Moving Towards a Finer Way of Light-Cured Resin-Based Restorative Dental Materials: Recent Advances in Photoinitiating Systems Based on Iodonium Salts
Abstract
:1. Introduction
- Short time of monomer/filler compositions curing (up to a few seconds);
- Conducting the reaction at room temperature;
- Low energy consumption;
- Spatial resolution (polymerization only in irradiated areas).
2. Monomers Used for the Production of Dental Composites
2.1. Monomers for Free Radical Photopolymerization Processes in Dental Adhesive Resin Application
2.2. Monomers for Cationic Photopolymerization.
3. Commonly Used Photoinitiating Systems for Dental Application
3.1. Bimolecular Photoinitiator System Containing Camphorquinone and Aromatic Amine
3.2. 1-Phenyl-1,2-propanedione as an Effective Alternative Photoinitiator
3.3. Phosphine Derivatives as Free radical Photoinitiators for in Visible Light Cure Polymerization
3.4. Germanium Derivatives—Extending the Scope of Visible Light Photoinitiators
3.5. Hexaarylbiimidazole Derivatives
3.6. Silane-Based Derivatives
3.7. Thioxanthone Derivatives (TX)
4. Onium Salts as an Innovative Component of Photoinitiating Systems for Photopolymerization Processes in Dental Applications
Two- or Three-Component Photoinitiating Systems Containing Iodonium Salt for Initiating Free Radical Photopolymerization Processes for an Obtained Dental Composites
- Increase conversion in short photo-activation time;
- Reduced inhibitory polymerization effect from an organic solvent;
- Improved dentin bonding performance;
- Improved reactivity and mechanical properties;
- Decreased sorption and water solubility;
- Reduced initial color and improved color stability.
5. Iodonium Salts as Photoinitiators for Cationic and IPN Photopolymerization to Obtain a Dental Composites
6. Challenges of Photoinitiator Systems for Dental Applications, Future Trends and Practical Aspects
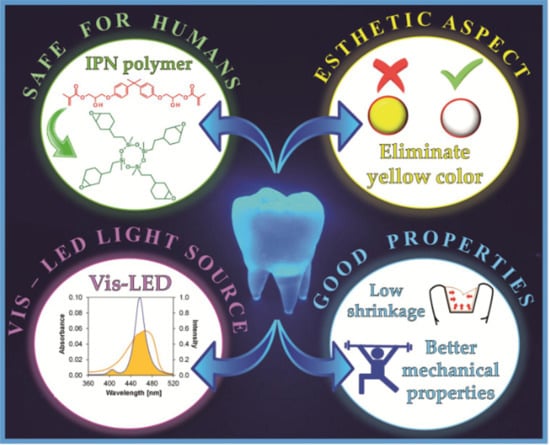
- Are entirely safe for humans, eliminating the cytotoxic amines and acrylate monomers that often cause severe allergies;
- Do not generate yellow color-eliminating camphorquinone, greater aesthetics, and quality of the final product;
- Have better and/or comparable mechanical properties and, due to the use of polymerizable monomers via to the cationic mechanism, have reduced polymerization shrinkage;
- Is possible to be used with dental lamps emitting radiation in the visible light range for the curing process, eliminating harmful UV radiation.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Funke, W. UV Curing: Science and Technology. Prog. Org. Coat. 1980, 8, 110. [Google Scholar] [CrossRef]
- Ligon, S.C.; Husár, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to reduce oxygen inhibition in photoinduced polymerization. Chem. Rev. 2014, 114, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-curing: Technology and applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Ortyl, J.; Popielarz, R. The performance of 7-hydroxycoumarin-3-carbonitrile and 7-hydroxycoumarin-3-carboxylic acid as fluorescent probes for monitoring of cationic photopolymerization processes by FPT. J. Appl. Polym. Sci. 2013, 128, 1974–1978. [Google Scholar] [CrossRef]
- Ortyl, J.; Galek, M.; Milart, P.; Popielarz, R. Aminophthalimide probes for monitoring of cationic photopolymerization by fluorescence probe technology and their effect on the polymerization kinetics. Polym. Test. 2012, 31, 466–473. [Google Scholar] [CrossRef]
- Noè, C.; Malburet, S.; Bouvet-Marchand, A.; Graillot, A.; Loubat, C.; Sangermano, M. Cationic photopolymerization of bio-renewable epoxidized monomers. Prog. Org. Coat. 2019, 133, 131–138. [Google Scholar] [CrossRef]
- Ortyl, J.; Galica, M.; Popielarz, R.; Bogdał, D. Application of a carbazole derivative as a spectroscopic fluorescent probe for real time monitoring of cationic photopolymerization. Polish J. Chem. Technol. 2014, 16, 75–80. [Google Scholar] [CrossRef] [Green Version]
- Ortyl, J.; Topa, M.; Kamińska-Borek, I.; Popielarz, R. Mechanism of interaction of aminocoumarins with reaction medium during cationic photopolymerization of triethylene glycol divinyl ether. Eur. Polym. J. 2019, 116, 45–55. [Google Scholar] [CrossRef]
- Schwalm, R. UV Coatings: Basics, Recent Developments and New Applications, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Schnabel, W. Polymers and Light: Fundamentals and Technical Applications; Wiley VCH: Hoboken, NJ, USA, 2007. [Google Scholar]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Hola, E.; Topa, M.; Chachaj-Brekiesz, A.; Pilch, M.; Fiedor, P.; Galek, M.; Ortyl, J. New, highly versatile bimolecular photoinitiating systems for free-radical, cationic and thiol-ene photopolymerization processes under low light intensity UV and visible LEDs for 3D printing application. RSC Adv. 2020, 10, 7509–7522. [Google Scholar] [CrossRef] [Green Version]
- Weems, A.C.; Delle Chiaie, K.R.; Yee, R.; Dove, A.P. Selective Reactivity of Myrcene for Vat Photopolymerization 3D Printing and Postfabrication Surface Modification. Biomacromolecules 2020, 21, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Noirbent, G.; Brunel, D.; Liu, F.; Gigmes, D.; Sun, K.; Zhang, Y.; Liu, S.; Morlet-Savary, F.; Xiao, P.; et al. Ketone derivatives as photoinitiators for both radical and cationic photopolymerizations under visible LED and application in 3D printing. Eur. Polym. J. 2020, 132, 109737. [Google Scholar] [CrossRef]
- Tang, L.; Nie, J.; Zhu, X. A high performance phenyl-free LED photoinitiator for cationic or hybrid photopolymerization and its application in LED cationic 3D printing. Polym. Chem. 2020, 11, 2855–2863. [Google Scholar] [CrossRef]
- Sivasankar, V.S.; Sachar, H.S.; Sinha, S.; Hines, D.R.; Das, S. 3D Printed Microdroplet Curing: Unravelling the Physics of On-Spot Photopolymerization. ACS Appl. Polym. Mater. 2020, 2, 966–976. [Google Scholar] [CrossRef]
- You, S.; Wang, P.; Schimelman, J.; Hwang, H.H.; Chen, S. High-fidelity 3D printing using flashing photopolymerization. Addit. Manuf. 2019, 30, 100834. [Google Scholar] [CrossRef]
- Lin, J.T.; Cheng, D.C.; Chen, K.T.; Liu, H.W. Dual-wavelength (UV and blue) controlled photopolymerization confinement for 3D-printing: Modeling and analysis of measurements. Polymers 2019, 11, 1819. [Google Scholar] [CrossRef] [Green Version]
- Malas, A.; Isakov, D.; Couling, K.; Gibbons, G.J. Fabrication of high permittivity resin composite for vat photopolymerization 3D printing: Morphology, thermal, dynamic mechanical and dielectric properties. Materials 2019, 12, 3818. [Google Scholar] [CrossRef] [Green Version]
- Sun, K.; Pigot, C.; Chen, H.; Nechab, M.; Gigmes, D.; Morlet-Savary, F.; Graff, B.; Liu, S.; Xiao, P.; Dumur, F.; et al. Free radical photopolymerization and 3D printing using newly developed dyes: Indane-1,3-dione and 1H-cyclopentanaphthalene-1,3-dione derivatives as photoinitiators in three-component systems. Catalysts 2020, 10, 463. [Google Scholar] [CrossRef] [Green Version]
- Sirrine, J.M.; Zlatanic, A.; Meenakshisundaram, V.; Messman, J.M.; Williams, C.B.; Dvornic, P.R.; Long, T.E. 3D printing amorphous polysiloxane terpolymers via vat photopolymerization. Macromol. Chem. Phys. 2019, 220, 1800425. [Google Scholar] [CrossRef]
- Zhao, T.; Yu, R.; Li, X.; Zhang, Y.; Yang, X.; Zhao, X.; Huang, W. A comparative study on 3D printed silicone-epoxy/acrylate hybrid polymers via pure photopolymerization and dual-curing mechanisms. J. Mater. Sci. 2019, 54, 5101–5111. [Google Scholar] [CrossRef]
- Wilts, E.M.; Pekkanen, A.M.; White, B.T.; Meenakshisundaram, V.; Aduba, D.C.; Williams, C.B.; Long, T.E. Vat photopolymerization of charged monomers: 3D printing with supramolecular interactions. Polym. Chem. 2019, 10, 1442–1451. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Aduba, D.C.; Margaretta, E.D.; Marnot, A.E.C.; Heifferon, K.V.; Surbey, W.R.; Chartrain, N.A.; Whittington, A.R.; Long, T.E.; Williams, C.B. Vat photopolymerization 3D printing of acid-cleavable PEG-methacrylate networks for biomaterial applications. Mater. Today Commun. 2019, 19, 204–211. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Liu, C.; Liu, Y.; Zhu, J.; Lao, C. Preparation of high solid loading and low viscosity ceramic slurries for photopolymerization-based 3D printing. Ceram. Int. 2019, 45, 11549–11557. [Google Scholar] [CrossRef]
