Ferrierite and Its Delaminated Forms Modified with Copper as Effective Catalysts for NH3-SCO Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.1.1. Synthesis of Zeolite Precursors
2.1.2. Synthesis of Al-FER and Ti-FER
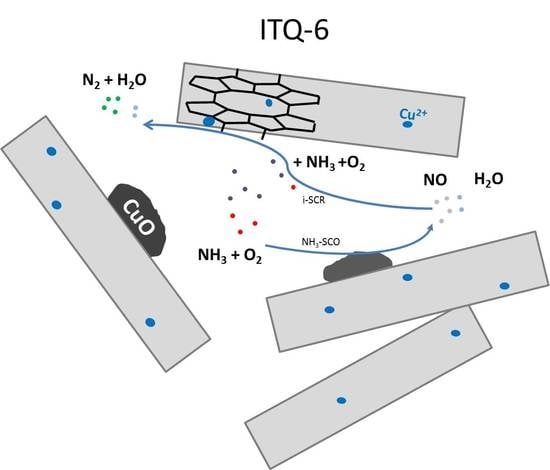
2.1.3. Swelling of PREFER and ITQ-6 Synthesis
2.1.4. Modification of Zeolites with Copper
2.2. Catalysts Characterization
2.3. Catalytic Studies
3. Results and Discussion
3.1. Characterization of Catalyst Precursors and Catalysts
3.1.1. X-ray Diffraction
3.1.2. N2 Sorption
3.1.3. UV-Vis DR Spectroscopy
3.1.4. H2-TPR
3.1.5. NH3-TPD
3.2. Catalytic Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO Global Estimates of Gaseous Emissions of NH3, NO and N2O from Agricultural Land. Available online: http://www.fao.org/docrep/004/Y2780E/y2780e00.htm (accessed on 30 September 2020).
- Zhao, D.; Wang, A. Estimation of anthropogenic ammonia emissions in Asia. Atmos. Environ. 1994, 28, 689–694. [Google Scholar] [CrossRef]
- Sutton, M.A.; Dragosits, U.; Tang, Y.S.; Fowler, D. Ammonia emissions from non-agricultural sources in the UK. Atmos. Environ. 2000, 34, 855–869. [Google Scholar] [CrossRef]
- ISO 22241-4:2009. Available online: https://www.iso.org/obp/ui/#iso:std:iso:22241:-4:ed-1:v1:en (accessed on 30 September 2020).
- Liu, C.; Wang, H.; Zhang, Z.; Liu, Q. The Latest Research Progress of NH3-SCR in the SO2 Resistance of the Catalyst in Low Temperatures for Selective Catalytic Reduction of NOx. Catalysts 2020, 10, 1034. [Google Scholar] [CrossRef]
- López-Hernández, I.; Mengual, J.; Palomares, A.E. The Influence of the Support on the Activity of Mn–Fe Catalysts Used for the Selective Catalytic Reduction of NOx with Ammonia. Catalysts 2020, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Górecka, S.; Pacultová, K.; Górecki, K.; Smýkalová, A.; Pamin, K.; Obalová, L. Cu-Mg-Fe-O-(Ce) Complex Oxides as Catalysts of Selective Catalytic Oxidation of Ammonia to Dinitrogen (NH3-SCO). Catalysts 2020, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Chmielarz, L.; Jabłońska, M. Advances in selective catalytic oxidation of ammonia to dinitrogen: A review. RSC Adv. 2015, 5, 43408–43431. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, D.W.; Chung, S.H.; Hong, Y.K.; Lee, S.H.; Oh, S.H.; Cho, I.H.; Lee, K.Y. Oxidation of ammonia to nitrogen over Pt/Fe/ZSM5 catalyst: Influence of catalyst support on the low temperature activity. J. Hazard. Mater. 2012, 237–238, 153–160. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Noble Metal (Pt, Rh, Pd) Promoted Fe-ZSM-5 for Selective Catalytic Oxidation of Ammonia to N2 at Low Temperatures. Catal. Lett. 2002, 78, 353–357. [Google Scholar] [CrossRef]
- Burch, R.; Southward, B.W.L. The Nature of the Active Metal Surface of Catalysts for the Clean Combustion of Biogas Containing Ammonia. J. Catal. 2001, 198, 286–295. [Google Scholar] [CrossRef]
- Basąg, S.; Piwowarska, Z.; Kowalczyk, A.; Węgrzyn, A.; Baran, R.; Gil, B.; Michalik, M.; Chmielarz, L. Cu-Mg-Al hydrotalcite-like materials as precursors of effective catalysts for selective oxidation of ammonia to dinitrogen - The influence of Mg/Al ratio and calcination temperature. Appl. Clay Sci. 2016, 129, 122–130. [Google Scholar] [CrossRef]
