Doping β-TCP as a Strategy for Enhancing the Regenerative Potential of Composite β-TCP—Alkali-Free Bioactive Glass Bone Grafts. Experimental Study in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Starting Metal-Doped β-TCP and FastOs®BG Powders
2.2. Preparation of Porous Granular Composite Bone Grafts
2.3. XRD, FT-IR and Particle Size Distribution Analyses
2.4. Surgical Procedure
2.5. Ex Vivo Studies
2.6. Direct Digital Radiography
2.7. Histological and Histomorphometric Analysis
2.8. Statistical Analysis
3. Results and Discussion
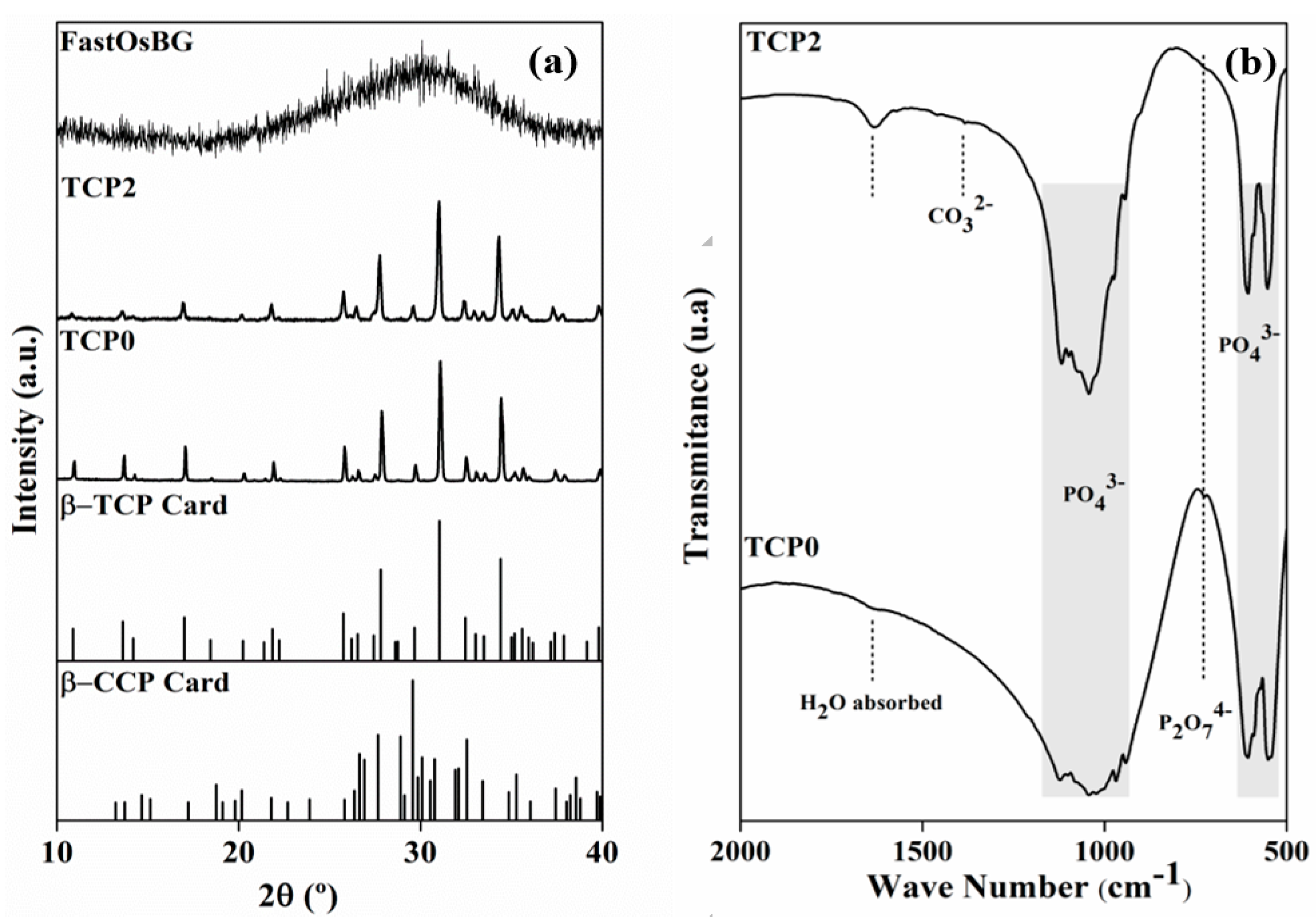
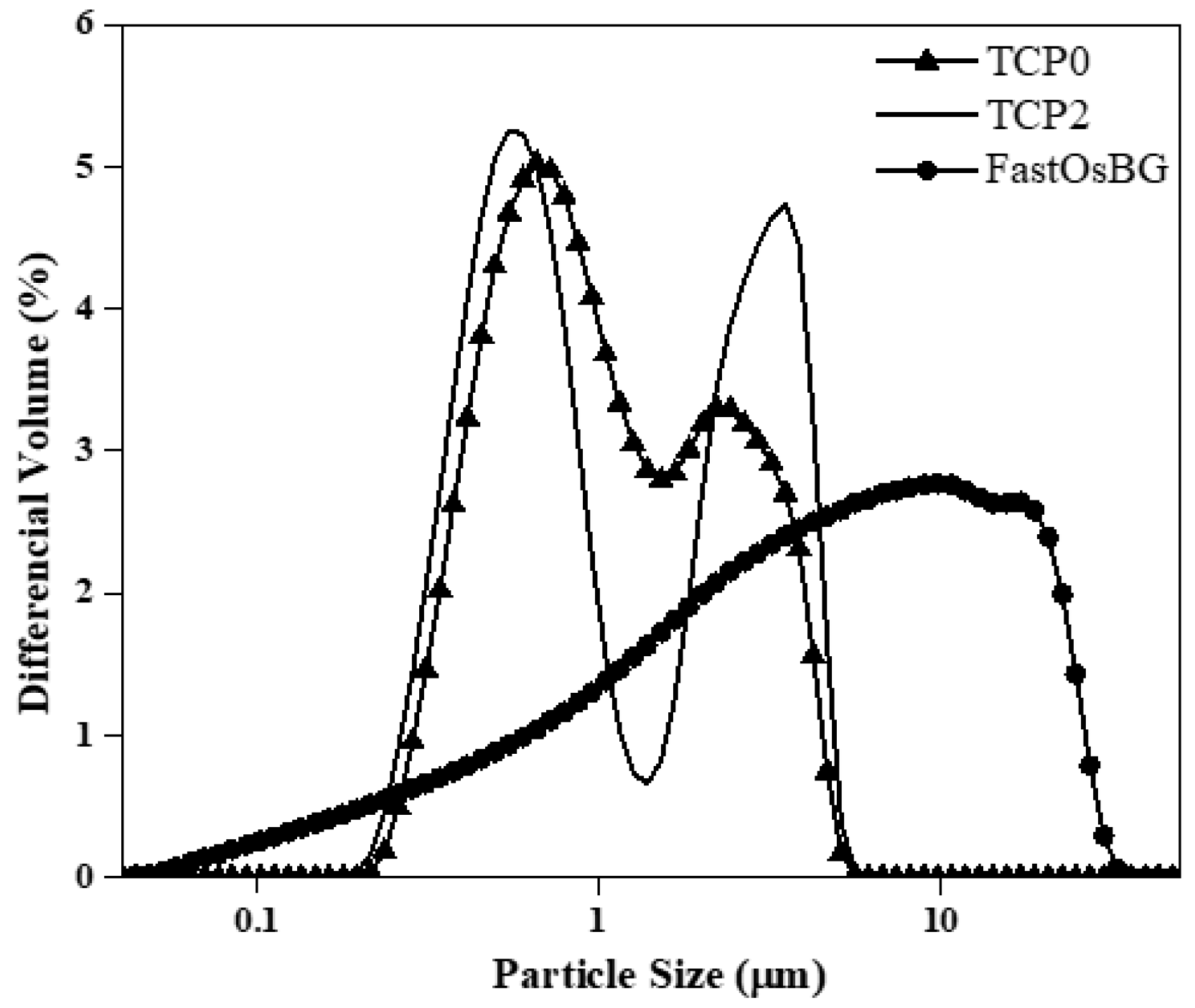
3.1. Characterization of the Starting Powders
3.2. Postoperative Animal Care
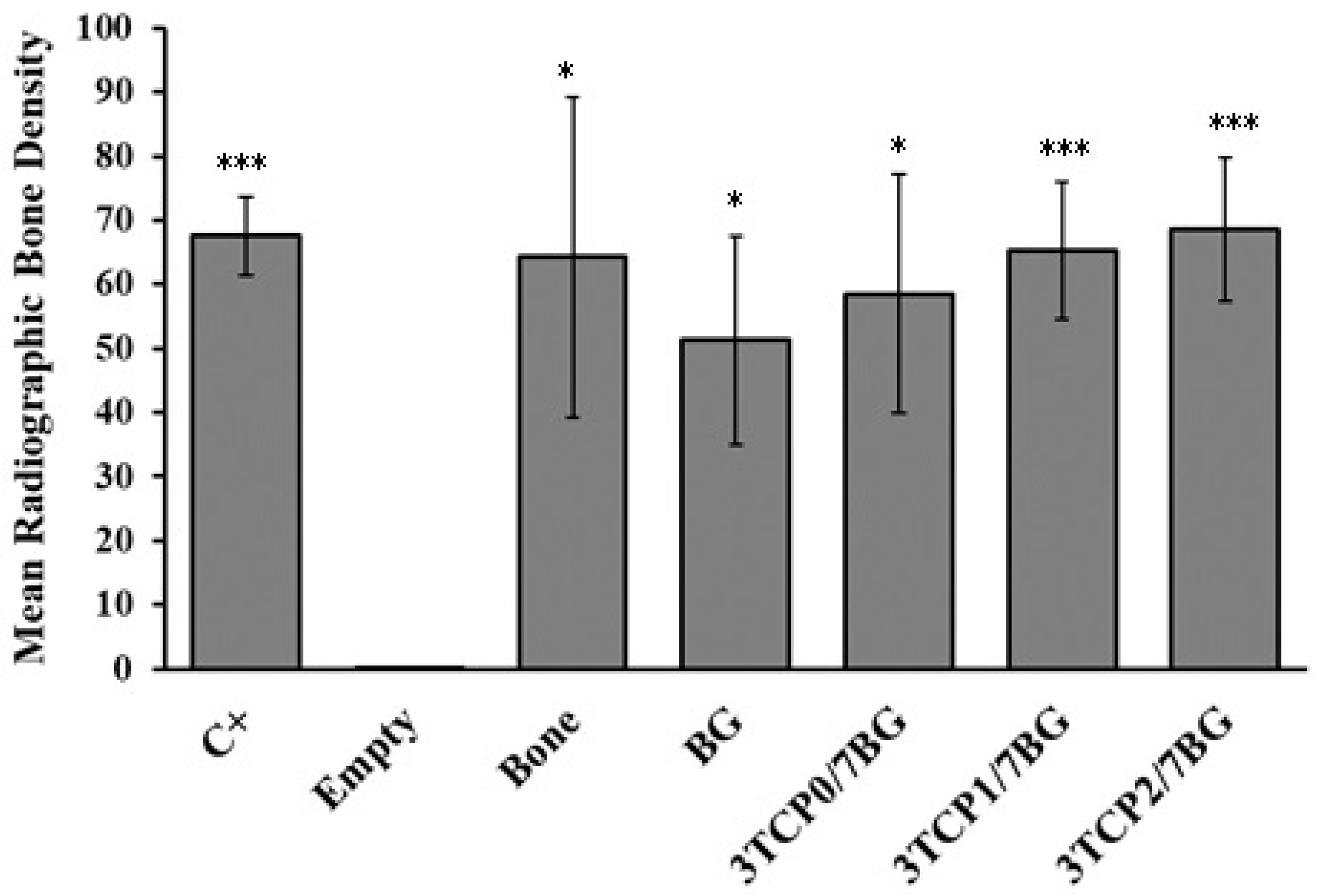
3.3. Digital Radiographic Evaluation
3.4. Histological Analysis
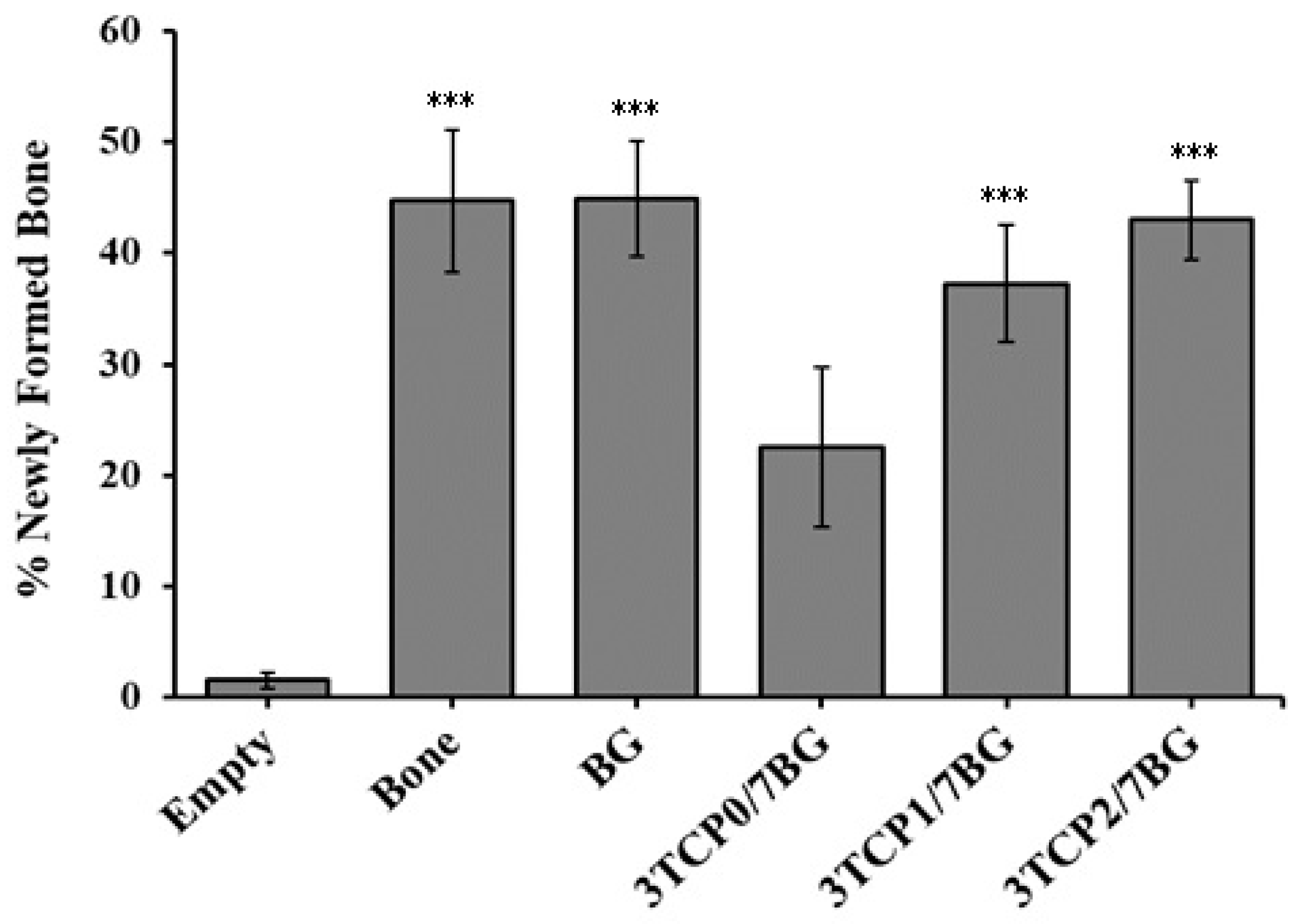
3.5. Histomorphometric Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Planell, J.A.; Navarro, M. Challenges of bone repair. In Bone Repair Biomaterials; Planell, J.A., Best, M., Lacroix, D., Merolli, A., Eds.; Woodhead Publishing Limited: Cambrigde, UK, 2009; pp. 3–6. ISBN 978-1-84569-385-5. [Google Scholar]
- Bohner, M. Resorbable biomaterials as bone graft substitutes. Mater. Today 2010, 13, 24–30. [Google Scholar] [CrossRef]
- Sheikh, Z.; Najeeb, S.; Khurshid, Z.; Verma, V.; Rashid, H.; Glogauer, M. Biodegradable materials for bone repair and tissue engineering applications. Materials 2015, 8, 5744–5794. [Google Scholar] [CrossRef] [PubMed]
- Schimandle, J.H.; Boden, S.D. Bone substitutes for lumbar fusion:present and future. Oper. Tech. Orthop. 1997, 7, 60–67. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Brown, W.E.; Chow, L.C. A new calcium phosphate water setting cement. Cem. Res. Progress Westerv. Am. Ceram. Soc. 1986, 352. [Google Scholar]