- Mokbel, H.; Anderson, D.; Plenderleith, R.; Dietlin, C.; Morlet-Savary, F.; Dumur, F.; Gigmes, D.; Fouassier, J.P.; Lalevée, J. Simultaneous initiation of radical and cationic polymerization reactions using the “G1” copper complex as photoredox catalyst: Applications of free radical/cationic hybrid photopolymerization in the composites and 3D printing fields. Prog. Org. Coat. 2019, 132, 50–61. [Google Scholar] [CrossRef]
- Vaut, L.; Zeng, G.; Tosello, G.; Boisen, A. Sacrificial polymer substrates in photopolymerization-based micro 3d printing for fabrication and release of complex micro components. Adv. Mater. Technol. 2019, 4, 1900378. [Google Scholar] [CrossRef] [Green Version]
- Metral, B.; Bischoff, A.; Ley, C.; Ibrahim, A.; Allonas, X. Photochemical study of a three-component photocyclic initiating system for free radical photopolymerization: Implementing a model for digital light processing 3d printing. ChemPhotoChem 2019, 3, 1109–1118. [Google Scholar] [CrossRef]
- Tomal, W.; Pilch, M.; Chachaj-Brekiesz, A.; Ortyl, J. Development of new high-performance biphenyl and terphenyl derivatives as versatile photoredox photoinitiating systems and their applications in 3D printing photopolymerization processes. Catalysts 2019, 9, 827. [Google Scholar] [CrossRef] [Green Version]
- Fiedor, P.; Pilch, M.; Szymaszek, P.; Chachaj-Brekiesz, A.; Galek, M.; Ortyl, J. Photochemical study of a new bimolecular photoinitiating system for vat photopolymerization 3D printing techniques under visible light. Catalysts 2020, 10, 284. [Google Scholar] [CrossRef] [Green Version]
- Corcione, C.E.; Greco, A.; Maffezzoli, A. Photopolymerization kinetics of an epoxy-based resin for stereolithography. J. Appl. Polym. Sci. 2004, 92, 3484–3491. [Google Scholar] [CrossRef]
- Lalevée, J.; Morlet-Savary, F.; Dietlin, C.; Graff, B.; Fouassier, J.P. Photochemistry and radical chemistry under low intensity visible light sources: Application to photopolymerization reactions. Molecules 2014, 19, 15026–15041. [Google Scholar] [CrossRef] [Green Version]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers: Part I: Comparison of the performance of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2017, 64, 313–320. [Google Scholar] [CrossRef]
- Nowak, D.; Ortyl, J.; Kamińska-Borek, I.; Kukuła, K.; Topa, M.; Popielarz, R. Photopolymerization of hybrid monomers, Part II: Determination of relative quantum efficiency of selected photoinitiators in cationic and free-radical polymerization of hybrid monomers. Polym. Test. 2018, 67, 144–150. [Google Scholar] [CrossRef]
- Topa, M.; Petko, F.; Galek, M.; Machowski, K.; Pilch, M.; Szymaszek, P.; Ortyl, J. Applicability of 1,6-Diphenylquinolin-2-one derivatives as fluorescent sensors for monitoring the progress of photopolymerisation processes and as photosensitisers for bimolecular photoinitiating systems. Polymers 2019, 11, 1756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortyl, J.; Milart, P.; Popielarz, R. Applicability of aminophthalimide probes for monitoring and acceleration of cationic photopolymerization of epoxides. Polym. Test. 2013, 32, 708–715. [Google Scholar] [CrossRef]
- Hola, E.; Ortyl, J.; Jankowska, M.; Pilch, M.; Galek, M.; Morlet-Savary, F.; Graff, B.; Dietlin, C.; Lalevée, J. New bimolecular photoinitiating systems based on terphenyl derivatives as highly efficient photosensitizers for 3D printing application. Polym. Chem. 2020, 11, 922–935. [Google Scholar] [CrossRef]
- Ortyl, J.; Wilamowski, J.; Milart, P.; Galek, M.; Popielarz, R. Relative sensitization efficiency of fluorescent probes/sensitizers for monitoring and acceleration of cationic photopolymerization of monomers. Polym. Test. 2015, 48, 151–159. [Google Scholar] [CrossRef]
- Kamińska, I.; Ortyl, J.; Popielarz, R. Applicability of quinolizino-coumarins for monitoring free radical photopolymerization by fluorescence spectroscopy. Polym. Test. 2015, 42, 99–107. [Google Scholar] [CrossRef]
- Kamińska, I.; Ortyl, J.; Popielarz, R. Mechanism of interaction of coumarin-based fluorescent molecular probes with polymerizing medium during free radical polymerization of a monomer. Polym. Test. 2016, 55, 310–317. [Google Scholar] [CrossRef]
- Topa, M.; Ortyl, J.; Chachaj-Brekiesz, A.; Kamińska-Borek, I.; Pilch, M.; Popielarz, R. Applicability of samarium(III) complexes for the role of luminescent molecular sensors for monitoring progress of photopolymerization processes and control of the thickness of polymer coatings. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2018, 199, 430–440. [Google Scholar] [CrossRef]
- Zuo, X.; Morlet-Savary, F.; Schmitt, M.; Le Nouën, D.; Blanchard, N.; Goddard, J.P.; Lalevée, J. Novel applications of fluorescent brighteners in aqueous visible-light photopolymerization: High performance water-based coating and LED-assisted hydrogel synthesis. Polym. Chem. 2018, 9, 3952–3958. [Google Scholar] [CrossRef]
- Staneva, D.; Grabchev, I.; Bosch, P. Fluorescent hydrogel-textile composite material synthesized by photopolymerization. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Xia, B.; Jiang, Z.; Debroy, D.; Li, D.; Oakey, J. Cytocompatible cell encapsulation via hydrogel photopolymerization in microfluidic emulsion droplets. Biomicrofluidics 2017, 11, 044102. [Google Scholar] [CrossRef]
- Larsen, E.K.U.; Larsen, N.B.; Almdal, K.; Larsen, E.K.U.; Larsen, N.B.; Almdal, K. Multimaterial hydrogel with widely tunable elasticity by selective photopolymerization of PEG diacrylate and epoxy monomers. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1195–1201. [Google Scholar] [CrossRef] [Green Version]
- Tseng, S.J.; Chien, C.C.; Liao, Z.X.; Chen, H.H.; Kang, Y.D.; Wang, C.L.; Hwu, Y.; Margaritondo, G. Controlled hydrogel photopolymerization inside live systems by X-ray irradiation. Soft Matter 2012, 8, 1420–1427. [Google Scholar] [CrossRef]
- Young, C.J.; Poole-Warren, L.A.; Martens, P.J. Combining submerged electrospray and UV photopolymerization for production of synthetic hydrogel microspheres for cell encapsulation. Biotechnol. Bioeng. 2012, 109, 1561–1570. [Google Scholar] [CrossRef]
- Patton, J.N.; Palmer, A.F. Photopolymerization of bovine hemoglobin entrapped nanoscale hydrogel particles within liposomal reactors for use as an artificial blood substitute. Biomacromolecules 2005, 6, 414–424. [Google Scholar] [CrossRef]
- Schmocker, A.; Khoushabi, A.; Farahi, S.; Pioletti, D.; Bourban, P.-E.; Manson, J.A.; Moser, C. Multi-scale modeling of photopolymerization for medical hydrogel-implant design. In Biomedical Applications of Light Scattering VII; SPIE: Bellingham, WA, USA, 2013; Volume 8592, p. 85921D. [Google Scholar]
- Zhou, Y.; Yang, D.; Ma, G.; Tan, H.; Jin, Y.; Nie, J. A pH-sensitive water-soluble N-carboxyethyl chitosan/poly(hydroxyethyl methacrylate) hydrogel as a potential drug sustained release matrix prepared by photopolymerization technique. Polym. Adv. Technol. 2008, 19, 1133–1141. [Google Scholar] [CrossRef]
- Lorusso, E.; Ali, W.; Hildebrandt, M.; Mayer-Gall, T.; Gutmann, J.S. Hydrogel functionalized polyester fabrics by UV-induced photopolymerization. Polymers 2019, 11, 1329. [Google Scholar] [CrossRef] [Green Version]
- Ingrosso, C.; Esposito Corcione, C.; Striani, R.; Comparelli, R.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Frigione, M. UV-Curable Nanocomposite Based on Methacrylic-Siloxane Resin and Surface-Modified TiO2 Nanocrystals. ACS Appl. Mater. Interfaces 2015, 7, 15494–15505. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, F.; He, J.; Lassila, L.V.J.; Vallittu, P.K. Synthesis of a novel tertiary amine containing urethane dimethacrylate monomer (UDMTA) and its application in dental resin. J. Mater. Sci. Mater. Med. 2013, 24, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Jia, F.; Xu, H.; Ji, B.; Liu, X. Properties of a new dental photocurable matrix resin with low shrinkage. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2011, 26, 236–241. [Google Scholar] [CrossRef]