- Basąg, S.; Kocoł, K.; Piwowarska, Z.; Rutkowska, M.; Baran, R.; Chmielarz, L. Activating effect of cerium in hydrotalcite derived Cu-Mg-Al catalysts for selective ammonia oxidation and the selective reduction of NO with ammonia. Reac. Kinet. Mech. Catal. 2017, 121, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Chmielarz, L.; Jabłońska, M.; Strumiński, A.; Piwowarska, Z.; Węgrzyn, A.; Witkowski, S.; Michalik, M. Selective Catalytic Oxidation of Ammonia to Nitrogen over Mg-Al, Cu-Mg-Al And Fe-Mg-Al Mixed Metal Oxides Doped with Noble Metals. Appl. Catal. B-Environ. 2013, 130–131, 152–162. [Google Scholar] [CrossRef]
- Jabłońska, M.; Król, A.; Kukulska-Zając, E.; Tarach, K.; Chmielarz, L.; Góra-Marek, K. Zeolite Y Modified with Palladium as Effective Catalyst for Selective Catalytic Oxidation of Ammonia to Nitrogen. J. Catal. 2014, 316, 36–46. [Google Scholar] [CrossRef]
- Campisi, S.; Palliggiano, S.; Gervasini, A.; Evangelisti, C. Finely Iron-Dispersed Particles on β Zeolite from Solvated Iron Atoms: Promising Catalysts for NH3-SCO. J. Phys. Chem. C 2019, 123, 11723–11733. [Google Scholar] [CrossRef]
- Rutkowska, M.; Pacia, I.; Basąg, S.; Kowalczyk, A.; Piwowarska, Z. Duda M.; Tarach, K.; Góra-Marek, K.; Michalik, M.; Díaz, U.; Chmielarz, L.; Catalytic performance of commercial Cu-ZSM-5 zeolite modified by desilication in NH3-SCR and NH3-SCO processes. Micropor. Mesopor. Mater. 2017, 246, 193–206. [Google Scholar] [CrossRef]
- Normann, F.; Cheng, M.; Zhao, D.; Li, Z.; Cai, N.; Leion, H. Oxidation of ammonia over a copper oxide-containing solid oxygen carrier with oxygen uncoupling capability. Comb. Flame 2016, 165, 445–452. [Google Scholar] [CrossRef]
- Święs, A.; Kowalczyk, A.; Rutkowska, M.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Ferrierite and its Delaminated and Silica-Intercalated Forms Modified with Copper as Effective Catalysts for NH3-SCR Process. Catalysts 2020, 10, 734. [Google Scholar] [CrossRef]
- Solsona, B.; Lopez Nieto, J.M.; Díaz, U. Siliceous ITQ-6: A new support for vanadia in the oxidative dehydrogenation of propane. Micropor. Mesopor. Mater. 2006, 94, 339–347. [Google Scholar] [CrossRef]
- Radko, M.; Rutkowska, M.; Kowalczyk, A.; Mikrut, P.; Święs, A.; Díaz, U.; Palomares, A.E.; Macyk, W.; Chmielarz, L. Catalytic oxidation of organic sulfides by H2O2 in the presence of titanosilicate zeolites. Micropor. Mesopor. Mater. 2020, 302, 110219–110227. [Google Scholar] [CrossRef]
- Maddalena, R.; Hall, C.; Hamilton, A. Effect of silica particle size on the formation of calcium silicate hydrate [C-S-H] using thermal analysis. Thermochim. Acta 2019, 672, 142–149. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.W.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M. Physical adsorption characterization of nanoporous materials. Chem. Ing. Tech. 2010, 82, 1059–1073. [Google Scholar] [CrossRef]
- Hu, H.; Ke, M.; Zhang, K.; Liu, Q.; Yu, P.; Liu, Y.; Li, C.; Liu, W. Designing ferrierite-based catalysts with improved properties for skeletal isomerization of n-butene to isobutene. RSC Adv. 2017, 7, 31535–31543. [Google Scholar] [CrossRef] [Green Version]
- Corma, A.; Díaz, U.; Domine, M.E.; Fornés, V. New aluminosilicate and titanosilicate delaminated materials active for acid catalysis, and oxidation reactions using H2O2. J. Am. Chem. Soc. 2000, 122, 2804–2809. [Google Scholar] [CrossRef]