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates in dentistry. J. Mater. Sci. Mater. Med. 2013, 24, 1335–1363. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Ceramics for medical applications. J. Chem. Soc. Dalton Trans. 2001, 97–108. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate bioceramics. Eurasian Chem. J. 2010, 12, 247–258. [Google Scholar] [CrossRef]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, 96–101. [Google Scholar] [CrossRef]
- Nandi, S.K.; Roy, S.; Mukherjee, P.; Kundu, B.; Basu, D. Orthopaedic applications of bone graft & graft substitutes: A review. Indian J. Med. Res. 2010, 132, 15–30. [Google Scholar]
- Sheikh, Z.; Abdallah, M.N.; Hanafi, A.A.; Misbahuddin, S.; Rashid, H.; Glogauer, M. Mechanisms of in vivo degradation and resorption of calcium phosphate based biomaterials. Materials 2015, 8, 7913–7925. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Fernandes, H.; Habibovic, P.; de Boer, J.; Barradas, A.M.C.; de Ruiter, A.; Walsh, W.R.; van Blitterswijk, C.A.; de Bruijn, J.D. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc. Natl. Acad. Sci. USA 2010, 107, 13614–13619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, K.J.; Ko, Y.B.; Jaiswal, S.; Whang, I.C. Comparison of osteoconductivity and absorbability of beta-tricalcium phosphate and hydroxyapatite in clinical scenario of opening wedge high tibial osteotomy. J. Mater. Sci. Mater. Med. 2016, 27, 1–7. [Google Scholar] [CrossRef]
- Ishack, S.; Mediero, A.; Wilder, T.; Ricci, J.L.; Cronstein, B.N. Bone regeneration in critical bone defects using three-dimensionally printed β-tricalcium phosphate/hydroxyapatite scaffolds is enhanced by coating scaffolds with either dipyridamole or BMP-2. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Hashemibeni, B.; Dehghani, L.; Sadeghi, F.; Esfandiari, E.; Gorbani, M.; Akhavan, A.; Tahani, S.T.; Bahramian, H.; Goharian, V. Bone repair with differentiated osteoblasts from adipose-derived stem cells in hydroxyapatite/tricalcium phosphate in vivo. Int. J. Prev. Med. 2016, 2016. [Google Scholar] [CrossRef]