- Ahn, K.D.; Han, D.K.; Lee, S.H.; Lee, C.W. New aromatic tert-amines for application as photoinitiator components in photocurable dental materials. Macromol. Chem. Phys. 2003, 204, 1628–1635. [Google Scholar] [CrossRef]
- Park, J.; Ye, Q.; Singh, V.; Kieweg, S.L.; Misra, A.; Spencer, P. Synthesis and evaluation of novel dental monomer with branched aromatic carboxylic acid group. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2012, 100B, 569–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzrati-Zerelli, M.; Maier, M.; Fik, C.P.; Dietlin, C.; Morlet-Savary, F.; Fouassier, J.P.; Klee, J.E.; Lalevée, J. A low migration phosphine to overcome the oxygen inhibition in new high performance photoinitiating systems for photocurable dental type resins. Polym. Int. 2017, 66, 504–511. [Google Scholar] [CrossRef]
- Gatti, A.; Rastelli, A.N.S.; Ribeiro, S.J.L.; Messaddeq, Y.; Bagnato, V.S. Polymerization of photocurable commercial dental methacrylate-based composites: Photocalorimetry study. J. Therm. Anal. Calorim. 2007, 87, 631–634. [Google Scholar] [CrossRef]
- Andreani, L.; Silva, L.L.; Witt, M.A.; Meier, M.M.; Joussef, A.C.; Soldi, V. Development of dental resinous systems composed of bisphenol a ethoxylated dimethacrylate and three novel methacrylate monomers: Synthesis and characterization. J. Appl. Polym. Sci. 2013, 128, 725–734. [Google Scholar] [CrossRef]
- Podgórski, M. Synthesis and characterization of acetyloxypropylene dimethacrylate as a new dental monomer. Dent. Mater. 2011, 27, 748–754. [Google Scholar] [CrossRef]
- Makvandi, P.; Ghaemy, M.; Ghadiri, A.A.; Mohseni, M. Photocurable, antimicrobial quaternary ammonium-modified nanosilica. J. Dent. Res. 2015, 94, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Ghaemy, M.; Mohseni, M. Synthesis and characterization of photo-curable bis-quaternary ammonium dimethacrylate with antimicrobial activity for dental restoration materials. Eur. Polym. J. 2016, 74, 81–90. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, Y.; Wang, X.; Li, Q.; Xiao, Y.; Li, P.; Wang, L.; Ye, Z.; Xing, X. Novel resin-based dental material with anti-biofilm activity and improved mechanical property by incorporating hydrophilic cationic copolymer functionalized nanodiamond. J. Mater. Sci. Mater. Med. 2018, 29, 162. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, Y.; Wang, X.; Chen, Y.; Li, Q.; Xing, X.; Xiao, Y.; Peng, X.; Ye, Z. Development of a novel resin-based dental material with dual biocidal modes and sustained release of Ag+ ions based on photocurable core-shell AgBr/cationic polymer nanocomposites. J. Mater. Sci. Mater. Med. 2017, 28, 103. [Google Scholar] [CrossRef]
- Fu, J.; Liu, W.; Liu, X.; Tuladhar, S.L.; Wan, Q.; Wang, H. Properties of a new dental photocurable resin based on the expanding monomer and three-component photoinitiator system. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2014, 29, 384–390. [Google Scholar] [CrossRef]
- He, J.; Garoushi, S.; Säilynoja, E.; Vallittu, P.K.; Lassila, L. The effect of adding a new monomer “Phene” on the polymerization shrinkage reduction of a dental resin composite. Dent. Mater. 2019, 35, 627–635. [Google Scholar] [CrossRef]
- Zhou, D.; Ito, Y. Visible light-curable polymers for biomedical applications. Sci. China Chem. 2014, 57, 510–521. [Google Scholar] [CrossRef]
- Hsu, S.H.; Chen, R.S.; Chang, Y.L.; Chen, M.H.; Cheng, K.C.; Su, W.F. Biphenyl liquid crystalline epoxy resin as a low-shrinkage resin-based dental restorative nanocomposite. Acta Biomater. 2012, 8, 4151–4161. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, J.; Yang, Y.; Nie, R.; Meng, X. Study on Preparation of Antibacterial Dental Resin Materials. J. Biomater. Tissue Eng. 2019, 8, 1580–1587. [Google Scholar] [CrossRef]
- Rodrigues, M.C.; Xavier, T.A.; Arana-Chavez, V.E.; Braga, R.R. Polymer-based material containing calcium phosphate particles functionalized with a dimethacrylate monomer for use in restorative dentistry. J. Biomater. Appl. 2017, 31, 871–877. [Google Scholar] [CrossRef]
- Rüttermann, S.; Dluzhevskaya, I.; Großsteinbeck, C.; Raab, W.H.M.; Janda, R. Impact of replacing Bis-GMA and TEGDMA by other commercially available monomers on the properties of resin-based composites. Dent. Mater. 2010, 26, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Ortyl, J. Chapter 3: Cationic Photoinitiators. In Photopolymerisation Initiating Systems; Lalevée, J., Fouassier, J.-P., Eds.; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 74–130. [Google Scholar]
- Crivello, J.V. Cationic polymerization—Iodonium and sulfonium salt photoinitiators. In Initiators—Poly-Reactions—Optical Activity; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–48. [Google Scholar]
- Jandt, K.D.; Sigusch, B.W. Future perspectives of resin-based dental materials. Dent. Mater. 2009, 25, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Rawls, H.R. Degree of conversion and color stability of the light curing resin with new photoinitiator systems. Dent. Mater. 2009, 25, 1030–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oxman, J.D.; Jacobs, D.W. Ternary Photoinitiator System for Curing of Epoxy Resns. U.S. Patent 6,043,295, 28 July 1999. [Google Scholar]
- Rabek, J.F. Photosensitized Processes in Polymer Chemistry: A Review. Photochem. Photobiol. 1968, 7, 5–57. [Google Scholar] [CrossRef]
- Marghalani, H.Y. Handbook of Bioceramics and Biocomposites, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9783319092300. [Google Scholar]
- Linden, L.A. Photocuring of polymericdental materials, plastics compositeresins. In Radiationcuring in Polymer Science and Technology; Fouassier, J.P., Rabek, J.F., Eds.; Elsevier Applied Science Publisher: Amsterdam, The Netherlands, 1993; Volume 4, pp. 387–466. [Google Scholar]
- Moszner, N.; Salz, U. New developments of polymeric dental composites. Prog. Polym. Sci. 2001, 26, 535–576. [Google Scholar] [CrossRef]
- Fouassier, J.P.; Rabek, J.F. Radiation Curing in Polymer Science and Technology: Practical Aspects and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Xu, X.; He, L.; Zhu, B.; Li, J.; Li, J. Advances in polymeric materials for dental applications. Polym. Chem. 2017, 8, 807–823. [Google Scholar] [CrossRef]
- Lovell, L.G.; Lu, H.; Elliott, J.E.; Stansbury, J.W.; Bowman, C.N. The effect of cure rate on the mechanical properties of dental resins. Dent. Mater. 2001, 17, 504–511. [Google Scholar] [CrossRef]
- Stansbury, J.W. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Zandinejad, A.A.; Atai, M.; Pahlevan, A. The effect of ceramic and porous fillers on the mechanical properties of experimental dental composites. Dent. Mater. 2006, 22, 382–387. [Google Scholar] [CrossRef]
- Lee, J.H.; Um, C.M.; Lee, I. Rheological properties of resin composites according to variations in monomer and filler composition. Dent. Mater. 2006, 22, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Leprince, J.G.; Palin, W.M.; Hadis, M.A.; Devaux, J.; Leloup, G. Progress in dimethacrylate-based dental composite technology and curing efficiency. Dent. Mater. 2013, 29, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Buruiana, T.; Melinte, V.; Stroea, L.; Buruiana, E.C. Urethane dimethacrylates with carboxylic groups as potential dental monomers. Synthesis and properties. Polym. J. 2009, 41, 978–987. [Google Scholar] [CrossRef]
- Nguyen, J.F.; Migonney, V.; Ruse, N.D.; Sadoun, M. Properties of experimental urethane dimethacrylate-based dental resin composite blocks obtained via thermo-polymerization under high pressure. Dent. Mater. 2013, 29, 535–541. [Google Scholar] [CrossRef]
- Polydorou, O.; König, A.; Hellwig, E.; Kümmerer, K. Uthethane dimethacrylate: A molecule that may cause confusion in dental research. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 1–4. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Charton, C.; Falk, V.; Marchal, P.; Pla, F.; Colon, P. Influence of Tg, viscosity and chemical structure of monomers on shrinkage stress in light-cured dimethacrylate-based dental resins. Dent. Mater. 2007, 23, 1447–1459. [Google Scholar] [CrossRef]
- Ellakwa, A.; Cho, N.; Lee, I.B. The effect of resin matrix composition on the polymerization shrinkage and rheological properties of experimental dental composites. Dent. Mater. 2007, 23, 1229–1235. [Google Scholar] [CrossRef]
