Amorphous Silica Nanoparticles Obtained by Laser Ablation Induce Inflammatory Response in Human Lung Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
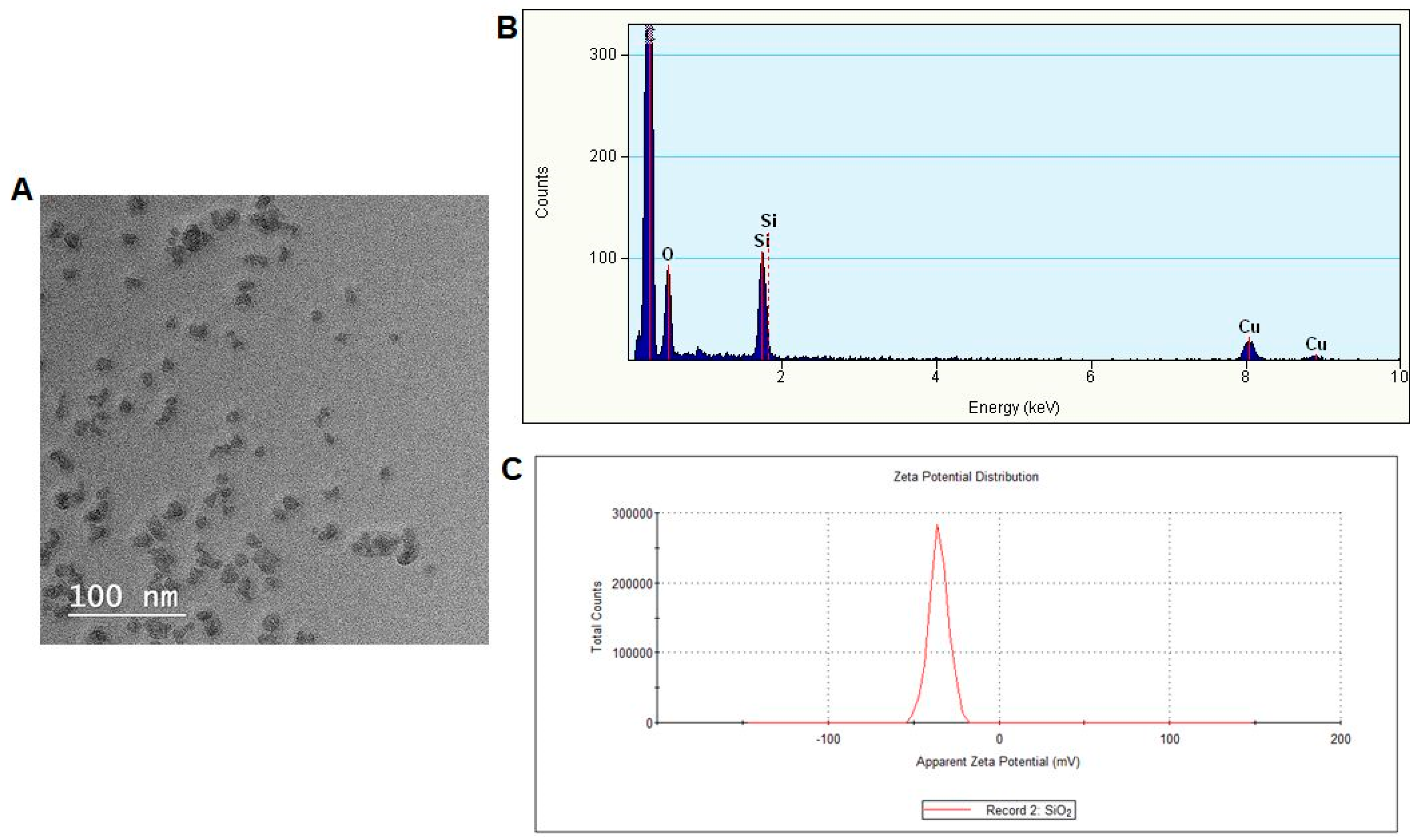
2.2. SiO2 NPs Preparation and Characterization
2.3. Cell Culture and Treatment
2.4. The Lysate Preparation
2.5. Protein Concentration Assessment
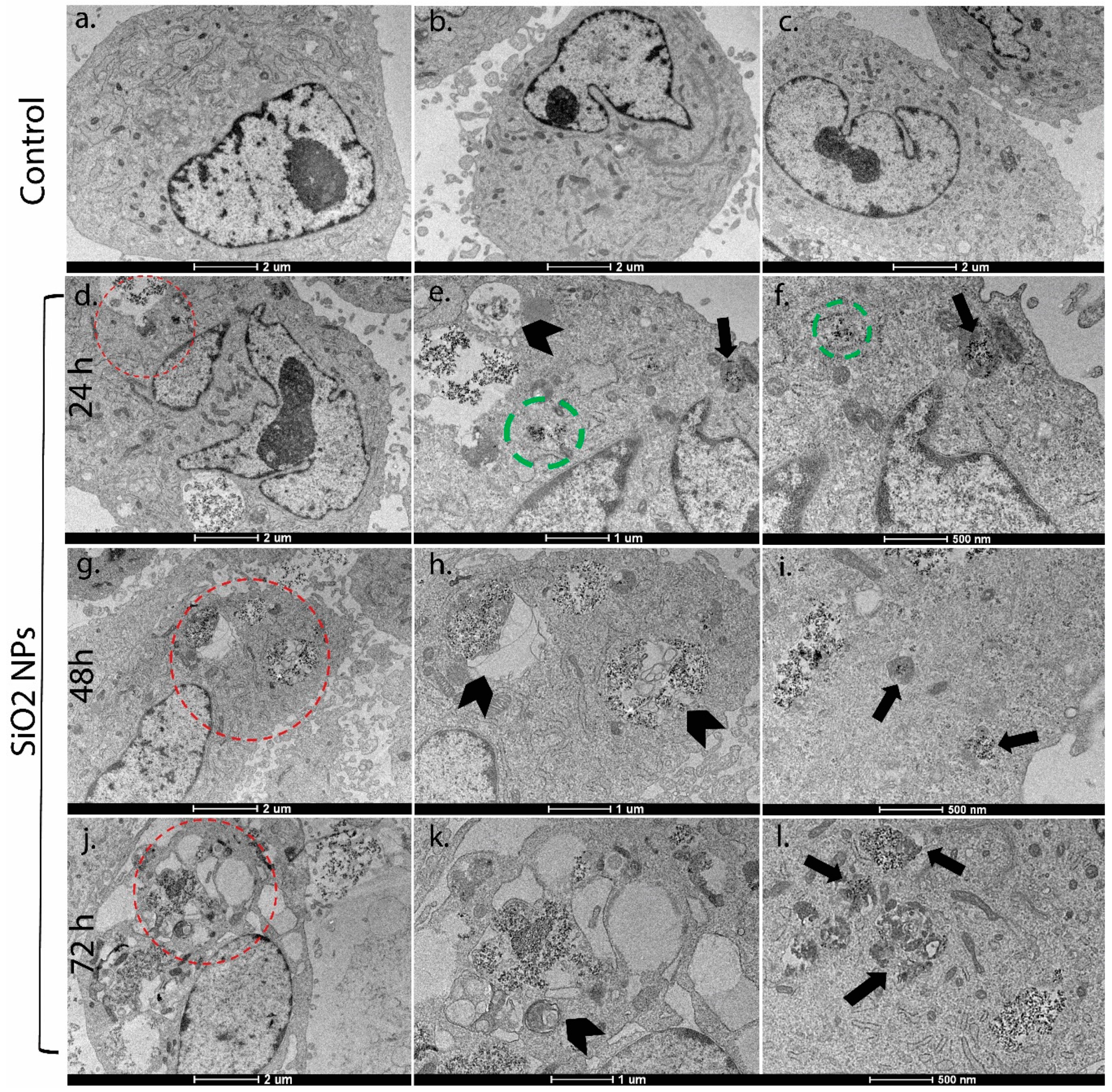
2.6. Electron Microscopy
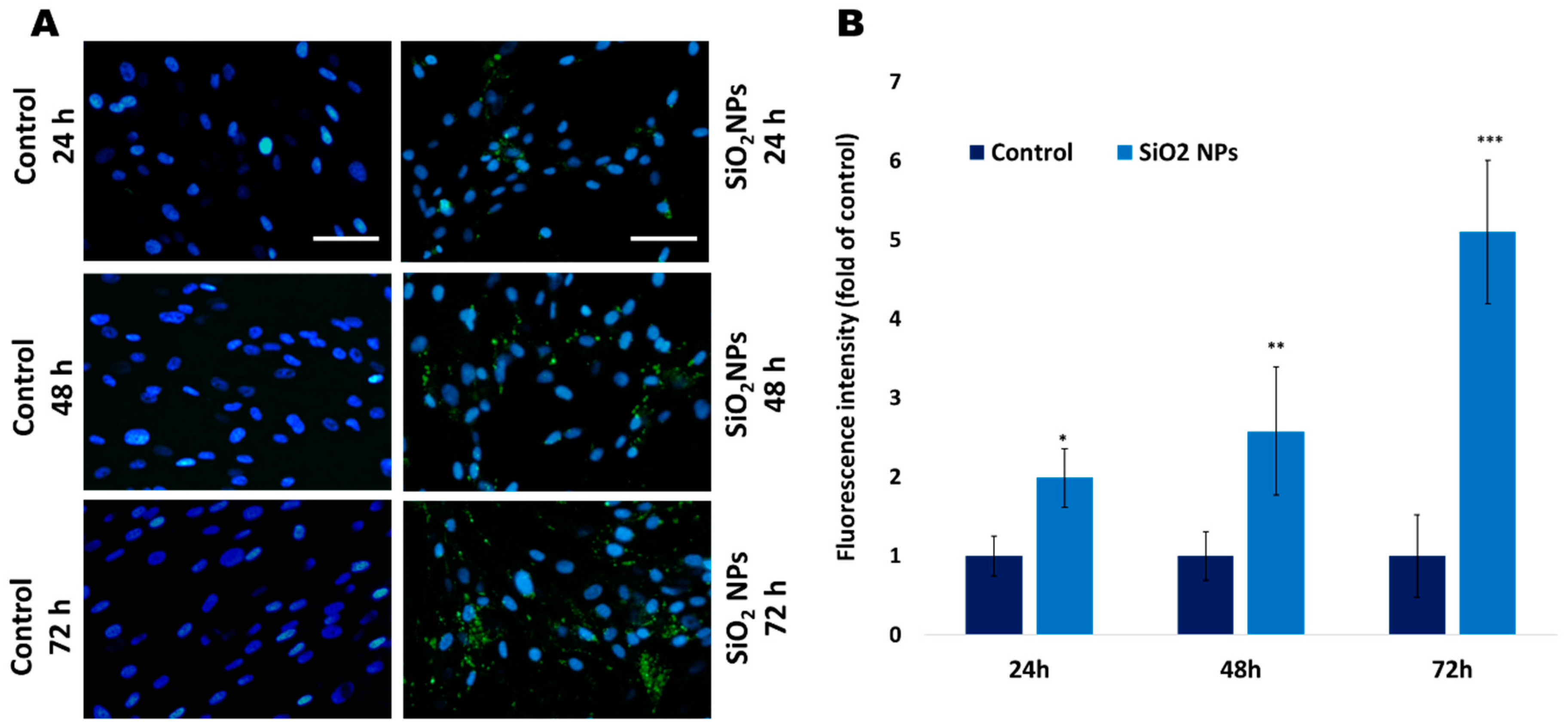
2.7. The Quantification of Lysosomes with Lyso Tracker Green DND-26
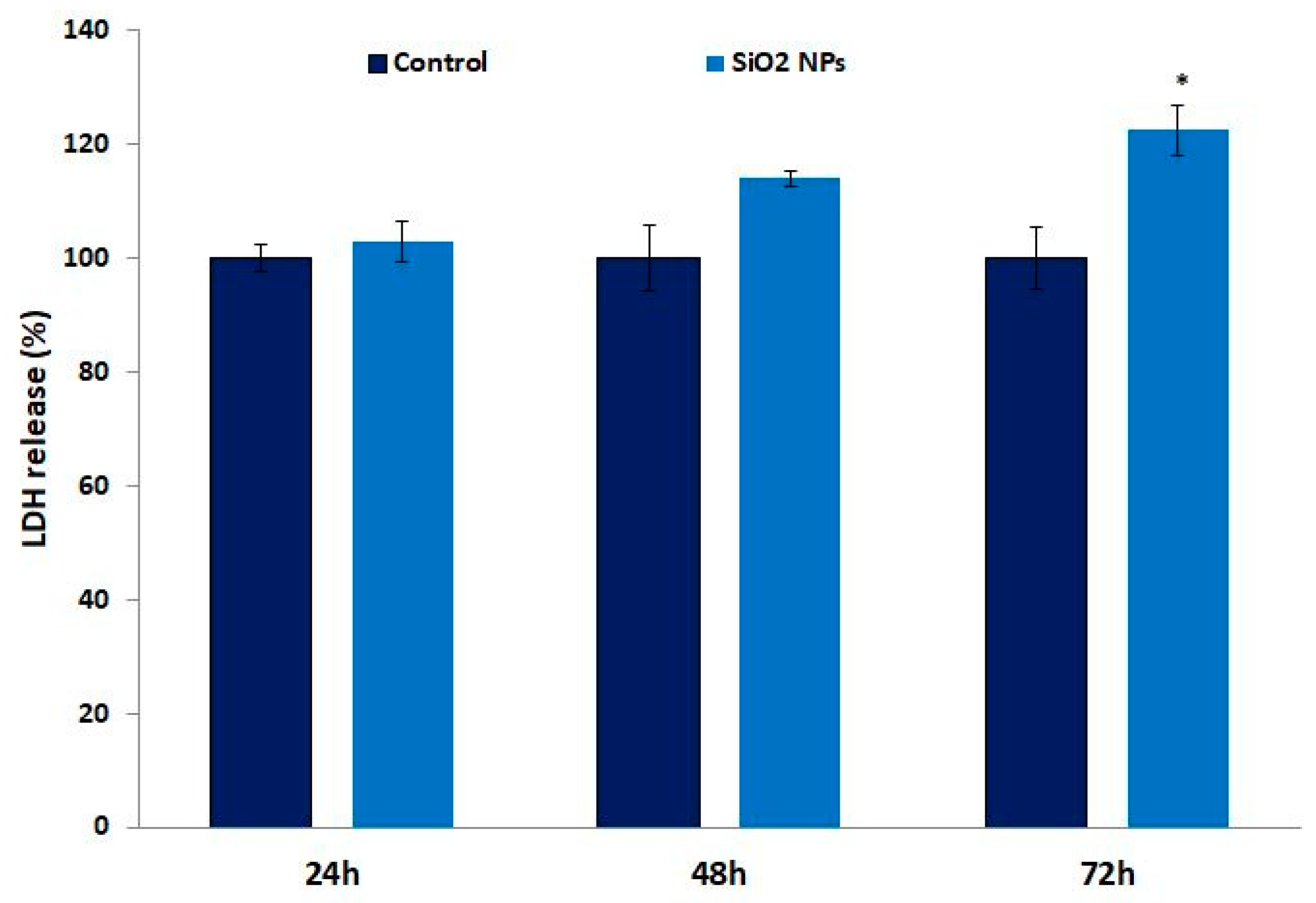
2.8. Lactate Dehydrogenase (LDH) Release Assay
2.9. Western Blot Analysis
2.10. Measurement of Nitric Oxide (NO) Concentration
2.11. SOD Zymography
2.12. Caspase-1 Activity Assay
2.13. Gelatin Zymography
2.14. Statistical Analysis
3. Results
3.1. Nanoparticles Characterization
3.2. Ultrastructural Studies
3.3. The Quantification of Lysosomes with Lyso Tracker Green in Human Lung Fibroblasts
3.4. Lactate Dehydrogenase (LDH) in Human Lung Fibroblasts
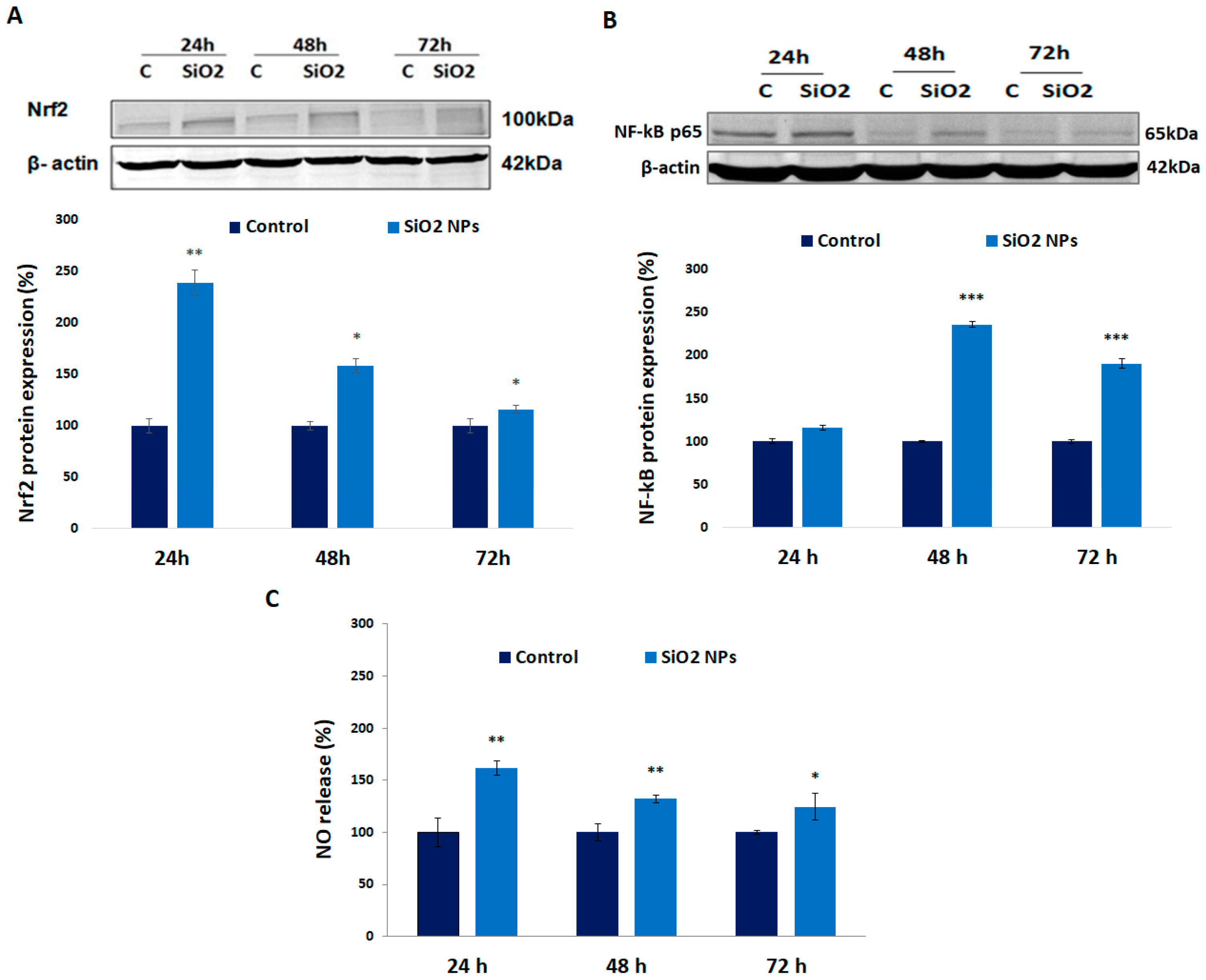
3.5. Expression of Nrf-2, NF-kB p-65 Proteins, and Nitric Oxide (NO) Release in Human Lung Fibroblasts
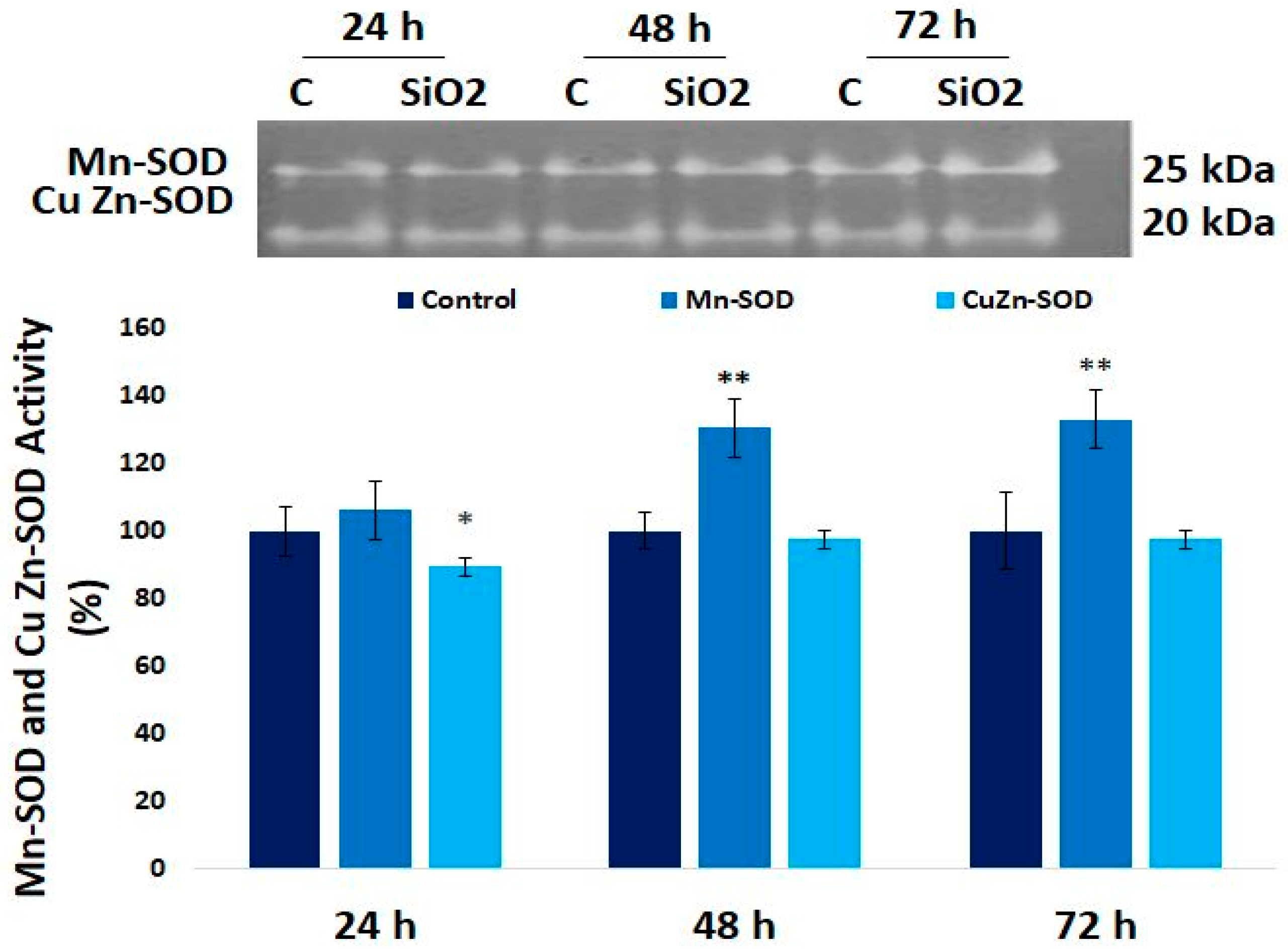
3.6. The Enzymatic MnSOD and CuZn-SOD Activities in Human Lung Fibroblast
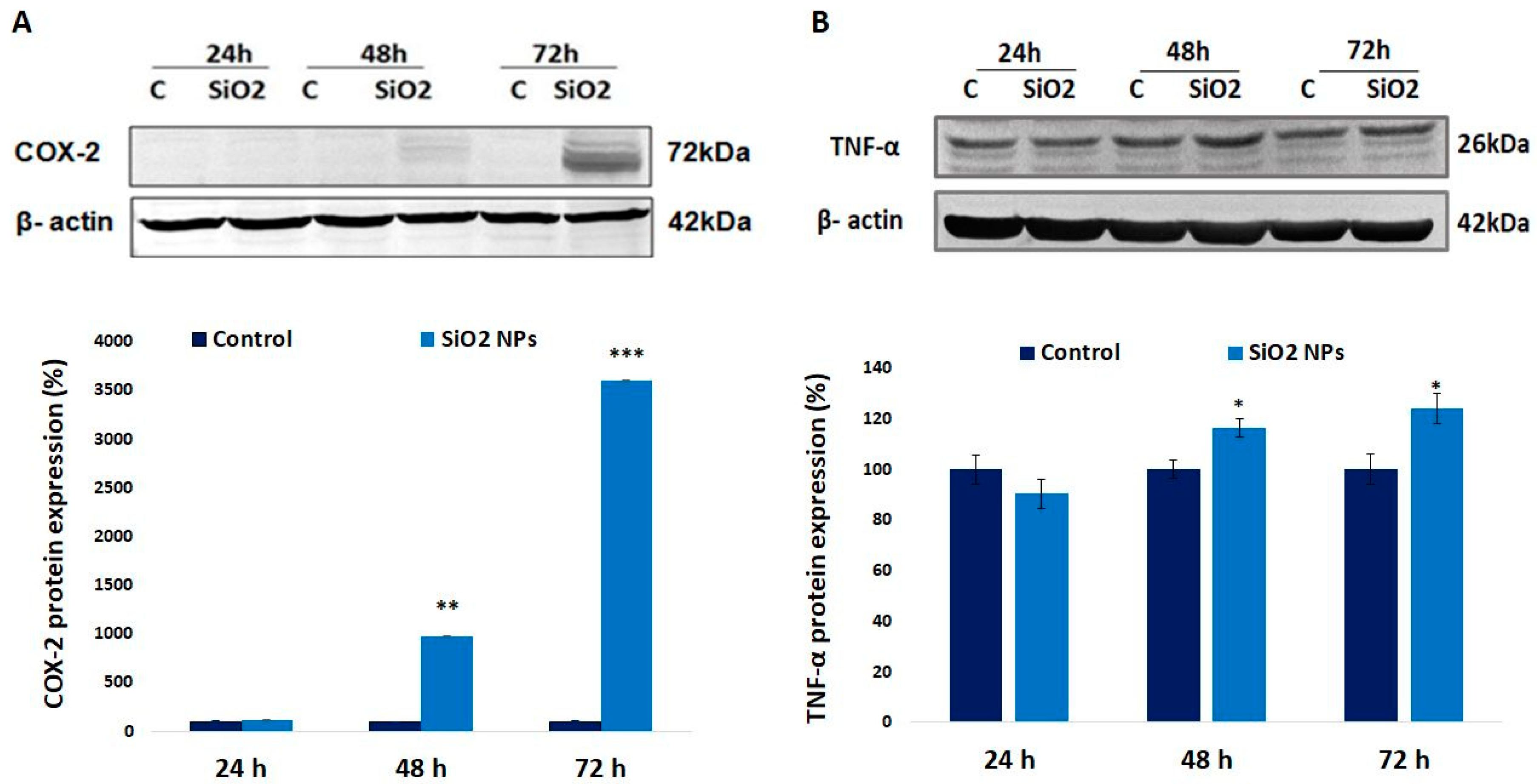
3.7. Cyclooxygenase-2 (COX-2) and Tumor Necrosis Factor Alpha (TNF-α) Proteins in Human Lung Fibroblasts
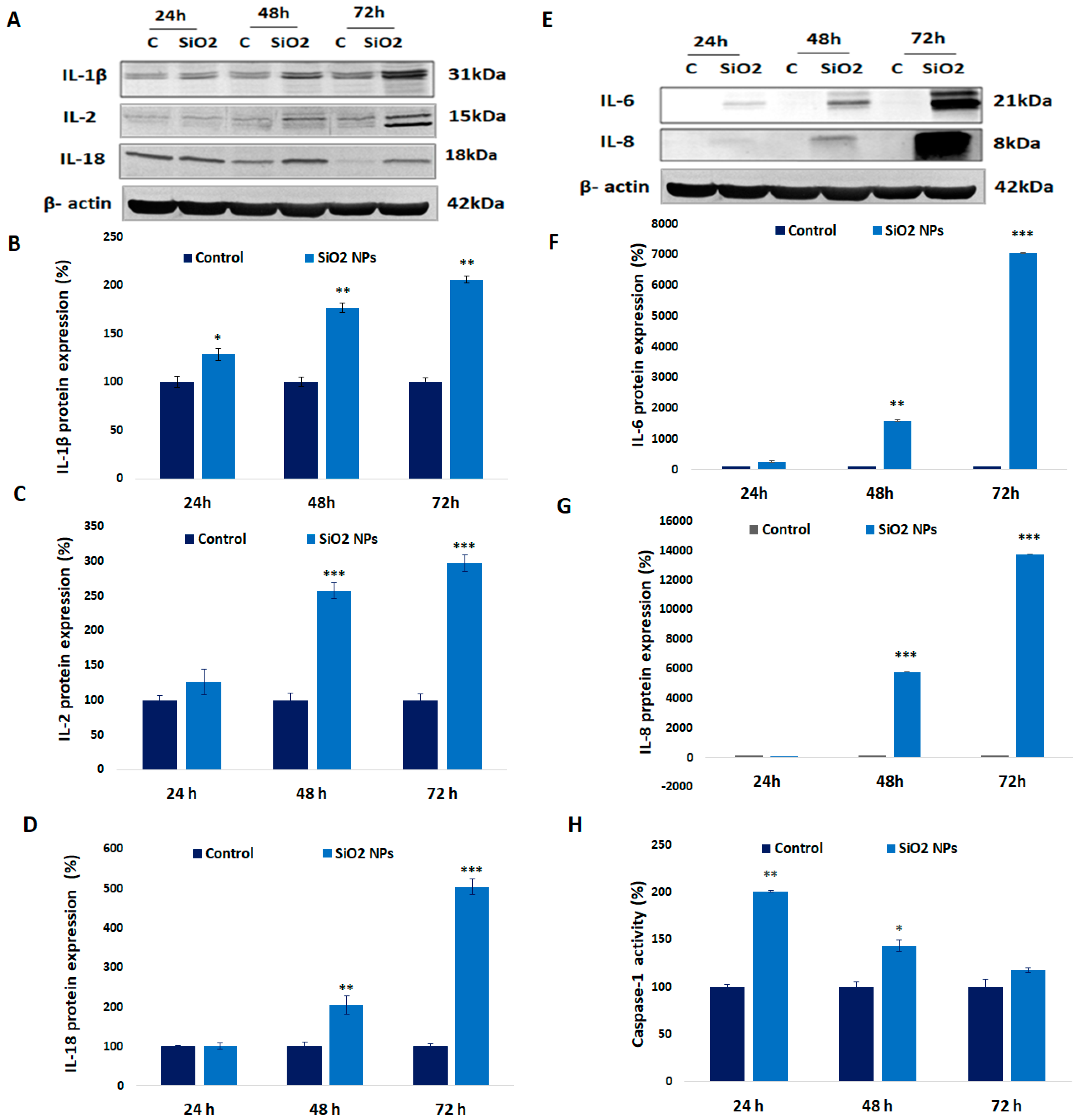
3.8. Expression of Interleukin (IL-1β, IL-2, IL-18, IL-6, and IL-8) and Caspase-1 Activity in Human Lung Fibroblasts
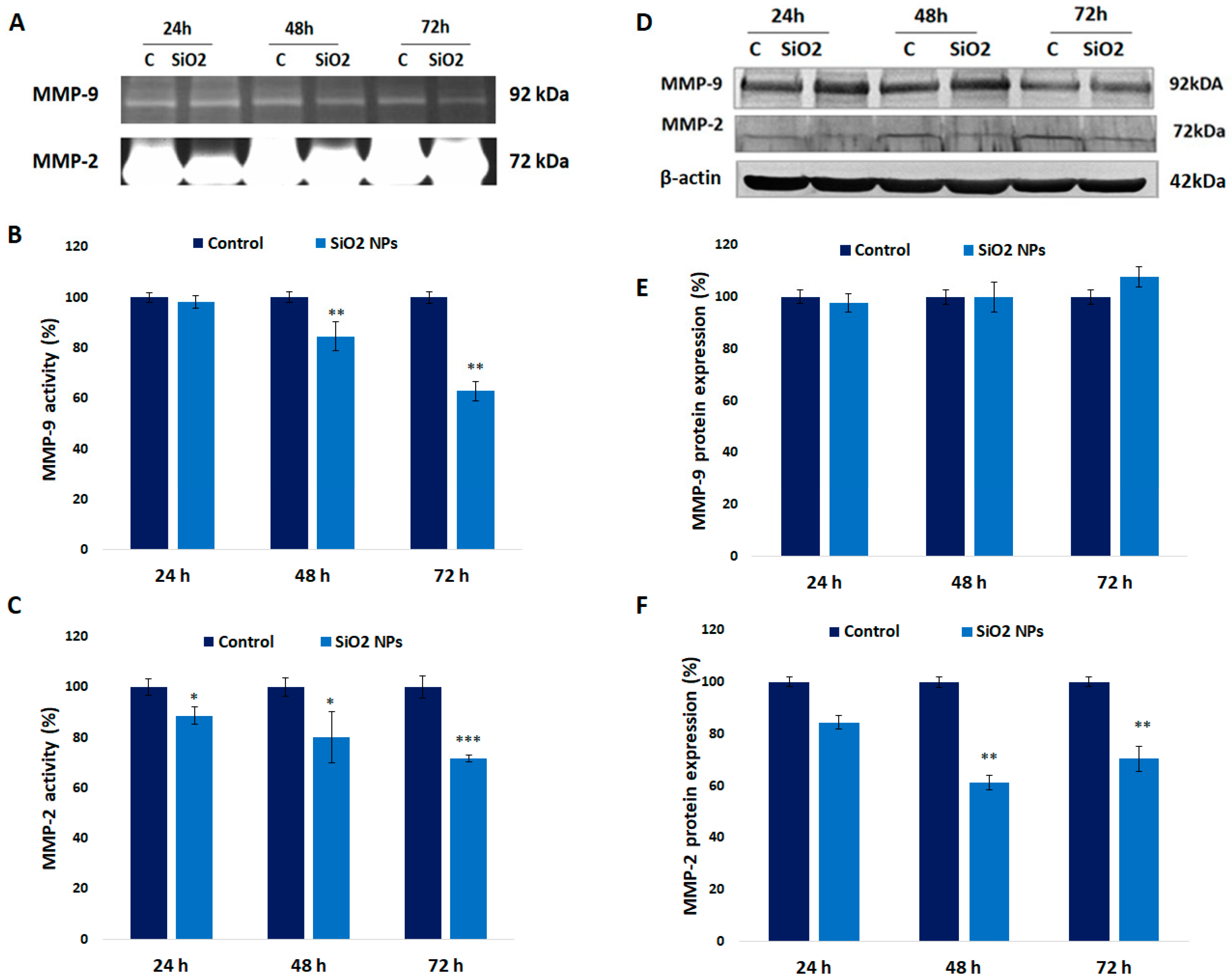
3.9. Activity and Expression of MMPs in Human Lung Fibroblasts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and Environmental Risks of Nanomaterials: Challenges and Future Needs. J. Environ. Sci. Health C 2009, 27, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Ogashawara, W.; Shenton, W.; Davis, S.A.; Mann, S. Template mineralization of ordered macroporous chitin-silica composites using a cuttlebone-derived organic matrix. Chem. Mat. 2000, 12, 2835–2837. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, Y.; Zhang, G.; Kang, Y.; Chen, A.; Feng, X.; Shao, L. The toxicity of silica nanoparticles to the immune system. Nanomedicine 2018, 13, 1939–1962. [Google Scholar] [CrossRef] [PubMed]
- Felix, D.G.; Kumar, G.S. Nanoparticles in Automobile Tires. IOSR J. Mech. Civil. Eng. 2014, 11, 7–11. [Google Scholar] [CrossRef]