- Li, B.; Liu, Z.; Yang, J.; Yi, Z.; Xiao, W.; Liu, X.; Yang, X.; Xu, W.; Liao, X. Preparation of bioactive β-tricalcium phosphate microspheres as bone graft substitute materials. Mater. Sci. Eng. C 2017, 70, 1200–1205. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, T.; Zhu, J.; Cai, P. Osteoinduction of Calcium Phosphate Ceramics in Four Kinds of Animals for 1 Year: Dog, Rabbit, Rat, and Mouse. Transplant. Proc. 2016, 48, 1309–1314. [Google Scholar] [CrossRef]
- Gan, Y.; Dai, K.; Zhang, P.; Tang, T.; Zhu, Z.; Lu, J. The clinical use of enriched bone marrow stem cells combined with porous beta-tricalcium phosphate in posterior spinal fusion. Biomaterials 2008, 29, 3973–3982. [Google Scholar] [CrossRef]
- Pina, S.; Vieira, S.I.; Rego, P.; Torres, P.M.C.; da Cruz E Silva, O.A.B.; da Cruz E Silva, E.F.; Ferreira, J.M.F. Biological responses of brushite-forming Zn- and ZnSr- substituted beta-tricalcium phosphate bone cements. Eur. Cell. Mater. 2010, 20, 162–177. [Google Scholar] [CrossRef]
- Ferreira, M.M.; Brito, A.F.; Marques, C.F.; Freitas, L.F.; Carrilho, E.; Abrantes, A.M.; Pires, A.S.; Aguiar, M.J.; Carvalho, L.; Botelho, M.F.; et al. Can the regenerative potential of an alkali-free bioactive glass composition be enhanced when mixed with resorbable β-TCP? Ceram. Int. 2017, 44, 5025–5031. [Google Scholar] [CrossRef]
- Goel, A.; Kapoor, S.; Rajagopal, R.R.; Pascual, M.J.; Kim, H.W.; Ferreira, J.M.F. Alkali-free bioactive glasses for bone tissue engineering: A preliminary investigation. Acta Biomater. 2012, 8, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Cortez, P.P.; Brito, A.F.; Kapoor, S.; Correia, A.F.; Atayde, L.M.; Dias-Pereira, P.; Maurício, A.C.; Afonso, A.; Goel, A.; Ferreira, J.M.F. The in vivo performance of an alkali-free bioactive glass for bone grafting, FastOs®BG, assessed with an ovine model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kansal, I.; Reddy, A.; Muñoz, F.; Choi, S.J.; Kim, H.W.; Tulyaganov, D.U.; Ferreira, J.M.F. Structure, biodegradation behavior and cytotoxicity of alkali-containing alkaline-earth phosphosilicate glasses. Mater. Sci. Eng. C 