- Gonçalves, F.; Pfeifer, C.S.; Ferracane, J.L.; Braga, R.R. Contraction stress determinants in dimethacrylate composites. J. Dent. Res. 2008, 87, 367–371. [Google Scholar] [CrossRef]
- Goņalves, F.; Kawano, Y.; Pfeifer, C.; Stansbury, J.W.; Braga, R.R. Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur. J. Oral Sci. 2009, 117, 442–446. [Google Scholar] [CrossRef]
- Pfeifer, C.S.; Silva, L.R.; Kawano, Y.; Braga, R.R. Bis-GMA co-polymerizations: Influence on conversion, flexural properties, fracture toughness and susceptibility to ethanol degradation of experimental composites. Dent. Mater. 2009, 25, 1136–1141. [Google Scholar] [CrossRef]
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent advances and developments in composite dental restorative materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, R.B.; Rizkalla, A.S.; Hall, G.C. Effect of stepped light exposure on the volumetric polymerization shrinkage and bulk modulus of dental composites and an unfilled resin. Am. J. Dent. 2000, 13, 176–180. [Google Scholar] [PubMed]
- Sheth, J.J.; Fuller, J.L.; Jensen, M.E. Cuspal deformation and fracture resistance of teeth with dentin adhesives and composites. J. Prosthet. Dent. 1988, 60, 560–569. [Google Scholar] [CrossRef]
- Schoerpf, S.; Catel, Y.; Moszner, N.; Gorsche, C.; Liska, R. Enhanced reduction of polymerization-induced shrinkage stress: Via combination of radical ring opening and addition fragmentation chain transfer. Polym. Chem. 2019, 10, 1357–1366. [Google Scholar] [CrossRef] [Green Version]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef]
- Vitale, A.; Sangermano, M.; Bongiovanni, R.; Burtscher, P.; Moszner, N. Visible light curable restorative composites for dental applications based on epoxy monomer. Materials 2014, 7, 554–562. [Google Scholar] [CrossRef] [Green Version]
- Oxman, J.D.; Jacobs, D.W. Ternary Photoinitiator System for Curing of Epoxy Resins. WO 1998047046 A1, 22 October 1998. [Google Scholar]
- Hamano, N.; Chiang, Y.C.; Nyamaa, I.; Yamaguchi, H.; Ino, S.; Hickel, R.; Kunzelmann, K.H. Repair of silorane-based dental composites: Influence of surface treatments. Dent. Mater. 2012, 28, 894–902. [Google Scholar] [CrossRef]
- Lavigueur, C.; Zhu, X.X. Recent advances in the development of dental composite resins. RSC Adv. 2012, 2, 59–63. [Google Scholar] [CrossRef]
- Weinmann, W.; Thalacker, C.; Guggenberger, R. Siloranes in dental composites. Dent. Mater. 2005, 21, 68–74. [Google Scholar] [CrossRef]
- Nuyken, O.; Böhner, R.; Erdmann, C. Oxetane photopolymerization—A system with low volume shrinkage. Macromol. Symp. 1996, 107, 125–138. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U. Composites for Dental Restoratives. In Polymers for Dental and Orthopedic Application, 1st ed.; Salz, U., Shalaby, W.S., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 13–68. [Google Scholar]
- Bailey, W.J. Polycyclic Ring-Opened Polymers. U.S. Patent 4,387,215, 15 September 1980. [Google Scholar]
- Stansbury, J.W.; Bailey, W.J. Evaluation of spiro orthocarbonate monomers capable of polymerization with expansion as ingredients in dental composite materials. In Progress in Biomedical Polymers; Gebelein, C.G., Dunn, R.L., Eds.; Springer: Boston, MA, USA, 1988; pp. 402–406. [Google Scholar]
- Eick, J.D.; Kotha, S.P.; Chappelow, C.C.; Kilway, K.V.; Giese, G.J.; Glaros, A.G.; Pinzino, C.S. Properties of silorane-based dental resins and composites containing a stress-reducing monomer. Dent. Mater. 2007, 23, 1011–1017. [Google Scholar] [CrossRef]
- Schneider, L.F.J.; Cavalcante, L.M.; Silikas, N. Shrinkage stresses generated during resin-composite applications: A review. J. Dent. Biomech. 2010, 2010, 131630. [Google Scholar] [CrossRef] [PubMed]
- Eick, J.D.; Kostoryz, E.L.; Rozzi, S.M.; Jacobs, D.W.; Oxman, J.D.; Chappelow, C.C.; Glaros, A.G.; Yourtee, D.M. In vitro biocompatibility of oxirane/polyol dental composites with promising physical properties. Dent. Mater. 2002, 18, 413–421. [Google Scholar] [CrossRef]
- Nie, J.; Andrzejewska, E.; Rabek, J.F.; Lindén, L.Å.; Fouassier, J.P.; Paczkowski, J.; Scigalski, F.; Wrzyszczynski, A. Effect of peroxides and hydroperoxides on the camphorquinoneinitiated photopolymerization. Macromol. Chem. Phys. 1999, 200, 1692–1701. [Google Scholar] [CrossRef]
- Neumann, M.G.; Miranda, W.G.; Schmitt, C.C.; Rueggeberg, F.A.; Correa, I.C. Molar extinction coefficients and the photon absorption efficiency of dental photoinitiators and light curing units. J. Dent. 2005, 33, 525–532. [Google Scholar] [CrossRef]
- Li, M.; Mondrinos, M.J.; Chen, X.; Gandhi, M.R.; Ko, F.K.; Lelkes, P.I. Elastin Blends for Tissue Engineering Scaffolds. J. Biomed. Mater. Res. Part A 2006, 79, 963–973. [Google Scholar] [CrossRef]
- Wei, J.; Lu, R.; Liu, F. Novel, highly efficient polymeric benzophenone photoinitiator containing coinitiator moieties for photopolymerization. Polym. Adv. Technol. 2010, 21, 656–662. [Google Scholar] [CrossRef]
- Cook, W.D. Photopolymerization kinetics of dimethacrylates using the camphorquinone/amine initiator system. Polymer 1992, 33, 600–609. [Google Scholar] [CrossRef]
- Jakubiak, J.; Allonas, X.; Fouassier, J.P.; Sionkowska, A.; Andrzejewska, E.; Linden, L.Å.; Rabek, J.F. Camphorquinone-amines photoinitating systems for the initiation of free radical polymerization. Polymer 2003, 44, 5219–5226. [Google Scholar] [CrossRef]
- Bi, Y.; Neckers, D.C. A Visible Light Initiating System for Free Radical Promoted Cationic Polymerization. Macromolecules 1994, 27, 3683–3693. [Google Scholar] [CrossRef]
- Nie, J.; Bowman, C.N. Synthesis and photopolymerization of N,N′-dimethyl,-N,N′-di(methacryloxy ethyl)-1,6-hexanediamine as a polymerizable amine coinitiator for dental restorations. Biomaterials 2002, 23, 1221–1226. [Google Scholar] [CrossRef]
- Guo, X.; Peng, Z.; Spencer, P.; Wang, Y. Effect of initiator on photopolymerization of acidic, aqueous dental model adhesives. J. Biomed. Mater. Res. Part A 2009, 90, 1120–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, J.; Liu, F. Novel highly efficient macrophotoinitiator comprising benzophenone, coinitiator amine, and thio moieties for photopolymerization. Macromolecules 2009, 42, 5486–5491. [Google Scholar] [CrossRef]
- Decker, C.; Jenkins, A.D. Kinetic Approach of o2 Inhibition in Ultraviolet and Laser-Induced Polymerizations. Macromolecules 1985, 18, 1241–1244. [Google Scholar] [CrossRef]
- Lin, Y.; Stansbury, J.W. Kinetics studies of hybrid structure formation by controlled photopolymerization. Polymer 2003, 44, 4781–4789. [Google Scholar] [CrossRef]
- Ogliari, F.A.; Ely, C.; Petzhold, C.L.; Demarco, F.F.; Piva, E. Onium salt improves the polymerization kinetics in an experimental dental adhesive resin. J. Dent. 2007, 35, 583–587. [Google Scholar] [CrossRef]
- Brömme, T.; Schmitz, C.; Moszner, N.; Burtscher, P.; Strehmel, N.; Strehmel, B. Photochemical Oxidation of NIR Photosensitizers in the Presence of Radical Initiators and Their Prospective Use in Dental Applications. ChemistrySelect 2016, 1, 524–532. [Google Scholar] [CrossRef]
- Münchow, E.A.; Valente, L.L.; Peralta, S.L.; Fernández, M.R.; Lima, G.D.S.; Petzhold, C.L.; Piva, E.; Ogliari, F.A. 1,3-Diethyl-2-thiobarbituric acid as an alternative coinitiator for acidic photopolymerizable dental materials. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2013, 101, 1217–1221. [Google Scholar] [CrossRef]
- Bowen, R.L.; Cobb, E.N.; Rapson, J.E. Adhesive Bonding of Various Materials to Hard Tooth Tissues: Improvement in Bond Strength to Dentin. J. Dent. Res. 1982, 61, 1070–1076. [Google Scholar] [CrossRef]
- Ikemura, K.; Endo, T. A review of our development of dental adhesives—Effects of radical polymerization initiators and adhesive monomers on adhesion. Dent. Mater. J. 2010, 29, 109–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali Tehfe, M.; El-Roz, M.; Lalevée, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.P. Bifunctional co-initiators: A new strategy for the design of efficient systems in radical photopolymerization reactions under air. Eur. Polym. J. 2012, 48, 956–962. [Google Scholar] [CrossRef]