- Fakoya, M.F.; Shah, S.N. Emergence of nanotechnology in the oil and gas industry: Emphasis on the application of silica nanoparticles. Petroleum 2017, 3, 391–405. [Google Scholar] [CrossRef]
- Witting, A.; Gehrke, H.; Del Favero, G.; Fritz, E.-M.; Al-Rawi, M.; Diabate, S.; Weiss, C.; Sami, H.; Ogris, M.; Marko, D. Amorphous Silica Particles Relevant in Food Industry Influence Cellular Growth and Associated Signaling Pathways in Human Gastric Carcinoma Cells. Nanomaterials 2017, 7, 18. [Google Scholar] [CrossRef]
- Sajid, M.; Ilyas, M.; Basheer, C.; Tariq, M.; Daud, M.; Baig, N.; Shehzad, F. Impact of nanoparticles on human and environment: Review of toxicity factors, exposures, control strategies and future prospects. Environ. Sci. Pollut. Res. Int. 2015, 22, 4122–4143. [Google Scholar] [CrossRef]
- Bakand, S.; Hayes, A.; Dechsakulthorn, F. Nanoparticles: A review of particle toxicology following inhalation exposure. Inhal. Toxicol. 2012, 24, 125–153. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Park, M.V.D.Z.; Gosens, I.; De Jong, W.H.; Cassee, F.R. Physicochemical characteristics of nanomaterials that affect pulmonary inflammation. Part. Fibre Toxicol. 2014, 11, 18. [Google Scholar] [CrossRef]
- Oberdorster, G.; Oberdorster, E.; Oberdorster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Frőhlich, E.; Salar-Behzadi, S. Toxicological Assessment of Inhaled Nanoparticles: Role of in Vivo, ex Vivo, in Vitro and in Silico Studies. Int. J. Mol. Sci. 2014, 15, 4795–4822. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.C.; Yu, I.T.S.; Chen, W. Silicosis. Lancet 2012, 379, 2008–2018. [Google Scholar] [CrossRef]
- Stone, V.; Johnston, H.; Schins, R.P.F. Development of in vitro systems for nanotoxicology: Methodological considerations. Crit. Rev. Toxicol. 2009, 39, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Shoeb, M.; Joseph, P.; Kodale, V.; Mustafa, G.; Farris, B.Y.; Umbright, C.; Roberts, J.R.; Erdely, A.; Antonini, J.M. Silica inhalation altered telomere lengh and gene expression of telomere regulatory proteins in lung tissue of rats. Sci. Rep. 2017, 7, 17284. [Google Scholar] [CrossRef] [PubMed]
- Turci, F.; Pavan, C.; Leinardi, R.; Tomatis, M.; Pastero, L.; Garry, D.; Anguissola, S.; Lison, D.; Fubini, B. Revisiting the paradigm of silica pathogenicity with synthetic quartz crystals: The role of crystallinity and surface disorder. Part. Fibre Toxicol. 2016, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–23010. [Google Scholar] [CrossRef] [PubMed]