2014, 44, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Semitela, Â.; Goel, A.; Xiang, Y.; Du, J.; Lourenço, A.H.; Sousa, D.M.; Granja, P.L.; Ferreira, J.M.F. Understanding the composition-structure-bioactivity relationships in diopside (CaO·MgO·2SiO2)-tricalcium phosphate (3CaO·P2O5) glass system. Acta Biomater. 2015, 15, 210–226. [Google Scholar] [CrossRef]
- Brito, A.F.; Antunes, B.; dos Santos, F.; Fernandes, H.R.; Ferreira, J.M.F. Osteogenic capacity of alkali-free bioactive glasses. In vitro studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2360–2365. [Google Scholar] [CrossRef]
- Stewart, G.J. The Skeletal and Muscular Systems; Chealsea House Publishers: New York, NY, USA, 2004; Volume 53, ISBN 9788578110796. [Google Scholar]
- Zofková, I.; Nemcikova, P.; Matucha, P. Trace elements and bone health. Clin Chem Lab Med 2013, 51, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.H.; Shepherd, D.V.; Best, S.M. Substituted hydroxyapatites for bone repair. J. Mater. Sci. Mater. Med. 2012, 23, 2335–2347. [Google Scholar] [CrossRef]
- LeGeros, R.; Lin, S.; LeGeros, J.; Cazalbou, S.; Combes, C.; Dupin-Roger, I. Strontium Ranelate Treatment Preserves Bone Crystal Characteristics and Bone Mineral Reactivity. Osteoporos. Int. 2004, 15, S116–S117. [Google Scholar]
- Luo, X.; Barbieri, D.; Davison, N.; Yan, Y.; De Bruijn, J.D.; Yuan, H. Zinc in calcium phosphate mediates bone induction: In vitro and in vivo model. Acta Biomater. 2014, 10, 477–485. [Google Scholar] [CrossRef]
- Meunier, P.J.; Roux, C.; Seeman, E.; Ortolani, S.; Badurski, J.E.; Spector, T.D.; Cannata, J.; Balogh, A.; Lemmel, E.-M.; Pors-Nielsen, S.; et al. The Effects of Strontium Ranelate on the Risk of Vertebral Fracture in Women with Postmenopausal Osteoporosis. N. Engl. J. Med. 2004, 350, 459–468. [Google Scholar] [CrossRef] [Green Version]