- Asmussen, S.; Vallo, C. Light absorbing products during polymerization of methacrylate monomers photoinitiated with phenyl-1,2-propanedione/amine. J. Photochem. Photobiol. A Chem. 2009, 202, 228–234. [Google Scholar] [CrossRef]
- Dart, E.C.; Cantwell, J.B.; Traynor, J.R.; Jaworzyn, J.F.; Nemeck, J. Method of Repairing Teeth Usinga Compostion Which Is Curable by Rradation with Wsble Light. U.S. Patent 4,089,763, 17 March 1976. [Google Scholar]
- Dressano, D.; Palialol, A.R.; Xavier, T.A.; Braga, R.R.; Oxman, J.D.; Watts, D.C.; Marchi, G.M.; Lima, A.F. Effect of diphenyliodonium hexafluorophosphate on the physical and chemical properties of ethanolic solvated resins containing camphorquinone and 1-phenyl-1,2-propanedione sensitizers as initiators. Dent. Mater. 2016, 32, 756–764. [Google Scholar] [CrossRef]
- Sun, G.J.; Chae, K.H. Properties of 2,3-butanedione and 1-phenyl-1,2-propanedione as new photosensitizers for visible light cured dental resin composites. Polymer 2000, 41, 6205–6212. [Google Scholar] [CrossRef]
- Brandt, W.C.; Silva, C.G.; Frollini, E.; Souza-Junior, E.J.C.; Sinhoreti, M.A.C. Dynamic mechanical thermal analysis of composite resins with CQ and PPD as photo-initiators photoactivated by QTH and LED units. J. Mech. Behav. Biomed. Mater. 2013, 24, 21–29. [Google Scholar] [CrossRef]
- Kunio, I.; Takeshi, E. A review of the development of radical photopolymerization initiators used for designing light-curing dental adhesives and resin composites. Dent. Mater. J. 2010, 29, 481–501. [Google Scholar] [CrossRef] [Green Version]
- Van Landuyt, K.L.; Snauwaert, J.; De Munck, J.; Peumans, M.; Yoshida, Y.; Poitevin, A.; Coutinho, E.; Suzuki, K.; Lambrechts, P.; Van Meerbeek, B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials 2007, 28, 3757–3785. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.; Wang, Y. Photo-polymerization efficiency of self-etch dental adhesives composed of camphorquinone or trimethylbenzoyl-diphenyl-phosphine oxide. Int. J. Adhes. Adhes. 2013, 45, 53–58. [Google Scholar] [CrossRef]
- Tomal, W.; Ortyl, J. Water-soluble photoinitiators in biomedical applications. Polymers 2020, 12, 1073. [Google Scholar] [CrossRef]
- Albuquerque, P.P.A.C.; Moreira, A.D.L.; Moraes, R.R.; Cavalcante, L.M.; Schneider, L.F.J. Color stability, conversion, water sorption and solubility of dental composites formulated with different photoinitiator systems. J. Dent. 2013, 41, e67–e72. [Google Scholar] [CrossRef] [PubMed]
- Moszner, N.; Salz, U.; Zimmermann, J. Chemical aspects of self-etching enamel-dentin adhesives: A systematic review. Dent. Mater. 2005, 21, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Meereis, C.T.W.; Leal, F.B.; Lima, G.S.; De Carvalho, R.V.; Piva, E.; Ogliari, F.A. BAPO as an alternative photoinitiator for the radical polymerization of dental resins. Dent. Mater. 2014, 30, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Popal, M.; Volk, J.; Leyhausen, G.; Geurtsen, W. Cytotoxic and genotoxic potential of the type I photoinitiators BAPO and TPO on human oral keratinocytes and V79 fibroblasts. Dent. Mater. 2018, 34, 1783–1796. [Google Scholar] [CrossRef] [PubMed]
- Ganster, B.; Fischer, U.K.; Moszner, N.; Liska, R. New photocleavable structures, 4a acylgermane-based photoinitiator for visible light curing. Macromol. Rapid Commun. 2008, 29, 57–62. [Google Scholar] [CrossRef]
- Ganster, B.; Fischer, U.K.; Moszner, N.; Liska, R. New photocleavable structures. Diacylgermane-based photoinitiators for visible light curing. Macromolecules 2008, 41, 2394–2400. [Google Scholar] [CrossRef]
- Ely, C.; Ottoboni, T.D.; Kumagai, R.Y.; de Souza, N.A.; da Ramos, T.S.; Arrais, C.A.G.; Piva, E.; Reis, A.F. Bond Strength of Methacrylate-based Blends Containing Elastomeric Monomers and Alternative Initiators after Thermomechanical Cycling. J. Adhes. Dent. 2019, 21, 281–286. [Google Scholar] [CrossRef]
- Moszner, N.; Fischer, U.K.; Ganster, B.; Liska, R.; Rheinberger, V. Benzoyl germanium derivatives as novel visible light photoinitiators for dental materials. Dent. Mater. 2008, 24, 901–907. [Google Scholar] [CrossRef]
- Hayashi, T.; Maeda, K. Preparation of a New Phototropic Substance. Bull. Chem. Soc. Jpn. 1960, 33, 565–566. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Chen, Y.C. Triphenylamine-hexaarylbiimidazole derivatives as hydrogen-acceptor photoinitiators for free radical photopolymerization under UV and LED light. Polym. Chem. 2020, 11, 1504–1513. [Google Scholar] [CrossRef]
- Berdzinski, S.; Strehmel, N.; Lindauer, H.; Strehmel, V.; Strehmel, B. Extended mechanistic aspects on photoinitiated polymerization of 1,6-hexanediol diacrylate by hexaarylbisimidazoles and heterocyclic mercapto compounds. Photochem. Photobiol. Sci. 2014, 13, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Berdzinski, S.; Horst, J.; Straßburg, P.; Strehmel, V. Recombination of lophyl radicals in pyrrolidinium-based ionic liquids. ChemPhysChem 2013, 14, 1899–1908. [Google Scholar] [CrossRef]
- Kawano, M.; Sano, T.; Abe, J.; Ohashi, Y. The first in situ direct observation of the light-induced radical pair from a hexaarylbiimidazolyl derivative by X-ray crystallography. J. Am. Chem. Soc. 1999, 121, 8106–8107. [Google Scholar] [CrossRef]
- Ahn, D.; Sathe, S.S.; Clarkson, B.H.; Scott, T.F. Hexaarylbiimidazoles as visible light thiol-ene photoinitiators. Dent. Mater. 2015, 31, 1075–1089. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Kuo, Y.T.; Ho, T.H. Photo-polymerization properties of type-II photoinitiator systems based on 2-chlorohexaaryl biimidazole (o-Cl-HABI) and various N-phenylglycine (NPG) derivatives. Photochem. Photobiol. Sci. 2019, 18, 190–197. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Liu, J.; He, S.; Zhang, L.; Polymers, F. Improved Crack Growth Resistance and Its Molecular Origin of Natural Rubber/Carbon Black by Nanodispersed Clay. Polym. Eng. Sci. 2012, 52, 1027–1036. [Google Scholar] [CrossRef]
- Shi, Y.; Yin, J.; Kaji, M.; Yori, H. Photopolymerization of acrylate derivatives initiated by hexaarylbiimidazole with ether groups. Polym. Int. 2006, 55, 330–339. [Google Scholar] [CrossRef]
- West, R. The polysilane high polymers. J. Organomet. Chem. 1986, 300, 327–346. [Google Scholar] [CrossRef]
- Wolff, A.R.; West, R. Photoinitiation of vinyl polymerization by polysilanes. Appl. Organomet. Chem. 1987, 1, 7–14. [Google Scholar] [CrossRef]
- Yagci, Y.; Kminek, I.; Schnabel, W. Long wavelength photoinitiated cationic polymerization using diphenyliodonium salt and catena-poly (phenyl-4-phenylphenylsilicon). Polymer 1993, 34, 426–428. [Google Scholar] [CrossRef]
- Gilman, H.; Atwell, W.H.; Sen, P.K.; Smith, C.L. Branched-chain organic polysilanes containing the silicon-hydrogen group. J. Organomet. Chem. 1965, 4, 163–167. [Google Scholar] [CrossRef]
- Kanabus-Kaminska, J.M.; Hawari, J.A.; Griller, D.; Chatgilialoglu, C. Reduction of Silicon-Hydrogen Bond Strengths. J. Am. Chem. Soc. 1987, 109, 5267–5268. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Griller, D.; Lesage, M. Tris(trimethylsilyl)silane. A new reducing agent. J. Org. Chem. 1988, 55, 3641–3642. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Ei-Roz, M.; Graff, B.; Allonas, X.; Fouassier, J.P. New photoinitiators based on the silyl radical chemistry: Polymerization ability, ESR spin trapping, and laser flash photolysis investigation. Macromolecules 2008, 41, 4180–4186. [Google Scholar] [CrossRef]
- Lalevée, J.; Dirani, A.; El-Roz, M.; Allonas, X.; Fouassier, J.P. Silanes as new highly efficient co-initiators for radical polymerization in aerated media. Macromolecules 2008, 41, 2003–2010. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Chany, A.C.; El-Roz, M.; Souane, R.; Graff, B.; Allonas, X.; Fouassier, J.P. Silyl radical chemistry and conventional photoinitiators: A route for the design of efficient systems. Macromolecules 2009, 42, 6031–6037. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Lalevée, J. Recent applications of the (TMS)3SiH radical-based reagent. Molecules 2012, 17, 527–555. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Stability and Utility of Pyridyl Disulfide Functionality in RAFT and Conventional Radical Polymerizations. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7207–7224. [Google Scholar] [CrossRef]