- Stan, M.S.; Sima, C.; Cinteza, L.O.; Dinischiotu, A. Silicon-based quantum dots induce inflammation in human lung cells and disrupt extracellular matrix homeostasis. FEBS J. 2015, 282, 2914–2929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.J.; Sohn, J.-H.; Kim, Y.-J.; Park, Y.H.; Han, H.; Park, K.H.; Lee, K.; Choi, H.; Um, K.; Choi, I.-H.; et al. Acute exposure to silica nanoparticles aggravate airway inflammation: Different effects according to surface characteristics. Exp. Mol. Med. 2015, 47, e173. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Han, Y.; Zou, X.; Zhu, K.; Wang, Z.; Ye, Z.; Liu, Y.; Dong, S.; Chen, X.; Liu, D.; et al. Silica nanoparticles are an enhancer in the IL-1β-induced inflammation cycle of A549 cells. Immunopharmacol. Immunotoxicol. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Guo, C.; Xia, X.; Niu, P.; Jiang, L.; Duan, J.; Yu, Y.; Zhou, X.; Li, Y.; Sun, Z. Silica nanoparticles induce oxidative strees, inflammation and endothelial dysfunction in vitro via activation of the MAPK/Nrf2 pathway and nuclear factor-kB signaling. Int. J. Nanomed. 2015, 10, 1463–1477. [Google Scholar] [CrossRef]
- Christen, V.; Tent, K. Silica nanoparticles reduce endoplasmic reticulum stress response and activate mitogen activated kinase (MAPK) signaling. Toxicol. Rep. 2016, 3, 832–840. [Google Scholar] [CrossRef]
- Breznan, D.; Das, D.D.; O’Brien, J.S.; Mackinnon-Roy, C.; Nimesh, S.; Vuong, N.Q.; Bernatchez, S.; DeSilva, N.; Hill, M.; Kumarathasan, P.; et al. Differential cytotoxic and inflammatory potency of amorphous silicon dioxide nanoparticles of similar size in multiple cell lines. Nanotoxicology 2017, 11, 223–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, E.-J.; Kim, X.; Choi, K. A Single Instillation of Amorphous Silica Nanoparticles Induced Inflammatory Responses and Tissue Damage until Day 28 after exposure. J. Health Sci. 2011, 57, 60–71. [Google Scholar] [CrossRef]
- Petrache Voicu, S.N.; Dinu, D.; Sima, C.; Hermenean, A.; Ardelean, A.; Codrici, E.; Stan, M.S.; Zarnescu, O.; Dinischiotu, A. Silica nanoparticles induce oxidative stress and autophagy but not apoptosis in the MRC-5 cell line. Int. J. Mol. Sci. 2015, 16, 29398–29416. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Griess, P. Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt Ueber einige Azoverbindungen. Ber. Deutsch. Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Kasper, J.; Hermanns, M.I.; Bantz, C.; Koshkina, O.; Lang, T.; Maskos, M.; Pohl, C.; Unger, R.E.; Kirkpatrick, C.J. Interactions of silica nanoparticles with lung epithelial cells and the association to flotillins. Arch. Toxicol. 