Synthetic Hydroxyapatite Inhibits Bisphosphonate Toxicity to the Oral Mucosa In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microscopy
2.2. Cell Culture
2.3. De-Cellularised Dermis Preparation
2.4. Bisphosphonates
2.5. Two-Dimensional Cell Viability Assays
2.6. Three-Dimensional Cell Culture
2.7. Hydroxyapatite Binding Assays
2.8. Statistics
3. Results
3.1. Microscopy of Hydroxyapatite Granules Confirmed Highly Porous Structure
3.2. Clinically Relevant Pamidronic Acid and Zoledronic Acid Concentrations are Toxic to Oral Mucosa Cells in 2D
3.3. Pamidronic Acid and Zoledronic Acid Reduce Epithelial Thickness and Cause Toxicity to the Oral Mucosa in 3D
3.4. Hydroxyapatite Granules Prevent Pamidronic Acid and Zoledronic Acid Toxicity in 2D
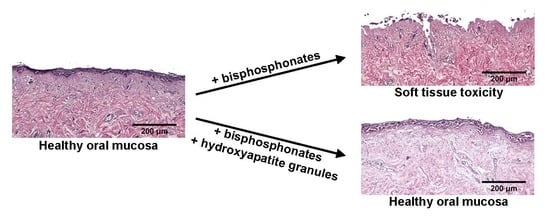
3.5. Hydroxyapatite Prevents Zoledronic Acid Toxicity in 3D
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dimopoulos, M.A.; Kastritis, E.; Anagnostopoulos, A.; Melakopoulos, I.; Gika, D.; Moulopoulos, L.A.; Bamia, C.; Terpos, E.; Tsionos, K.; Bamias, A. Osteonecrosis of the jaw in patients with multiple myeloma treated with bisphosphonates: Evidence of increased risk after treatment with zoledronic acid. Haematologica 2006, 91, 968–971. [Google Scholar] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Burr, D.; Cauley, J.; Dempster, D.W.; Ebeling, P.R.; Felsenberg, D.; Gagel, R.F.; Gilsanz, V.; Guise, T.; Koka, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2007, 22, 1479–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebetino, F.H.; Hogan, A.-M.L.; Sun, S.; Tsoumpra, M.K.; Duan, X.; Triffitt, J.T.; Kwaasi, A.A.; Dunford, J.E.; Barnett, B.L.; Oppermann, U.; et al. The relationship between the chemistry and biological activity of the bisphosphonates. Bone 2011, 49, 20–33. [Google Scholar] [CrossRef]
- Russell, R.G.G. Bisphosphonates: From bench to bedside. Ann. N. Y. Acad. Sci. 2006, 1068, 367–401. [Google Scholar] [CrossRef] [Green Version]
- Paulo, S.; Abrantes, A.M.; Laranjo, M.; Carvalho, L.; Serra, A.; Botelho, M.F.; Ferreira, M.M. Bisphosphonate-related osteonecrosis of the jaw: Specificities. Oncol. Rev. 2014, 8, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Barba-Recreo, P.; de Vera, J.L.d.P.; Georgiev-Hristov, T.; Bravo-Burguillos, E.R.; Abarrategi, A.; Burgueño, M.; García-Arranz, M. Adipose-derived stem cells and platelet-rich plasma for preventive treatment of bisphosphonate-related osteonecrosis of the jaw in a murine model. J. Cranio-Maxillofac. Surg. 2015, 43, 1161–1168. [Google Scholar] [CrossRef] [Green Version]
- Galis, B.; Zajko, J.; Hirjak, D.; Vanko, L.; Kupcova, I.; Jurkemik, J.; Gengelova, P.; Mikuskova, K.; Halmova, K.; Riznic, M.; et al. Is the Prevalence of the Medication-Related Osteonecrosis of the Jaws Underestimated, Evaluation in Oncological and Non-Oncological Disease. Bratisl. Med. J. 2017, 118, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Reid, I.R.; Bolland, M.J.; Grey, A.B. Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone 2007, 41, 318–320. [Google Scholar] [CrossRef]
- Cozin, M.; Pinker, B.M.; Solemani, K.; Zuniga, J.M.; Dadaian, S.C.; Cremers, S.; Landesberg, R.; Raghavan, S. Novel therapy to reverse the cellular effects of bisphosphonates on primary human oral fibroblasts. J. Oral Maxillofac. Surg. 2011, 69, 2564–2578. [Google Scholar] [CrossRef] [Green Version]