- Lalevée, J.; Blanchard, N.; Graff, B.; Allonas, X.; Fouassier, J.P. Tris(trimethylsilyl)silyl versus tris(trimethylsilyl)germyl: Radical reactivity and oxidation ability. J. Organomet. Chem. 2008, 693, 3643–3649. [Google Scholar] [CrossRef]
- Lalevée, J.; Allonas, X.; Fouassier, J.P. Tris(trimethylsilyl)silane (TTMSS)-derived radical reactivity toward alkenes: A combined quantum mechanical and laser flash photolysis study. J. Org. Chem. 2007, 72, 6434–6439. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Allonas, X.; Fouassier, J.P. Radical photopolymerization reactions under air upon lamp and diode laser exposure: The input of the organosilane radical chemistry. Prog. Org. Coatings 2011, 70, 83–90. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Allonas, X.; Fouassier, J.P. Oxygen mediated and wavelength tunable cationic photopolymerization reactions under air and low intensity: A new concept. Prog. Org. Coat. 2011, 70, 23–31. [Google Scholar] [CrossRef]
- Chatgilialoglu, C. Organosilanes as Radical-Based Reducing Agents in Synthesis. Acc. Chem. Res. 1992, 25, 188–194. [Google Scholar] [CrossRef]
- Bouzrati-Zerelli, M.; Kirschner, J.; Fik, C.P.; Maier, M.; Dietlin, C.; Morlet-Savary, F.; Fouassier, J.P.; Becht, J.M.; Klee, J.E.; Lalevée, J. Silyl Glyoxylates as a New Class of High Performance Photoinitiators: Blue LED Induced Polymerization of Methacrylates in Thin and Thick Films. Macromolecules 2017, 50, 6911–6923. [Google Scholar] [CrossRef]
- Kirschner, J.; Bouzrati-Zerelli, M.; Fouassier, J.P.; Becht, J.M.; Klee, J.E.; Lalevée, J. Silyl glyoxylates as high-performance photoinitiators for cationic and hybrid polymerizations: Towards better polymer mechanical properties. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1420–1429. [Google Scholar] [CrossRef]
- Kirschner, J.; Baralle, A.; Graff, B.; Becht, J.M.; Klee, J.E.; Lalevée, J. 1-Aryl-2-(triisopropylsilyl)ethane-1,2-diones: Toward a New Class of Visible Type I Photoinitiators for Free Radical Polymerization of Methacrylates. Macromol. Rapid Commun. 2019, 40, 2–7. [Google Scholar] [CrossRef]
- Kirschner, J.; Paillard, J.; Graff, B.; Becht, J.M.; Klee, J.E.; Lalevée, J. 2-Oxo-2(tert-butyldimethylsilyl)Acetic Acid (DKSi-COOH) as a New Water-Soluble Visible Light Type I Photoinitiator for Free Radical Polymerization. Macromol. Chem. Phys. 2020, 221, 5–9. [Google Scholar] [CrossRef]
- Hayakawa, T.; Horie, K. Effect of water-soluble photoinitiator on the adhesion between composite and tooth substrate. Dent. Mater. 1992, 8, 351–353. [Google Scholar] [CrossRef]
- Breloy, L.; Losantos, R.; Sampedro, D.; Marazzi, M.; Malval, J.-P.; Heo, Y.; Akimoto, J.; Ito, Y.; Brezová, V.; Versace, D.-L. Allyl amino-thioxanthone derivatives as highly efficient visible light H-donors and co-polymerizable photoinitiators. Polym. Chem. 2020, 11, 4297–4312. [Google Scholar] [CrossRef]
- Ely, C.; Schneider, L.F.J.; Ogliari, F.A.; Schmitt, C.C.; Corrêa, I.C.; Lima, G.D.S.; Samuel, S.M.W.; Piva, E. Polymerization kinetics and reactivity of alternative initiators systems for use in light-activated dental resins. Dent. Mater. 2012, 28, 1199–1206. [Google Scholar] [CrossRef]
- Kabatc, J.; Ortyl, J.; Kostrzewska, K. New kinetic and mechanistic aspects of photosensitization of iodonium salts in photopolymerization of acrylates. RSC Adv. 2017, 7, 41619–41629. [Google Scholar] [CrossRef] [Green Version]
- Baralle, A.; Garra, P.; Morlet-Savary, F.; Dietlin, C.; Fouassier, J.P.; Lalevée, J. Polymeric Iodonium Salts to Trigger Free Radical Photopolymerization. Macromol. Rapid Commun. 2020, 41. [Google Scholar] [CrossRef] [PubMed]
- Abedin, F.; Ye, Q.; Song, L.; Ge, X.; Camarda, K.; Spencer, P. Effect of Partition of Photo-Initiator Components and Addition of Iodonium Salt on the Photopolymerization of Phase-Separated Dental Adhesive. JOM 2016, 68, 1090–1099. [Google Scholar] [CrossRef]
- Shiraishi, A.; Ueda, Y.; Schläpfer, M.; Schmitz, C.; Brömme, T.; Oprych, D.; Strehmel, B. Nir-sensitized photopolymerization with iodonium salts bearing weak coordinating anions. J. Photopolym. Sci. Technol. 2016, 29, 609–615. [Google Scholar] [CrossRef] [Green Version]
- Cook, W.D.; Chen, F. Enhanced visible radiation photopolymerization of dimethacrylates with the three component thioxanthone (CPTXO)-amine-iodonium salt system. Polym. Chem. 2015, 6, 1325–1338. [Google Scholar] [CrossRef]
- Podsiadły, R.; Maruszewska, A.; Michalski, R.; Marcinek, A.; Kolińska, J. Naphthoylenebenzimidazolone dyes as electron transfer photosensitizers for iodonium salt induced cationic photopolymerizations. Dye. Pigment. 2012, 95, 252–259. [Google Scholar] [CrossRef]
- Brömme, T.; Oprych, D.; Horst, J.; Pinto, P.S.; Strehmel, B. New iodonium salts in NIR sensitized radical photopolymerization of multifunctional monomers. RSC Adv. 2015, 5, 69915–69924. [Google Scholar] [CrossRef]
- Asmussen, S.; Arenas, G.; Vallo, C. Photopolymerization of pyrrole/methacrylate mixtures using α-cleavage type photoinitiators in combination with iodonium salt. Synth. Met. 2015, 209, 304–312. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lam, J.H.W. Diaryliodonium Salts. A New Class of Photoinitiators for Cationic Polymerization. Macromolecules 1977, 10, 1307–1315. [Google Scholar] [CrossRef]
- Topa, M.; Petko, F.; Galek, M.; Ortyl, J. Double role of diphenylpyridine derivatives as fluorescent sensors for monitoring photopolymerization and the determination of the efficiencies of the generation of superacids by cationic photoinitiators. Sensors 2020, 20, 3043. [Google Scholar] [CrossRef]
- Topa, M.; Hola, E.; Galek, M.; Petko, F.; Pilch, M.; Popielarz, R.; Morlet-Savary, F.B.G.; Lalevée, J.; Ortyl, J. One-component cationic photoinitiators based on coumarin scaffold iodonium salts as highly sensitive photoacid generators for 3D printing IPN photopolymers under visible LED sources. Polym. Chem. 2020, 11, 5261–5278. [Google Scholar] [CrossRef]
- Green, W.A. Industrial Photoinitiators, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Dadashi-Silab, S.; Aydogan, C.; Yagci, Y. Shining a light on an adaptable photoinitiator: Advances in photopolymerizations initiated by thioxanthones. Polym. Chem. 2015, 6, 6595–6615. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Dietlin, C.; Graff, B.; Morlet-Savary, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.P.; Chachaj-Brekiesz, A.; Ortyl, J.; Lalevée, J. Meta-Terphenyl Derivative/Iodonium Salt/9H-Carbazole-9-ethanol Photoinitiating Systems for Free Radical Promoted Cationic Polymerization upon Visible Lights. Macromol. Chem. Phys. 2016, 217, 1955–1965. [Google Scholar] [CrossRef]
- Karaca, N.; Ocal, N.; Arsu, N.; Jockusch, S. Thioxanthone-benzothiophenes as photoinitiator for free radical polymerization. J. Photochem. Photobiol. A Chem. 2016, 331, 22–28. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application. Arab. J. Chem. 2016, 9, 745–754. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.F.; Salvador, M.V.O.; Dressano, D.; Saraceni, C.H.C.; Gonçalves, L.S.; Hadis, M.; Palin, W.M. Increased rates of photopolymerisation by ternary type II photoinitiator systems in dental resins. J. Mech. Behav. Biomed. Mater. 2019, 98, 71–78. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Spencer, P.; Ye, Q.; Yao, X. Effects of water content and initiator composition on photopolymerization of a model BisGMA/HEMA resin. Dent. Mater. 2008, 24, 824–831. [Google Scholar] [CrossRef] [Green Version]
- Dressano, D.; Salvador, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875. [Google Scholar] [CrossRef]
- Baena Lopes, M.; Tirado Dos Santos, A.M.; Coelho, D.; Gonini Júnior, A.; Aulo Ogliari, F.; Ratto De Moraes, R. Influence of diphenyliodonium hexafluorophosphate on the bond strength and mechanical properties of model resin cements. Int. J. Adhes. Adhes. 2013, 47, 125–128. [Google Scholar] [CrossRef]
- Leal, F.B.; Lima, G.S.; Collares, F.M.; Samuel, S.M.; Petzhold, C.L.; Piva, E.; Ogliari, F.A. Iodonium salt improves the dentin bonding performance in an experimental dental adhesive resin. Int. J. Adhes. Adhes. 2012, 38, 1–4. [Google Scholar] [CrossRef]