2013, 87, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Napierska, D.; Thomassen, L.C.J.; Rabolli, V.; Lison, D.; Gonzalez, L.; Kirsch-Volders, M.; Martens, J.A.; Hoet, P.H. Size-dependent cytotoxicity of monodisperse silica nanoparticles in human endothelial cells. Small 2009, 5, 846–853. [Google Scholar] [CrossRef]
- Hsiao, I.-L.; Gramatke, A.M.; Joksimovic, M.; Sokolovski, M.; Gradzielski, M.; Haase, A. Size and Cell type Dependent Uptake of Silica nanoparticles. J. Nanomed. Nanotechnol. 2014, 5, 1. [Google Scholar] [CrossRef]
- Kuhn, D.A.; Vanheche, D.; Michen, B.; Blank, F.; Gehr, P.; Petri-Fink, A.; Rotehn-Rutishauser, B. Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein. J. Nanotechnol. 2014, 5, 1625–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- Verma, A.; Stelacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Shannahan, J. The biocorona: A chalange for the biomedical application of nanoparticles. Nanotechnol. Rev. 2017, 6, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, L.; Yu, D.; Gao, C. Influence of silica particle internalization on adhesion and migration of human dermal fibroblasts. Biomaterials 2010, 31, 8465–8475. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tang, W.; Wang, P.; Liu, C.; Yuan, Y.; Qian, J. Cytotoxicity and Cellular Uptake of Amorphous Silica Nanoparticles in Human Cancer Cells. Part. Part. Syst. Characteriz. 2015, 32, 779–787. [Google Scholar] [CrossRef]
- Lehman, S.E.; Morris, A.S.; Mueller, P.S.; Salem, A.K.; Grassian, V.H.; Larsen, S.C. Silica Nanoparticle-Generated ROS as a Predictor of Cellular Toxicity: Mechanistic Insights and Safety by Design. Environ. Sci. Nano 2016, 3, 56–66. [Google Scholar] [CrossRef]
- Patwardhan, S.V.; Emami, F.S.; Berry, R.J.; Jones, S.E.; Naik, R.R.; Deschaume, O.; Heinz, H.; Perry, C.C. Chemistry of aqueous silica nanoparticle surfaces and the mechanism of selective peptide adsorption. J. Am. Chem. Soc. 2012, 134, 6244–6256. [Google Scholar] [CrossRef]
- Zhang, H.; Dunphy, D.R.; Jiang, X.; Meng, H.; Sun, B.; Tarn, D.; Xue, M.; Wang, X.; Lin, S.; Ji, Z.; et al. Processing Pathway Dependence of Amorphous Silica Nanoparticle Toxicity: Colloidal vs. Pyrolytic. J. Am. Chem. Soc. 2012, 134, 15790–15804. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Watsky, M.A. Chloride channel activity in human lung fibroblasts and myofibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L1110–L1116. [Google Scholar] [CrossRef] [Green Version]