- Hagelauer, N.; Ziebart, T.; Pabst, A.M.; Walter, C. Bisphosphonates inhibit cell functions of HUVECs, fibroblasts and osteogenic cells via inhibition of protein geranylgeranylation. Clin. Oral Investig. 2015, 19, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.M.; Ziebart, T.; Koch, F.P.; Taylor, K.Y.; Al-Nawas, B.; Walter, C. The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes-in vitro study. Clin. Oral Investig. 2012, 16, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Scheper, M.A.; Badros, A.; Chaisuparat, R.; Cullen, K.J.; Meiller, T.F. Effect of zoledronic acid on oral fibroblasts and epithelial cells: A potential mechanism of bisphosphonate-associated osteonecrosis. Br. J. Haematol. 2009, 144, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Renò, F.; Migliario, M.; Rizzi, M.; Invernizzi, M.; Cisari, C.; Cannas, M. Low concentration amino-bisphosphonates stimulate human keratinocyte proliferation and in vitro wound healing. Int. Wound J. 2012, 9, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Sun, S.; Aghaloo, T.; Oh, J.-E.; McKenna, C.E.; Kang, M.K.; Shin, K.-H.; Tetradis, S.; Park, N.-H.; Kim, R.H. Development of oral osteomucosal tissue constructs in vitro and localization of fluorescently-labeled bisphosphonates to hard and soft tissue. Int. J. Mol. Med. 2014, 34, 559–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.H.; Lee, R.S.; Williams, D.; Bae, S.; Woo, J.; Lieberman, M.; Oh, J.-E.; Dong, Q.; Shin, K.-H.; Kang, M.K.; et al. Bisphosphonates Induce Senescence in Normal Human Oral Keratinocytes. J. Dent. Res. 2011, 90, 810–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLeod, N.M.H.; Moutasim, K.A.; Brennan, P.A.; Thomas, G.; Jenei, V. In vitro effect of bisphosphonates on oral keratinocytes and fibroblasts. J. Oral Maxillofac. Surg. 2014, 72, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Izumi, K.; Shiomi, A.; Uenoyama, A.; Ohnuki, H.; Kato, H.; Terada, M.; Nozawa-Inoue, K.; Kawano, Y.; Takagi, R.; et al. Zoledronic acid impairs re-epithelialization through down-regulation of integrin αvβ6 and transforming growth factor beta signalling in a three-dimensional in vitro wound healing model. Int. J. Oral Maxillofac. Surg. 2014, 43, 373–380. [Google Scholar] [CrossRef]
- Elsayed, R.; Abraham, P.; Awad, M.E.; Kurago, Z.; Baladhandayutham, B.; Whitford, G.M.; Pashley, D.H.; McKenna, C.E.; Elsalanty, M.E. Removal of matrix-bound zoledronate prevents post-extraction osteonecrosis of the jaw by rescuing osteoclast function. Bone 2018, 110, 141–149. [Google Scholar] [CrossRef]
- Oizumi, T.; Yamaguchi, K.; Sato, K.; Takahashi, M.; Yoshimura, G.; Otsuru, H.; Tsuchiya, M.; Hagiwara, Y.; Itoi, E.; Sugawara, S.; et al. A Strategy against the Osteonecrosis of the Jaw Associated with Nitrogen-Containing Bisphosphonates (N-BPs): Attempts to Replace N-BPs with the Non-N-BP Etidronate. Biol. Pharm. Bull. 2016, 39, 1549–1554. [Google Scholar] [CrossRef] [Green Version]
- Paulo, S.; Laranjo, M.; Abrantes, A.M.; Casalta-Lopes, J.; Santos, K.; Gonçalves, A.C.; Paula, A.B.; Marto, C.M.; Sarmento-Ribeiro, A.B.; Carrilho, E.; et al. Synthetic Calcium Phosphate Ceramics as a Potential Treatment for Bisphosphonate-Related Osteonecrosis of the Jaw. Materials 2019, 12, 1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Dickson, M.A.; Hahn, W.C.; Ino, Y.; Ronfard, V.; Wu, J.Y.; Weinberg, R.A.; Louis, D.N.; Li, F.P.; Rheinwald, J.G. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 2000, 20, 1436–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindberg, K.; Rheinwald, J.G. Three distinct keratinocyte subtypes identified in human oral epithelium by their patterns of keratin expression in culture and in xenografts. Differentiation 1990, 45, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Scheper, M.A.; Badros, A.; Salama, A.R.; Warburton, G.; Cullen, K.J.; Weikel, D.S.; Meiller, T.F. A novel bioassay model to determine clinically significant bisphosphonate levels. Supportive Care Cancer 2009, 17, 1553–1557. [Google Scholar] [CrossRef]
- Grayson, A.K.; Hearnden, V.; Bolt, R.; Jebreel, A.; Colley, H.E.; Murdoch, C. Use of a Rho kinase inhibitor to increase human tonsil keratinocyte longevity for three-dimensional, tissue engineered tonsil epithelium equivalents. J. Tissue Eng. Regen. Med. 2018, 12, e1636–e1646. [Google Scholar] [CrossRef] [Green Version]