- Marie, P.J.; Ammann, P.; Boivin, G.; Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 2001, 69, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yamaguchi, R. Action of zinc on bone metabolism in rats. Increases in alkaline phosphatase activity and DNA content. Biochem. Pharmacol. 1986, 35, 773–777. [Google Scholar] [CrossRef]
- Ito, A.; Otsuka, M.; Kawamura, H.; Ikeuchi, M.; Ohgushi, H.; Sogo, Y.; Ichinose, N. Zinc-containing tricalcium phosphate and related materials for promoting bone formation. Curr. Appl. Phys. 2005, 5, 402–406. [Google Scholar] [CrossRef]
- Kawamura, H.; Ito, A.; Miyakawa, S.; Layrolle, P.; Ojima, K.; Ichinose, N.; Tateishi, T. Stimulatory effect of zinc-releasing calcium phosphate implant on bone formation in rabbit femora. J. Biomed. Mater. Res. 2000, 50, 184–190. [Google Scholar] [CrossRef]
- Chen, D.; Waite, L.C.; Pierce, W.M. In vitro effects of zinc on markers of bone formation. Biol. Trace Elem. Res. 1999, 68, 225–234. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Bizios, R. Hydroxylapatite with substituted magnesium, zinc, cadmium, and yttrium. II. Mechanisms of osteoblast adhesion. J. Biomed. Mater. Res. 2002, 59, 312–317. [Google Scholar] [CrossRef]
- Lang, C.; Murgia, C.; Leong, M.; Tan, L.; Perozzi, G.; Knight, D.; Ruffin, R.; Zalewski, P. Anti-inflammatory effects of zinc and alterations in zinc transporter mRNA in mouse models of allergic inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, 577–584. [Google Scholar] [CrossRef]
- Lansdown, A.B.G.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Ågren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef]
- Söderberg, T.A.; Sunzel, B.; Holm, S.; Elmros, T.; Hallmans, G.; Sjöberg, S. Antibacterial effect of zinc oxide in vitro. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1990, 24, 193–197. [Google Scholar] [CrossRef]
- Armulik, A.; Svineng, G.; Wennerberg, K.; Fässler, R.; Johansson, S. Expression of integrin subunit beta1B in integrin beta1-deficient GD25 cells does not interfere with alphaVbeta3 functions. Exp. Cell Res. 2000, 254, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.; Flynn, A. Trace elements and bone metabolism. Bibl Nutr Dieta 1998, 54, 150–164. [Google Scholar]