- Fischer, A.B. Redox Signaling across Cell Membranes. Antioxid. Redox Signal. 2009, 11, 1349–1356. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-kB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Mitsuishi, Y.; Taguchi, K.; Kawatani, Y.; Shibata, T.; Nukiwa, T.; Aburatani, H.; Yamamoto, M.; Motahashi, H. Nrf-2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 2012, 22, 66–79. [Google Scholar] [CrossRef]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.L. The Keap-Nrf2 pathway mechanisms of activation and dysregulation in cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, Y.; Katsuoka, F.; Funayama, R.; Nagashyma, T.; Nishida, Y.; Nakayama, K.; Engel, J.D.; Yamamoto, M. Nrf2-MafG heterodimers contribute globally to antioxidant and metabolic networks. Nucleic Acids Res. 2012, 40, 10228–10239. [Google Scholar] [CrossRef]
- Angsten, M.; Sjöberg, E.; Frings, O.; Vorrink, S.U.; Frijhoff, J.; Olsson, E.; Borg, A.; Östman, A. Cancer-associated fibroblasts expressing CXCL 14 rely upon NOS-1 derived nitric oxide signaling for their tumor-suppoting properties. Cancer Res. 2014, 74, 2999–3010. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, M.C.; Radu, M.; Hermenean, A.; Sima, C.; Dinu, D.; Costache, M.; Grigoriu, C.; Dinischiotu, A. Antioxidative response induced by SiO2 nanoparticles in MRC5 cell line. Rom. Biotechnol. Lett. 2010, 15, 5000–5007. [Google Scholar]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vuyaskovic, Z.; St. Clair, D.; Batinic-Haberle, J. Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta 2012, 1822, 794–814. [Google Scholar] [CrossRef]
- Buettner, G.R.; Ng, C.F.; Wang, M.; Rodgers, V.G.; Schafer, F.Q. A new paradigm: Manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic. Biol. Med. 2006, 41, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.L.; Kamata, H.; Karin, M. IKK/NF-kB signaling balancing life and death-a new approach to cancer therapy. J. Clin. Investig. 2005, 115, 2625–2632. [Google Scholar] [CrossRef]
- Buelna-Chontal, M.; Zazueta, C. Redox activation of Nrf2 &NF-kB: A double end sword? Cell Signal. 2013, 25, 2548–2557. [Google Scholar] [CrossRef]
- Gegotek, A.; Skrzydlewska, E. The role of transcription factor Nrf2 in skin cells metabolism. Arch. Dermatol. Res. 2015, 307, 385–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.H.; Qu, J.; Shen, X. NF-kB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to Maf k. Biochem. Biophys. Acta 2008, 1783, 713–727. [Google Scholar] [CrossRef]