- Minchev, C.; Köhn, R.; Tsonsheva, T.; Dimitrov, M.; Fröba, M. Preparation and characterization of copper oxide modified MCM-41 molecular sieves. Stud. Surf. Sci. Catal. 2001, 135, 253. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Qiao, X.; Jin, Y.; Fan, B. Effect of hydrothermal aging treatment on decomposition of NO by Cu-ZSM-5 and modified mechanism of doping Ce against this influence. Materials 2020, 13, 888. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, J.A.; Cunningham, J. Selective catalytic reduction of NO with C2H4 over Cu/ZSM-5: Influences of oxygen partial pressure and incorporated rhodia. Appl. Catal. B Environ. 1998, 15, 275–289. [Google Scholar] [CrossRef]
- Shtepliuk, I.; Vagin, M.; Yakimova, R. Electrochemical Deposition of Copper on Epitaxial Graphene. Appl. Sci. 2020, 10, 1405. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, A.; Święs, A.; Gil, B.; Rutkowska, M.; Piwowarska, Z.; Borcuch, A.; Michalik, M.; Chmielarz, L. Effective catalysts for the low-temperature NH3-SCR process based on MCM-41 modified with copper by template ion-exchange (TIE) method. Appl. Catal. B Environ. 2018, 237, 927–937. [Google Scholar] [CrossRef]
- Petrov, L.; Soria, J.; Dimitrov, L.; Cataluna, R.; Spasov, L.; Dimitrov, P. Cu exchanged microporous titanium silicalite (TS-1) coated on polycrystalline mullite fibres as catalyst for the CO and NO conversion. Appl. Catal. B Environ. 2000, 28, 175–185. [Google Scholar] [CrossRef]
- Wichterlova, B.; Tvaruzkova, Z.; Sobalık, Z.; Sarv, P. Determination and properties of acid sites in H-ferrierite: A comparison of ferrierite and MFI structures. Micropor. Mesopor. Mater. 1998, 24, 223–233. [Google Scholar] [CrossRef]
- Starzyk, F.T.; Stan, I.; Abello, S.; Bonilla, A.; Thomas, K.; Fernandez, C.; Gilson, J.P.; Ramırez, J.P. Quantification of enhanced acid site accessibility in hierarchical zeolites—The accessibility index. J. Catal. 2009, 264, 11–14. [Google Scholar] [CrossRef]
- Borgida, C.; Boscherini, F.; Caluccia, S.; Genoni, F.; Lamberti, C.; Marchese, L.; Petrini, G.; Vlaich, C.; Zecchina, A. XAFS study of Ti-silicalite: Structure of framework Ti(IV) in presence and in absence of reactive molecules (H2O, NH3). Catal. Lett. 1994, 26, 195–208. [Google Scholar] [CrossRef]









| Sample | SBET (m2/g) | Vmicro (cm3/g) | Vmeso (cm3/g) | Ca (µmol/g) | Da (µmol/m2) | Cu (wt%) | Si/Al (mol/mol) | Si/Ti (mol/mol) |
|---|---|---|---|---|---|---|---|---|
| Ti-FER | 368 | 0.14 | 0.09 | 49 | 0.13 | - | 60 | |
| Al-FER | 377 | 0.13 | 0.10 | 49 | 0.13 | 64 | ||
| Ti-ITQ-6 | 615 | 0.02 | 1.38 | 70 | 0.11 | - | 80 | |
| Al-ITQ-6 | 372 | 0.02 | 1.12 | 37 | 0.10 | 111 | ||
| Cu/Ti-FER | 330 | 0.12 | 0.09 | 54 | 0.16 | 2.7 | 68 | |
| Cu/Al-FER | 359 | 0.12 | 0.11 | 79 | 0.22 | 0.9 | 74 | |
| Cu/Ti-ITQ-6 | 523 | 0.02 | 0.78 | 141 | 0.27 | 2.8 | 85 | |
| Cu/Al-ITQ-6 | 281 | 0.02 | 0.47 | 162 | 0.58 | 2.7 | 124 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Święs, A.; Rutkowska, M.; Kowalczyk, A.; Díaz, U.; Palomares, A.E.; Chmielarz, L. Ferrierite and Its Delaminated Forms Modified with Copper as Effective Catalysts for NH3-SCO Process. Materials 2020, 13, 4885. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13214885
Święs A, Rutkowska M, Kowalczyk A, Díaz U, Palomares AE, Chmielarz L. Ferrierite and Its Delaminated Forms Modified with Copper as Effective Catalysts for NH3-SCO Process. Materials. 2020; 13(21):4885. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13214885
Chicago/Turabian StyleŚwięs, Aneta, Małgorzata Rutkowska, Andrzej Kowalczyk, Urbano Díaz, Antonio E. Palomares, and Lucjan Chmielarz. 2020. "Ferrierite and Its Delaminated Forms Modified with Copper as Effective Catalysts for NH3-SCO Process" Materials 13, no. 21: 4885. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13214885