- Arai, N.; Inoue, S.; Tomihara, K.; Tsuno, H.; Noguchi, M. In vitro synergistic effects of zoledronic acid and calcium on viability of human epithelial cells. Oral Dis. 2013, 19, 200–205. [Google Scholar] [CrossRef]
- Açil, Y.; Arndt, M.L.; Gülses, A.; Wieker, H.; Naujokat, H.; Ayna, M.; Wiltfang, J. Cytotoxic and inflammatory effects of alendronate and zolendronate on human osteoblasts, gingival fibroblasts and osteosarcoma cells. J. Cranio-Maxillofac. Surg. 2018, 46, 538–546. [Google Scholar] [CrossRef]
- Agis, H.; Blei, J.; Watzek, G.; Gruber, R. Is Zoledronate Toxic to Human Periodontal Fibroblasts? J. Dent. Res. 2010, 89, 40–45. [Google Scholar] [CrossRef]
- Draenert, G.F.; Huetzen, D.O.; Kämmerer, P.W.; Palarie, V.; Nacu, V.; Wagner, W. Dexrazoxane shows cytoprotective effects in zoledronic acid-treated human cells in vitro and in the rabbit tibia model in vivo. J. Cranio-Maxillofac. Surg. 2012, 40, e369–e374. [Google Scholar] [CrossRef]
- Jung, J.; Park, J.S.; Righesso, L.; Pabst, A.M.; Al-Nawas, B.; Kwon, Y.D.; Walter, C. Effects of an oral bisphosphonate and three intravenous bisphosphonates on several cell types in vitro. Clin. Oral Investig. 2018, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, H.; Izumi, K.; Terada, M.; Saito, T.; Kato, H.; Suzuki, A.; Kawano, Y.; Nozawa-Inoue, K.; Takagi, R.; Maeda, T. Zoledronic acid induces S-phase arrest via a DNA damage response in normal human oral keratinocytes. Arch. Oral Biol. 2012, 57, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Ravosa, M.J.; Ning, J.; Liu, Y.; Stack, M.S. Bisphosphonate effects on the behaviour of oral epithelial cells and oral fibroblasts. Arch. Oral Biol. 2011, 56, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Soydan, S.S.; Araz, K.; Senel, F.V.; Yurtcu, E.; Helvacioglu, F.; Dagdeviren, A.; Tekindal, M.A.; Sahin, F. Effects of alendronate and pamidronate on apoptosis and cell proliferation in cultured primary human gingival fibroblasts. Hum. Exp. Toxicol. 2015, 34, 1073–1082. [Google Scholar] [CrossRef]
- Marolt, D.; Cozin, M.; Vunjak-Novakovic, G.; Cremers, S.; Landesberg, R. Effects of pamidronate on human alveolar osteoblasts in vitro. J. Oral Maxillofac. Surg. 2012, 70, 1081–1092. [Google Scholar] [CrossRef] [Green Version]
- Donetti, E.; Gualerzi, A.; Sardella, A.; Lodi, G.; Carrassi, A.; Sforza, C. Alendronate impairs epithelial adhesion, differentiation and proliferation in human oral mucosa. Oral Dis. 2014, 20, 466–472. [Google Scholar] [CrossRef]
- Ikebe, T. Pathophysiology of BRONJ: Drug-related osteoclastic disease of the jaw. Oral Sci. Int. 2013, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Khominsky, A.; Lim, M. “Spontaneous” medication-related osteonecrosis of the jaw; two case reports and a systematic review. Aust. Dent. J. 2018, 63, 441–454. [Google Scholar] [CrossRef]
- Micallef, L.; Belaubre, F.; Pinon, A.; Jayat-Vignoles, C.; Delage, C.; Charveron, M.; Simon, A. Effects of extracellular calcium on the growth-differentiation switch in immortalized keratinocyte HaCaT cells compared with normal human keratinocytes. Exp. Dermatol. 2009, 18, 143–151. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bullock, G.; Miller, C.; McKechnie, A.; Hearnden, V. Synthetic Hydroxyapatite Inhibits Bisphosphonate Toxicity to the Oral Mucosa In Vitro. Materials 2020, 13, 2086. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13092086
Bullock G, Miller C, McKechnie A, Hearnden V. Synthetic Hydroxyapatite Inhibits Bisphosphonate Toxicity to the Oral Mucosa In Vitro. Materials. 2020; 13(9):2086. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13092086
Chicago/Turabian StyleBullock, George, Cheryl Miller, Alasdair McKechnie, and Vanessa Hearnden. 2020. "Synthetic Hydroxyapatite Inhibits Bisphosphonate Toxicity to the Oral Mucosa In Vitro" Materials 13, no. 9: 2086. https://0-doi-org.brum.beds.ac.uk/10.3390/ma13092086