- Mayer, I.; Diab, H.; Reinen, D.; Albercht, C. Manganese in apatites, chemical, ligand-field and electron paramagnetic resonance spectroscopy studies. J. Mater. Sci. 1993, 28, 2428–2432. [Google Scholar] [CrossRef]
- Torres, P.; Vieira, S.; Cerqueira, R.; Pina, S.; Da Cruz Silva, B.; Abrantes, J.; Ferreira, J. Effects of Mn-doping on the structure and biological properties of β-tricalcium phosphate. J. Inorg. Biochem. 2014, 136, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L.; Day, D.E.; Höland, W.; Rheinberger, V.M. Glass and Medicine. Int. J. Appl. Glass Sci. 2010, 1, 104–117. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. In vitro dissolution of melt derived 45 S5 and sol–gel derived 58 S bioactive glasses. Biomed. Mater. Res. 2002, 61, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef] [PubMed]
- Clupper, D.C.; Hench, L.L. Crystallization kinetics of tape cast bioactive glass 45S5. J. Non-Cryst. Solids 2003, 318, 43–48. [Google Scholar] [CrossRef]
- Lefebvre, L.; Chevalier, J.; Gremillard, L.; Zenati, R.; Thollet, G.; Bernache-Assolant, D.; Govin, A. Structural transformations of bioactive glass 45S5 with thermal treatments. Acta Mater. 2007, 55, 3305–3313. [Google Scholar] [CrossRef] [Green Version]
- Bretcanu, O.; Chatzistavrou, X.; Paraskevopoulos, K.; Conradt, R.; Thompson, I.; Boccaccini, A.R. Sintering and crystallisation of 45S5 Bioglass® powder. J. Eur. Ceram. Soc. 2009, 29, 3299–3306. [Google Scholar] [CrossRef]
- Kapoor, S.; Goel, A.; Pascual, M.J.; Ferreira, J.M.F. Alkali-free bioactive diopside-tricalcium phosphate glass-ceramics for scaffold fabrication: Sintering and crystallization behaviours. J. Non-Cryst. Solids 2016, 432, 81–89. [Google Scholar] [CrossRef]
- Goel, A.; Kapoor, S.; Tilocca, A.; Rajagopal, R.R.; Ferreira, J.M.F. Structural role of zinc in biodegradation of alkali-free bioactive glasses. J. Mater. Chem. B 2013, 1, 3073–3082. [Google Scholar] [CrossRef]
