Energy Effects in Confined Diblock Copolymers. Phys. Rev. Lett. 1996, 76, 2503–2506. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Huang, H.; Hu, Z.; Chen, Y.; Chen, D.; Wang, Z.; He, T. Inverted to Normal Phase Transition in Solution-Cast Polystyrene−Poly(methyl methacrylate) Block Copolymer Thin Films. Macromolecules 2006, 39, 3369–3376. [Google Scholar] [CrossRef]
- Knoll, A.; Horvat, A.; Lyakhova, K.S.; Krausch, G.; Sevik, G.J.A.; Zvelindovsky, A.V.; Magerle, R. Phase Behavior in Thin Films of Cylinder-Forming Block Copolymers. Phys. Rev. Lett. 2002, 89, 035501. [Google Scholar] [CrossRef] [PubMed]
- Konrad, M.; Knoll, A.; Krausch, G.; Magerle, R. Volume Imaging of an Ultrathin SBS Triblock Copolymer Film. Macromolecules 2000, 33, 5518–5523. [Google Scholar] [CrossRef]
- Harrison, C.; Park, M.; Chaikin, P.; Register, R.A.; Adamson, D.H.; Yao, N. Depth Profiling Block Copolymer Microdomains. Macromolecules 1998, 31, 2185–2189. [Google Scholar] [CrossRef]
- Wu, J.; Soucek, M.D.; Cakmak, M. Investigation of Electron Beam Initiated Reactions of Styrenic Block Copolymers. Prog. Org. Coat. 2016, 100, 141–152. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, F.; Hu, Z.; Du, B.; He, T.; Kay Lee, F.; Wang, Y.; Tsui, O.K.C. Study on the Origin of Inverted Phase in Drying Solution-Cast Block Copolymer Films. Macromolecules 2003, 36, 4084–4092. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Tsui, O.K.C.; Du, B.; Zhang, F.; Tang, T.; He, T. Observation of Inverted Phases in Poly(styrene-b-butadiene-b-styrene) Triblock Copolymer by Solvent-Induced Order–Disorder Phase Transition. Macromolecules 2000, 33, 9561–9567. [Google Scholar] [CrossRef]
- Zhang, Q.; Hua, W.; Ren, Q.; Feng, J. Regulation of Physical Networks and Mechanical Properties of Triblock Thermoplastic Elastomer through Introduction of Midblock Similar Crystalline Polymer with Multiblock Architecture. Macromolecules 2016, 49, 7379–7386. [Google Scholar] [CrossRef]
- Alharbe, L.G.; Register, R.A.; Hobbs, J.K. Orientation Control and Crystallization in a Soft Confined Phase Separated Block Copolymer. Macromolecules 2017, 50, 987–996. [Google Scholar] [CrossRef] [Green Version]








| Dispersion Parameters | δd (MPa1/2) a | δp (MPa1/2) a | δh (MPa1/2) a | V (cm3/mol) b |
|---|---|---|---|---|
| Solvents | ||||
| Toluene | 18.0 | 1.4 | 2.0 | 106.8 |
| Cyclohexane | 16.8 | 0.0 | 0.2 | 108.7 |
| Tetrahydrofuran (THF) | 16.8 | 5.7 | 8.0 | 81.7 |
| Polymeric blocks | ||||
| Polystyrene (PS) | 17.6 | 6.1 | 4.1 | - |
| Polybutadiene (PB) | 18.0 | 5.1 | 2.5 | - |
| Poly(isoprene) (PI) | 17.4 | 3.1 | 3.1 | - |
| Poly(ethylene/propylene) (PEP) | 16.7 | 0.0 | 5.2 | - |
| Poly(ethylene) (PE) | 18.1 | 0.0 | 0.0 | - |
| Polymers | Toluene | Cyclohexane | THF | Affinity |
|---|---|---|---|---|
| PS | 0.170 | 0.360 | 0.088 | THF > Toluene> Cyclohexane |
| PB | 0.091 | 0.241 | 0.178 | Toluene > THF > Cyclohexane |
| PI | 0.036 | 0.127 | 0.160 | Toluene > Cyclohexane > THF |
| PEP | 0.120 | 0.160 | 0.200 | Toluene > Cyclohexane > THF |
| PE | 0.039 | 0.044 | 0.506 | Toluene > Cyclohexane > THF |
| Morphologies | SEPS | SIS | SEES | SBS | ||||
|---|---|---|---|---|---|---|---|---|
| 1% | 3% | 1% | 3% | 1% | 3% | 1% | 3% | |
| Disordered | - | - | 4C,3C 4T,3T 2F,3F 4F | 4C,3C 4T,3T 3F | 1C,2C 3C,1T 2T,3T 1F,2F 3F | 1C,2C 3C,1T 2T,3T 4T,1F 2F | 2C,3C 4C,2T 4T,1F 2F,3F 4F | 2C,3C 4C,3T 4T,1F 2F,3F 4F |
| Worm-like | 4C,3C 4T,1F 2F,3F 4F | 4T,1F 2F,3F 4F | 2T,1F | - | 4C,4T 4F | 4C,4F 3F | 1C,3T | - |
| Worm-like + crystals | 2C,3T 2T | - | - | - | - | - | - | - |
| Worm-like + perpendicular cylinders | - | 1C,2C 3C,4C 1T,2T | - | - | - | - | - | 2T |
| Parallel cylinders | - | - | 1C | 1C,1T | - | - | - | 1C |
| Perpendicular cylinders + crystal-like domains | - | - | 2C | 2C,2T 4F,2F | - | - | - | - |
| Perpendicular cylinders (disordered) | - | - | - | - | - | - | 1T | 1T |
| Perpendicular + parallel cylinders | 1C,1T | 3T | 1T | 1F | - | - | - | - |
| Parameters | ||||
|---|---|---|---|---|
| Concentration | 1% | 3% | - | - |
| Solvent | Toluene (T) | THF (F) | Cyclohexane (C) | - |
| Temperature | RT (1) | 60 °C (2) | 80 °C (3) | 100 °C (4) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politakos, N.; Kortaberria, G. Exploring the Self-Assembly Capabilities of ABA-Type SBS, SIS, and Their Analogous Hydrogenated Copolymers onto Different Nanostructures Using Atomic Force Microscopy. Materials 2018, 11, 1529. https://0-doi-org.brum.beds.ac.uk/10.3390/ma11091529
Politakos N, Kortaberria G. Exploring the Self-Assembly Capabilities of ABA-Type SBS, SIS, and Their Analogous Hydrogenated Copolymers onto Different Nanostructures Using Atomic Force Microscopy. Materials. 2018; 11(9):1529. https://0-doi-org.brum.beds.ac.uk/10.3390/ma11091529
Chicago/Turabian StylePolitakos, Nikolaos, and Galder Kortaberria. 2018. "Exploring the Self-Assembly Capabilities of ABA-Type SBS, SIS, and Their Analogous Hydrogenated Copolymers onto Different Nanostructures Using Atomic Force Microscopy" Materials 11, no. 9: 1529. https://0-doi-org.brum.beds.ac.uk/10.3390/ma11091529