- Yu, M.; Li, H.; Liu, Q.; Liu, F.; Tang, L.; Li, C.; Yuan, Y.; Zhan, Y.; Xu, W.; Li, W.; et al. Nuclear factor p65 interacts with Keap 1 to repress the Nrf2-ARE pathway. Cell. Signal. 2011, 23, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Falvo, J.V.; Tsytsykova, A.V.; Goldfeld, A.E. Transcriptional control of the TNF Gene. Curr. Dir. Autoimmun. 2010, 11, 27–60. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, A.B.; Cheng, Z.; Vargas, J.D.; Birnbaum, H.A.; Hoffmann, A. Network dynamics determine the autocrine and paracrine signaling functions of TNF. Genes Dev. 2014, 28, 2120–2133. [Google Scholar] [CrossRef] [Green Version]
- Sha, Q.; Truong-Tran, A.Q.; Plitt, J.R.; Beck, L.A.; Schleimer, R.P. Activation of airway epithelial cells by toll like receptor agonists. Am. J. Respir. Cell Mol. Biol. 2004, 31, 358–364. [Google Scholar] [CrossRef]
- Chen, P.; Kanehira, K.; Taniguchi, A. Role of toll-like receptors 3, 4 and 7 in cellular uptake and response to titanium dioxide nanoparticles. Sci. Technol. Adv. Mater. 2013, 14, 015008. [Google Scholar] [CrossRef] [Green Version]
- Nakao, S.; Ogtata, Y.; Shimizu, E.; Yamazaki, M.; Furuyama, S.; Sugiya, H. Tumor necrosis factor alpha (TNF-alpha)-induced prostaglandin E2 release is mediated by the activation of cyclooxygenase 2 (COX-2) transcription via NF-kappa B in human gingival fibroblasts. Mol. Cell. Biochem. 2002, 238, 11–18. [Google Scholar] [CrossRef]
- Cogswell, J.P.; Godlevski, M.M.; Wisely, G.B.; Clay, W.C.; Leesnitzer, L.M.; Ways, J.P.; Gray, J.G. NF-kappa B regulates IL-1beta transcription through a consensus NF-kappa B binding site and a nonconsensus CRE-like site. J. Immunol. 1994, 153, 712–723. [Google Scholar]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-kB signaling in inflammation. Signal. Transd. Targ. Ther. 2017, 2, e17023. [Google Scholar] [CrossRef]
- Son, Y.H.; Jeong, Y.T.; Lee, K.A.; Choi, K.H.; Kim, S.M.; Rhim, B.Y.; Kim, K. Roles of MAPK and NF-kappa B in interleukin-6 induction by lipopolysaccharide in vascular smooth muscle cells. J. Cardiovasc. Pharmacol. 2008, 51, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kunsch, C.; Rosen, C.A. NF-kappa B subunit-specific regulation of the interleukin-8 promoter. Mol. Cell. Biol. 1993, 13, 6137–6146. [Google Scholar] [CrossRef]