- Augusto, C.R.; Leitune, V.C.B.; Ogliari, F.A.; Collares, F.M. Influence of an iodonium salt on the properties of dual-polymerizing self-adhesive resin cements. J. Prosthet. Dent. 2017, 118, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Chloride, D.; Padon, K.S.; Scranton, A.B. A mechanistic investigation of the three-component radical photoinitiator system Eosin Y spirit soluble, n-methyldiethanolamine. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 715–723. [Google Scholar] [CrossRef]
- Sauro, S.; Vijay, S.; Deb, S. Development and assessment of experimental dental polymers with enhanced polymerisation, crosslink density and resistance to fluid permeability based on ethoxylated-Bisphenol-A-dimethacrylates and 2-Hydroxyethyl methacrylate. Eur. Polym. J. 2012, 48, 1466–1474. [Google Scholar] [CrossRef]
- Bouzrati-Zerelli, M.; Maier, M.; Dietlin, C.; Fabrice, M.S.; Fouassier, J.P.; Klee, J.E.; Lalevée, J. A novel photoinitiating system producing germyl radicals for the polymerization of representative methacrylate resins: Camphorquinone/R3GeH/iodonium salt. Dent. Mater. 2016, 32, 1226–1234. [Google Scholar] [CrossRef]
- Kirschner, J.; Szillat, F.; Bouzrati-Zerelli, M.; Becht, J.M.; Klee, J.E.; Lalevée, J. Iodonium sulfonates as high-performance coinitiators and additives for CQ-based systems: Toward aromatic amine-free photoinitiating systems. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1664–1669. [Google Scholar] [CrossRef]
- Kirschner, J.; Paillard, J.; Bouzrati-Zerelli, M.; Becht, J.M.; Klee, J.E.; Chelli, S.; Lakhdar, S.; Lalevée, J. Aryliodonium ylides as novel and efficient additives for radical chemistry: Example in Camphorquinone (CQ)/Amine based photoinitiating systems. Molecules 2019, 24, 2913. [Google Scholar] [CrossRef] [Green Version]
- Kirschner, J.; Szillat, F.; Bouzrati-Zerelli, M.; Becht, J.M.; Klee, J.E.; Lalevée, J. Sulfinates and sulfonates as high performance co-initiators in CQ based systems: Towards aromatic amine-free systems for dental restorative materials. Dent. Mater. 2020, 36, 187–196. [Google Scholar] [CrossRef]
- Kirschner, J.; Becht, J.M.; Klee, J.E.; Lalevée, J. A New Phosphine for Efficient Free Radical Polymerization under Air. Macromol. Rapid Commun. 2020, 41, 2000053. [Google Scholar] [CrossRef]
- Ogliari, F.A.; Ely, C.; Lima, G.S.; Conde, M.C.M.; Petzhold, C.L.; Demarco, F.F.; Piva, E. Onium salt reduces the inhibitory polymerization effect from an organic solvent in a model dental adhesive resin. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2008, 86, 113–118. [Google Scholar] [CrossRef]
- Borges, B.C.D.; de Sousa-Lima, R.X.; Moreno, G.B.P.; Moreira, D.G.L.; Oliveira, D.C.R.S.; Souza-Junior, E.J.C.; Sinhoreti, M.A.C. Polymerization and adhesion behavior of experimental dental bonding materials with different initiator systems. J. Adhes. Sci. Technol. 2018, 32, 239–246. [Google Scholar] [CrossRef]
- Gonçalves, L.S.; Moraes, R.R.; Ogliari, F.A.; Boaro, L.; Braga, R.R.; Consani, S. Improved polymerization efficiency of methacrylate-based cements containing an iodonium salt. Dent. Mater. 2013, 29, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.S. Photoinitiators for UV and visible curing of coatings: Mechanisms and properties. J. Photochem. Photobiol. A Chem. 1996, 100, 101–107. [Google Scholar] [CrossRef]
- Danso, R.; Hoedebecke, B.; Whang, K.; Sarrami, S.; Johnston, A.; Flipse, S.; Wong, N.; Rawls, H.R. Development of an oxirane/acrylate interpenetrating polymer network (IPN) resin system. Dent. Mater. 2018, 34, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S.; Lalevée, J.; Morlet-Savary, F.; Lam, E.S.-H.; Graff, B.; Liu, J.; Xing, F.; Xiao, P. 1,2-Diketones as photoinitiators of both cationic and free-radical photopolymerization under UV (392 nm) or Blue (455 nm) LEDs. J. Polym. Sci. 2020, 58, 792–802. [Google Scholar] [CrossRef]
- Moszner, N.; Zeuner, F.; Lamparth, I.; Fischer, U.K. Benzoylgermanium derivatives as novel visible-light photoinitiators for dental composites. Macromol. Mater. Eng. 2009, 294, 877–886. [Google Scholar] [CrossRef]






















| Monomer | Molecular Weight [g/mol] | ρmona [g/cm3] | ρpolb [g/cm3] | ΔVp [%] | Viscosity [mPa·s] |
|---|---|---|---|---|---|
| TEGDMA | 286 | 1.072 | 1.250 | −14.3 | 100 |
| UDMA | 470 | 1.110 | 1.190 | −6.7 | 5000–10,000 |
| Bis-GMA | 512 | 1.151 | 1.226 | −6.1 | 500,000–800,000 |
| Acronym of Photoinitiator | Structure, Together with a Scheme of Photoinduced Cleavage of Photoinitiator | Maximum Absorbance/Characteristic of Absorbance | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| CQ |  | λmax = 468nm | wide absorption range based on the visible range | molar extinction coefficient in the range of 400–500 nm is only 40 [dm3 · mol–1 · cm–1], strongly yellow color | [118,119] |
| PPD |  | λmax = 400nm | improve the color stability | necessary to use LED light sources with two violet emission bands (380–420 nm and blue 420–520 nm), dual peak LEDs | [136,138,139] |
| TPO |  | λmax = 382nm | high efficiency of generating radicals, improve the color stability | low initiation efficiency, the need for UV light sources | [141,142,143] |
| IVO |  | λmax = 445nm | no cytotoxicity, high initiation rate and excellent bleaching | initiators only for free radical polymerization | [149,150,151] |
| HABI |  | display extended absorption tails well into the visible spectral region | effective in initiating thiol-ene photopolymerization | poor absorption in the visible spectrum, sometimes requiring a photosensitizer, low solubility in standard resins used in a dental application and low solubility in organic solvents | [154,160,161] |
| Silane derivatives |  | ultraviolent strong absorption in the 300–350 nm region | very effective in free radical photopolymerization, witch iodonium salts or pyridinium can be used for cationic photopolymerization | need for UV light sources | [162,163,164] |
 | λmax = 425 nm | excellent bleaching properties, in combination with an iodonium salt, can be useful for initiating cationic photopolymerization | low value of molar extinction coefficient ε: 120 dm3 · mol–1 · cm–1 in toluene and 100 dm3 · mol–1 · cm–1 in acetonitrile | [178,179] | |
 | λmax = 486nm for SED1 λmax = 468 nm for SED2 in toluene | suitable for free radical photopolymerization exposure to blue (@ 455 nm) and even green (@ 520 nm) LED | not suitable for methacrylate photopolymerization | [180] | |
 | λmax = 442 nm in acetonitirle | excellent bleaching properties, a high water solubility, and a very good stability in acidic | not suitable for methacrylate photopolymerization | [181] | |
| TX |  | λmax = ∼378 nm | water-soluble co-initiators, instant bond strength to dentin minimize the effects of concentration stress and phase separation in the aquatic environment | generally, less reactive than CQ/amine system | [151,182,183] |
| Photoinitiator | Structure | Wavelength of Maximum Absorbance (λmax) [nm] |
|---|---|---|
| Iodonium Photoinitiators Generating Benzene | ||
| Hycure-810 (ChemFine) |  | 230–260 nm |
| Uvacure 1600 (Surface Specialties [UCB]) |  | 240 nm |
| Sarcat CD-1012 (Sartomer) |  | 240 nm |
| Iodonium Photoinitiators not Generating Benzene | ||
| OMNICAT 440 (IGM) or Hycure-820 (ChemFine) |  | 267 nm |
| Irgacure 250 (Ciba) |  | 240–245 nm |
| UV 9310 (GE) |  | 240 nm |
| Rhodorsil 2076 (Rhodia) |  | 240 nm |
| Rhodorsil 2074 (Rhodia) |  | 240–250 nm |
| Sylanto-7MP |  | 350 nm |
| Sylanto-7MS |  | 349 nm |


| Photoinitiating Systems Based on Iodonium Salts | Reference of Photoinitiating System | Monomers/ Solution | Influence of Addition Iodonium Salt/Properties of the Dental Composition with Iodonium Salt | Ref. |
|---|---|---|---|---|
| 1 mol% CQ + 0.25 mol% DPIHFP 1 mol% CQ + 0.5 mol% DPIHFP 1 mol% CQ + 1 mol% DPIHFP 1 mol% CQ + 2 mol% DPIHFP 1 mol% CQ + 4 mol% DPIHFP 1 mol% CQ + 2 mol% EDAB + 0.25 mol% DPIHFP 1 mol% CQ + 2 mol% EDAB + 0.5 mol% DPIHFP 1 mol% CQ + 2 mol% EDAB + 1 mol% DPIHFP 1 mol% CQ + 2 mol% EDAB + 2 mol% DPIHFP 1 mol% CQ + 2 mol% EDAB + 4 mol% DPIHFP | 1 mol% CQ 1 mol% CQ + 0.25 mol% EDAB 1 mol% CQ + 0.5 mol% EDAB 1 mol% CQ + 1 mol% EDAB 1 mol% CQ + 2 mol% EDAB 1 mol% CQ + 4 mol% EDAB | 50 wt.% Bis-GMA 25 wt.% TEGDMA 25 wt.% HEMA | increase conversion in short photo-activation time | [130] |