- Kapoor, S.; Goel, A.; Tilocca, A.; Dhuna, V.; Bhatia, G.; Dhuna, K.; Ferreira, J.M.F. Role of glass structure in defining the chemical dissolution behavior, bioactivity and antioxidant properties of zinc and strontium co-doped alkali-free phosphosilicate glasses. Acta Biomater. 2014, 10, 3264–3278. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Goel, A.; Pascual, M.J.; Ferreira, J.M.F. Thermo-mechanical behaviour of alkali free bioactive glass-ceramics co-doped with strontium and zinc. J. Non-Cryst. Solids 2013, 375, 74–82. [Google Scholar] [CrossRef]
- Olhero, S.M.; Fernandes, H.R.; Marques, C.F.; Silva, B.C.G.; Ferreira, J.M.F. Additive manufacturing of 3D porous alkali-free bioactive glass scaffolds for healthcare applications. J. Mater. Sci. 2017, 52, 12079–12088. [Google Scholar] [CrossRef]
- Kannan, S.; Goetz-Neunhoeffer, F.; Neubauer, J.; Pina, S.; Torres, P.M.C.; Ferreira, J.M.F. Synthesis and structural characterization of strontium- and magnesium-co-substituted β-tricalcium phosphate. Acta Biomater. 2010, 6, 571–576. [Google Scholar] [CrossRef]
- Jillavenkatesa, A.; Condrate, R.A. The Infrared and Raman Spectra of β-and α-Tricalcium Phosphate (Ca3(Po4)2). Spectrosc. Lett. 1998, 31, 1619–1634. [Google Scholar] [CrossRef]
- Berzina-Cimdina, L.; Borodajenko, N. Research of Calcium Phosphates Using Fourier Transform Infrared Spectroscopy. In Infrared Spectroscopy—Materials Science, Engineering and Technology; IntechOpen: London, UK, 2012; pp. 123–148. ISBN 978-953-51-0537-4. [Google Scholar]
- Meejoo, S.; Maneeprakorn, W.; Winotai, P. Phase and thermal stability of nanocrystalline hydroxyapatite prepared via microwave heating. Thermochim. Acta 2006, 447, 115–120. [Google Scholar] [CrossRef]




| Features | References |
|---|---|
| Moderate degradation rate and fast bio mineralization in vitro, with HA formation detected after 1 h of immersion in simulated body fluid (SBF) | [23,56,57] |
| Ability to reduce oxidative stress | [56,57] |
| Excellent bone bonding ability in vivo | [24] |