- Lee, D.-J.; Du, F.; Chen, S.W.; Nakasaki, M.; Rana, I.; Shih, V.F.S.; Hoffmann, A.; Jamora, C. Regulation and function of the caspase 1 in an inflammatory microenvironment. J. Investig. Dermatol. 2015, 135, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Meissner, F.; Molawi, K.; Zychlinsky, A. Superoxide dismutase 1 regulates caspase 1 in endotoxic shock. Nat. Immunol. 2008, 9, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1 β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L.A. The IL-1 cytokine family and its role in inflammation and fibrosis in the lung. Semin. Immunopathol. 2016, 38, 517–534. [Google Scholar] [CrossRef] [Green Version]
- Afonina, I.S.; Mūller, C.; Martin, S.J.; Beyaert, R. Proteolytic Processing of Interleukine-1 Family Cytokines. Variation on a Common Theme. Immunity 2015, 42, 991–1004. [Google Scholar] [CrossRef]
- Kahari, V.M.; Heino, J.; Vuorio, E. Interleukin-1 increases collagen production and mRNA levels in cultured skin fibroblasts. Biochim. Biophys. Acta 1987, 929, 142–147. [Google Scholar] [CrossRef]
- Hoshino, T.; Kato, S.; Oka, N.; Imaoka, H.; Kinoshita, T.; Takei, S.; Kitasato, Y.; Kawayama, T.; Imaizumi, T.; Yamada, K.; Young, H.A.; Aizawa, H. Pulmonary inflammation and emphysema: Role of the cytokines IL-18 and IL-13. Am. J. Respir. Crit. Care Med. 2007, 176, 49–62. [Google Scholar] [CrossRef]
- Czuszak, C.A.; Sutherland, D.E.; Billman, M.A.; Stein, S.H. Prostaglandin E2 potentiates interleukin-1 beta induced interleukin-6 production by human gingival fibroblasts. J. Clin. Periodontol. 1996, 23, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Eskan, M.A.; Benakanakere, M.R.; Rose, B.G.; Zhang, P.; Zhao, J.; Stathopoulou, P.; Fujioka, D.; Kinane, D.F. Interleukin-1β Modulates Proinflammatory Cytokine Production in Human Epithelial Cells. Infect. Immun. 2008, 76, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, S.L.; Standiford, T.; Kasahara, K.; Strieter, R.M. Interleukin-8 (IL-8): The major neutrophil chemotactic factor in the lung. Exp. Lung Res. 1991, 17, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Sharma, R.; Sung, S.S.; Gaskin, F.; Fu, S.M.; Ju, S.T. A novel function of IL-2: Chemokine/chemoattractant/retention receptor genes induction in Th subset for skin and lung inflammation. J. Autoimmun. 2012, 38, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Pascual, V.; O’Garra, A. From IL-2 to IL-37: The expanding spectrum of anti-inflammatory cytokines. Nat. Immunol. 2012, 13, 925–931. [Google Scholar] [CrossRef]