| 1 mol% CQ + 1 mol% EDAB + 1 mol% DPIHFP | 1 mol% CQ + 1 mol% EDAB | 50 wt.% Bis-GMA 25 wt.% TEGDMA 25 wt.% HEMA (0, 10, 20, 30 and 40 wt.% ethanol) | reduce the inhibitory polymerization effect from an organic solvent | [214] |
| 1 mol% CQ + 1 mol% DPIHFP 1 mol% CQ + 1 mol% EDAB + 1 mol% DPIHFP | 1 mol% CQ 1 mol% CQ + 1 mol% EDAB | 50 wt.% Bis-GMA 25 wt.% TEGDMA 25 wt.% HEMA | improve dentin bonding performance | [205] |
| 1 mol% CQ + 1 mol% EDAB + 1 mol% DPIHFP 0.5 mol% CQ + 1 mol% EDAB + 0.5 mol% BAPO + 1 mol% DPIHFP 0.5 mol% CQ + 1 mol% EDAB + 0.5 mol% PPD + 1 mol% DPIHFP 1 mol% BAPO + 1 mol% EDAB + 1 mol% DPIHFP 1 mol% PPD + 1 mol% EDAB + 1 mol% DPIHFP | 1 mol% CQ + 1 mol% EDAB 0.5 mol% CQ + 1 mol% EDAB + 0.5 mol% BAPO 0.5 mol% CQ + 1 mol% EDAB + 0.5 mol% PPD 1 mol% BAPO + 1 mol% EDAB 1 mol% PPD + 1 mol% EDAB | BisGMA:HEMA (60:40 wt.%) 20 wt.% ethanol | increase conversion, does not affect the dentin bond strength | [215] |
| 1 mol% BAPO + 1 mol% DPIHFP 1 mol% BAPO + 2 mol% EDAB + 1 mol% DPIHFP 1 mol% CQ + 2 mol% EDAB + 1 mol% DPIHFP 1 mol% BAPO + 1 mol% CQ + 2 mol% EDAB + 1 mol% DPIHFP | 1 mol% BAPO 1 mol% CQ 1 mol% BAPO + 2 mol% EDAB 1 mol% CQ + 2 mol% EDAB 1 mol% BAPO + 1 mol% CQ + 1 mol% EDAB | 50 wt.% Bis-GMA 50 wt.% TEGDMA | highest polymerization and conversion rate for 1 mol% BAPO/1 mol% EDAB/1 mol% DPIHFP in short photo-activation time | [147] |
| QTX + DPIHFP QTX + EDAB + DPIHFP QTX + DPIHFP + BARB QTX + DPIHFP + SULF | QTX CQ + EDAB QTX + EDAB QTX + BARB QTX + SULF CQ + QTX + EDAB QTX + EDAB + BARB OTX + EDAB + SULF | 50 wt.% Bis-GMA, 25 wt.% TEGDMA 25 wt.% HEMA | similar conversion rates as in the case of the standard two-component system (CQ + EDAB); lower reactivity | [184] |
| 1 mol% CQ + 2 mol% DMAEMA/ 0.25, 0.5, 1, 2 or 4 mol% DPI | 1 mol% CQ/2 mol% DMAEMA | 20 wt.% Bis-GMA 20 wt.% TEGDMA 60 wt.% of silanated barium borosilicate glass fillers | improve the reactivity and mechanical properties | [216] |
| 0.5, 1 or 2 mol% DPIHFP | commercially available dual-polymerizing self-adhesive resin cements: RelyX U100 (3M ESPE) and BisCem (Bisco Inc.) | increase the degree of conversion, microhardness and push-out bond strength | [206] | |
| 0.5 mol% CQ + 0.5 mol% DPI 1.0 mol% CQ + 0.5 mol% DPI 0.5 mol% PPD + 0.5 mol% DPI 1.0 mol% PPD + 0.5 mol% DPI 0.5 mol% CQ + 1 mol% DPI 1.0 mol% CQ + 1 mol% DPI 0.5 mol% PPD + 1 mol% DPI 1.0 mol% PPD + 1 mol% DPI 0.5 mol% CQ + 0.5 mol% PPD + 0.5 mol% DPI 0.5 mol% CQ + 1.0 mol% PPD + 0.5 mol% DPI 1.0 mol% CQ + 0.5 mol% PPD + 0.5 mol% DPI 1.0 mol% CQ + 1.0 mol% PPD + 0.5 mol% DPI 0.5 mol% CQ + 0.5 mol% PPD + 1 mol% DPI 0.5 mol% CQ + 1.0 mol% PPD + 1 mol% DPI 1.0 mol% CQ + 0.5 mol% PPD + 1 mol% DPI 1.0 mol% CQ + 1.0 mol% PPD + 1 mol% DPI | 0.5 mol% CQ 1.0 mol% CQ 0.5 mol% PPD 1.0 mol% PPD 0.5 mol% CQ + 0.5 mol% PPD 0.5 mol% CQ + 1.0 mol% PPD 1.0 mol% CQ + 0.5 mol% PPD 1.0 mol% CQ + 1.0 mol% PPD | 25 wt.% BisGMA 20 wt.% TEGDMA 10 wt.% GDMA 25 wt.% HEMA | improve flexural strength and modulus of elasticity, cohesive strength, as well as lower sorption and water solubility | [138] |
| 0.25 wt.% CQ + 1 wt.% ETDA + 1 wt.% DPIHP | 0.25 wt.% CQ + 1 wt.% ETDA | 37 wt.% E-BisADM 25 wt.% TEGDMA 28% HEMA 10 wt.% ethanol 65 wt.% E-BisADM 25% TEGDMA 10 wt.% ethanol | enhance the degree of conversion, glass transition temperature (Tg) as well as resin permeability (rP). | [208] |
| 1 part of CQ and 2 parts of OPPI Equally proportioned CQ, OPPI, and DMAEMA Total concentrations of 1 wt.% and 3 wt.% | CQ only 1 part of CQ and 2 parts of DMAEMA Total concentrations of 1 wt.% and 3wt.% | 37.5 wt.% BisGMA 37.5 wt.% BisEMA 25 wt.% TEGDMA | reduce initial color and improve color stability | [79] |
| CQ + Ph3GeH + DPI in diffrent mass ratio | CQ + EDB + DPI CQ + EDB | 70 wt.% Bis-GMA 30 wt.% TEGDMA 100 wt.% UDMA | excellent bleaching properties, | [209] |
| 0.5 wt.% CQ + 1 wt.% IS1 0.5 wt.% CQ + 1 wt.% IS2 0.5 wt.% CQ + 1 wt.% IS3 0.5 wt.% CQ + 1 wt.% IS4 0.5 wt.% CQ + 1 wt.% IS5 0.2 wt.% CQ + 0.2 wt.% EDB +1 wt.% IS3 | 0.5 wt.% CQ + 1 wt.% EDB 0.2 wt.% CQ + 0.2 wt.% EDB 0.2 wt.% CQ + 0.2 wt.% EDB + 1 wt.% SC938 | 30 wt.% BisGMA 70 wt.% TEGDMA 10 wt.% MA 63 wt.% BisGMA 27 wt.% TEGDMA 10 wt.% HEMA 63 wt.% BisGMA 27 wt.% TEGDMA | excellent bleaching properties, very good performance, | [210] |
| 0.2 wt.% CQ + 0.5 wt.% EDB + 1 wt.% S5 0.2 wt.% CQ + 0.5 wt.% EDB + 1 wt.% S6 1 wt.% CQ + 1 wt.% EDB + 1 wt.% S5 1.5 wt.% CQ + 0.6 wt.% DMABN + 0.75 wt.% S5 1.5 wt.% CQ + 0.6 wt.% DMABN + 0.75 wt.% S6 | 0.2 wt.% CQ + 0.5 wt.% EDB 0.2 wt.% CQ + 0.5 wt.% EDB + 1 wt.% SC938 1 wt.% CQ + 1 wt.% EDB 1.5 wt.% CQ + 0.6 wt.% DMABN | Spectrum® TPH®3 resin received from Dentsply Sirona consisting of a mixture of modified BisGMA, TEGDMA and other methacrylate monomers 30 wt.% BisGMA 70 wt.% TEGDMA Prime&Bond Active® resin | strongly oxygen-inhibited conditions, excellent bleaching properties | [211] |
| 0.5 wt.% CQ + 1 wt.% NaMeSP + 1 wt.% SC938 0.5 wt.% CQ + 1 wt.% ZnBuS + 1 wt.% SC938 0.5 wt.% CQ + 1 wt.% NaAcABS + 1 wt.% SC938 0.5 wt.% CQ + 1 wt.% ZniPrS + 1 wt.% SC938 0.5 wt.% CQ + 1 wt.% NaBuNS + 1 wt.% SC938 0.5 wt.% CQ + 1 wt.% NapTSo + 1 wt.% SC938 0.5 wt.% CQ + 1 wt.% DPIpTS 0.2 wt.% CQ + 0.5 wt.% EDB + 1 wt.% NaMeSP+ 1 wt.% SC938 0.2 wt.% CQ + 0.5 wt.% EDB + 1 wt.% NapTSo+ 1 wt.% SC938 0.2 wt.% CQ + 0.5 wt.% EDB + 1 wt.% SC938 | 0.5 wt.% CQ + 1 wt.% NaMeSP 0.5 wt.% CQ + 1 wt.% ZnBuS 0.5 wt.% CQ + 1 wt.% NaAcABS 0.5 wt.% CQ + 1 wt.% ZniPrS 0.5 wt.% CQ + 1 wt.% NaBuNS 0.5 wt.% CQ + 1 wt.% NapTSo 0.5 wt.% CQ + 1 wt.% EDB 0.2 wt.% CQ + 0.5 wt.% EDB | 30 wt.% BisGMA 70 wt.% TEGDMA 10 wt.% MA 63 wt.% BisGMA 27 wt.% TEGDMA 10 wt.% HEMA 63 wt.% BisGMA 27 wt.% TEGDMA | excellent bleaching properties, color stability, excellent mechanical properties | [212] |
| 0.5 wt.% CQ + 1 wt.% DPPBS + 1 wt.% Iod 0.5 wt.% CQ + 1 wt.% Iod-DPPBS 0.4 wt.% CQ + 0.1 wt.% Iod-DPPBS 0.4 wt.% CQ + 0.4 wt.% Iod-DPPBS 0.4 wt.% CQ + 0.6 wt.% Iod-DPPBS 0.4 wt.% CQ + 1 wt.% Iod-DPPBS 0.5 wt.% CQ + 0.2 wt.% EDB + 1 wt.% DPPBS 0.5 wt.% CQ + 0.2 wt.% EDB + 1 wt.% DPPBS + 1 wt.% Iod 0.5 wt.% CQ + 0.1 wt.% EDB + 1 wt.% DPPBS + 1 wt.% Iod 0.5 wt.% CQ + 1 wt.% DPPBS + 1 wt.% Iod | 0.5 wt.% CQ + 1 wt.% EDB 0.5 wt.% CQ + 0.2 wt.% EDB 0.4 wt.% CQ + 1 wt.% Iod 0.5 wt.% CQ + 0.2 wt.% EDB + 1 wt.% Iod | 30 wt.% BisGMA 70 wt.% TEGDMA | oxygen inhibition | [213] |
| Iodonium Salt | Other Co-initiators | Monomers | Properties | Ref. | ||
|---|---|---|---|---|---|---|
 |  |  |  | lowering of shrinkage | [106] | |
| Rhodosil 2074 | CQ | EMBO | UV 30 | |||
 DPIHFP |  |  | greater decrease of volumetric shrinkage and better mechanical properties | [68] | ||
| CQ | MTOSN | |||||
 |  | |||||
| DMAEMA | BisS-GMA | |||||
 OPPI |  |  | lower shrinkage stress more hydrophobic mechanically weaker | [218] | ||
| CQ | DPHA | |||||
 |  | |||||
| Diol | EPS5000 | |||||
 |  |  |  | exceptional bleaching properties better mechanical properties | [179] | |
| DPI | (TMS)3SiH | DVE-3 | DEGVE | |||
 |  |  |  | |||
| SC938 | Ph3GeH | CHDVE | DODECYL VINYL ETHER | |||
 |  |  |  | |||
| PI 2074 | EDB | DEGDVE | VEEM | |||
 IOD |  |  | higher initiation ability than the well-known CQ-based systems, but cytotoxicity | [219] | ||
| ANPQ | BisGMA | |||||
 |  | |||||
| AATQ | TEGDMA | |||||
 |  | |||||
| PANQ | EPOX | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topa, M.; Ortyl, J. Moving Towards a Finer Way of Light-Cured Resin-Based Restorative Dental Materials: Recent Advances in Photoinitiating Systems Based on Iodonium Salts. Materials 2020, 13, 4093. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13184093
Topa M, Ortyl J. Moving Towards a Finer Way of Light-Cured Resin-Based Restorative Dental Materials: Recent Advances in Photoinitiating Systems Based on Iodonium Salts. Materials. 2020; 13(18):4093. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13184093
Chicago/Turabian StyleTopa, Monika, and Joanna Ortyl. 2020. "Moving Towards a Finer Way of Light-Cured Resin-Based Restorative Dental Materials: Recent Advances in Photoinitiating Systems Based on Iodonium Salts" Materials 13, no. 18: 4093. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13184093