| Osteogenic ability | [26] |
| Excellent sintering behavior | [25,58] |
| Strong mechanical properties | [25,58] |
| Easiness of 3D scaffolds fabrication | [59] |
| Description of the Samples | Codes of the Samples |
|---|---|
| Component materials | |
| β-TCP non-doped | TCP0 |
| β-TCP doped with 5Sr, 1Zn, 0.5Mn (mol%) | TCP1 |
| β-TCP doped with 10Sr, 2Zn, 0.5Mn (mol%) | TCP2 |
| FastOs®BG | BG |
| Composites (volume ratio) | |
| 3β-TCP0/7FastOs®BG | 3TCP0/7BG |
| 3β-TCP1/7FastOs®BG | 3TCP1/7BG |
| 3β-TCP2/7FastOs®BG | 3TCP2/7BG |
| Control groups used in the in vivo experiments | |
| Non-manipulated bone (Positive control) | C+ |
| Empty defect (Negative control) | Empty |
| Bone-filled defect | Bone |
| FastOs®BG | BG |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.M.; Brito, A.F.; Brazete, D.; Pereira, I.C.; Carrilho, E.; Abrantes, A.M.; Pires, A.S.; Aguiar, M.J.; Carvalho, L.; Botelho, M.F.; et al. Doping β-TCP as a Strategy for Enhancing the Regenerative Potential of Composite β-TCP—Alkali-Free Bioactive Glass Bone Grafts. Experimental Study in Rats. Materials 2019, 12, 4. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12010004
Ferreira MM, Brito AF, Brazete D, Pereira IC, Carrilho E, Abrantes AM, Pires AS, Aguiar MJ, Carvalho L, Botelho MF, et al. Doping β-TCP as a Strategy for Enhancing the Regenerative Potential of Composite β-TCP—Alkali-Free Bioactive Glass Bone Grafts. Experimental Study in Rats. Materials. 2019; 12(1):4. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12010004
Chicago/Turabian StyleFerreira, Manuel M., Ana F. Brito, Daniela Brazete, Inês C. Pereira, Eunice Carrilho, Ana M. Abrantes, Ana S. Pires, Maria J. Aguiar, Lina Carvalho, Maria F. Botelho, and et al. 2019. "Doping β-TCP as a Strategy for Enhancing the Regenerative Potential of Composite β-TCP—Alkali-Free Bioactive Glass Bone Grafts. Experimental Study in Rats" Materials 12, no. 1: 4. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12010004