- Hubbard, A.K.; Timblin, C.R.; Shukla, A.; Rincón, M.; Mossman, B.T. Activation of NF-kB dependent gene expression by silica in lungs of luciferase reporter mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L968–L975. [Google Scholar] [CrossRef]
- Manicone, A.M.; McGuire, J.K. Matrix Metalloproteinases as Modulators of Inflammation. Semin. Cell Dev. Biol. 2008, 19, 34–41. [Google Scholar] [CrossRef]
- Lindner, D.; Zietsch, C.; Becker, P.M.; Schulze, K.; Schultheiss, H.-P.; Tschöpe, C.; Westermann, D. Differential Expression of Matrix Metalloproteases in Human Fibroblasts with Different Origins. Biochem. Res. Int. 2012, 2012, 10. [Google Scholar] [CrossRef]
- Mytych, J.; Wnuk, M. Nanoparticles Technology as a Double-Edged Sword: Cytotoxic, Genotoxic and Epigenetic Effects on Living Cells. J. Biomat. Nanobiotech. 2013, 4, 53–63. [Google Scholar] [CrossRef]
- Fasciglione, G.F.; Marini, S.; D’Alessio, S.; Politi, V.; Coletta, M. pH- and Temperature- Dependence of Functional Modulation in Metalloproteinases. A comparison between Neutrophil Collagenase and Gelatinases A and B. Biophys. J. 2000, 79, 2138–2149. [Google Scholar] [CrossRef]
- Anseau, M.R.; Leung, J.P.; Sahai, N.; Swaddle, T.W. Interactions of Silicate Ions with Zinc (II) and Aluminium (III) in Alkaline Aqueous Solution. Inorg. Chem. 2005, 44, 8023–8032. [Google Scholar] [CrossRef] [PubMed]
- Dagonassat, M.; Lanone, S.; Boczkowski, J. Interaction of matrix metalloproteinases with pulmonary pollutants. Eur. Respir. J. 2012, 39, 1021–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voicu, S.N.; Balas, M.; Stan, M.S.; Trică, B.; Serban, A.I.; Stanca, L.; Hermenean, A.; Dinischiotu, A. Amorphous Silica Nanoparticles Obtained by Laser Ablation Induce Inflammatory Response in Human Lung Fibroblasts. Materials 2019, 12, 1026. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12071026
Voicu SN, Balas M, Stan MS, Trică B, Serban AI, Stanca L, Hermenean A, Dinischiotu A. Amorphous Silica Nanoparticles Obtained by Laser Ablation Induce Inflammatory Response in Human Lung Fibroblasts. Materials. 2019; 12(7):1026. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12071026
Chicago/Turabian StyleVoicu, Sorina Nicoleta, Mihaela Balas, Miruna Silvia Stan, Bogdan Trică, Andreea Iren Serban, Loredana Stanca, Anca Hermenean, and Anca Dinischiotu. 2019. "Amorphous Silica Nanoparticles Obtained by Laser Ablation Induce Inflammatory Response in Human Lung Fibroblasts" Materials 12, no. 7: 1026. https://0-doi-org.brum.beds.ac.uk/10.3390/ma12071026





