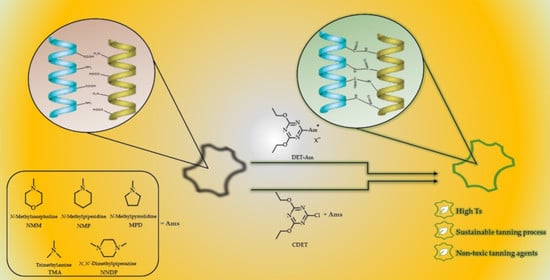
Efficient Triazine Derivatives for Collagenous Materials Stabilization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. General Procedure for Dehydro-Condensation Reactions with CDET/tert-Amine System
2.2.2. Synthesis of 4-(4,6-diethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium Perchlorate (DET-MM(ClO4)) and 4-(4,6-diethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium Tetrafluoroborate (DET-MM(BF4))
2.2.3. Cross-Linking of Bovine Collagen Powder with CDET/NMM
2.2.4. Tanning Tests
2.2.5. Shrinking Temperature
2.2.6. Physical–Mechanical Tests
3. Results and Discussion
3.1. Amidation Reaction
3.2. Cross-Linking Reaction of Collagen by CDET/NMM
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.Q.; Ciacci, L.; Sun, N.N.; Yoshioka, T. Sustainable cycles and management of plastics: A brief review of RCR publications in 2019 and early 2020. Resour. Conserv. Recycl. 2020, 159, 104822–104828. [Google Scholar] [CrossRef]
- Kabir, E.; Kaur, R.; Lee, J.; Kim, K.H.; Kwon, E.E. Prospects of biopolymer technology as an alternative option for non-degradable plastics and sustainable management of plastic wastes. J. Clean. Prod. 2020, 258, 120536–120550. [Google Scholar] [CrossRef]
- Mercado, G.; Dominguez, M.; Herrera, I.; Melgoza, R.M. Are Polymers Toxic? Case Study: Environmental Impact of a Biopolymer. J. Environ. Sci. Eng. B. 2017, 6, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Nkanga, U.J.; Joseph, J.J.; Adams, F.V.; Uche, O.U. Characterization of Bitumen/Plastic Blends for Flexible Pavement Application. Procedia Manuf. 2016, 7, 490–496. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Marine Pollution. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Nesic, A.; Castillo, C.; Castano, P.; Cabrera-Barjas, G.; Serrano, J. Bio-based packaging materials. In Biobased Products and Industries; Galanakis, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 279–309, Chapter 8. [Google Scholar] [CrossRef]
- Walker, S.; Rothman, R. Life cycle assessment of bio-based and fossil-based plastic: A review. J. Clean. Prod. 2020, 261, 121158–121172. [Google Scholar] [CrossRef]
- Beghetto, V.; Gatto, V.; Conca, S.; Bardella, N.; Buranello, C.; Gasparetto, G.; Sole, R. Development of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride cross-linked carboxymethyl cellulose films. Carbohydr. Polym. 2020, 249, 116810. [Google Scholar] [CrossRef]
- Haider, T.; Völker, C.; Kramm, J.; Landfester, K.; Wurm, F.R. Plastics of the future? The impact of biodegradable polymers on the environment and on society. Angew. Chem. Int. Ed. 2019, 58, 50–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sole, R.; Taddei, L.; Franceschi, C.; Beghetto, V. Efficient Chemo-Enzymatic Transformation of Animal Biomass Waste for Eco-Friendly Leather Production. Molecules 2019, 24, 2979. [Google Scholar] [CrossRef] [Green Version]
- BCC Research. Sustainable Biopolymers: A BCC Research Outlook. Available online: https://www.bccresearch.com/market-research/plastics/biopolymers-market-report.html (accessed on 12 December 2020).
- Markets and Markets. Available online: https://www.marketsandmarkets.com/Market-Reports/synthetic-leather-market-6616309.html?gclid=CjwKCAjwnPOEBhA0EiwA609ReSL8J8q0ZySPxTfPe1DMWeQ9EACRCzD_IfZfrskDHGc3F6EMdMmkmRoCgGMQAvD_BwE (accessed on 10 May 2021).
- Euroleather. Available online: https://www.euroleather.com (accessed on 30 April 2021).
- Yu, L.; Qiang, X.; Cui, L.; Chen, B.; Wang, X.; Wu, X. Preparation of a syntan containing active chlorine groups for chrome-free tanned leather. J. Clean. Prod. 2020, 270, 122351. [Google Scholar] [CrossRef]
- Zhang, Y.; Mansel, B.W.; Naffa, R.; Cheong, Y.Y.; Holmes, G.; Chen, H.L.; Prabakar, S. Revealing molecular level indicators of collagen stability: Minimizing chrome usage in leather processing. ACS Sustain. Chem. Eng. 2018, 6, 7096–7104. [Google Scholar] [CrossRef]
- Cao, S.; Liu, B.; Cheng, B.; Lu, F.; Wang, Y.; Li, Y. Mechanisms of Zn(II) binded to collagen and its effect on the capacity of eco-friendly Zn-Cr combination tanning system. J. Hazard. Mater. 2017, 321, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhou, J.; Hua, L.; Cheng, F.; Zhou, L.; Qiao, X. Chromium recovery from tannery sludge by bioleaching and its reuse in tanning process. J. Clean. Prod. 2017, 142, 2752–2760. [Google Scholar] [CrossRef]
- Ye, Y.; Jiang, Z.; Xu, Z.; Zhang, X.; Wang, D.; Lv, L.; Pan, B. Efficient removal of Cr(III)-organic complexes from water using UV/Fe(III) system: Negligible Cr(VI) accumulation and mechanism. Water Res. 2017, 126, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xia, F.; Long, J.; Peng, B. An integrated technology to minimize the pollution of chromium in wet-ed of leather manufacture. J. Clean. Prod. 2017, 154, 276–283. [Google Scholar] [CrossRef]
- Liu, M.; Ma, J.; Lyu, B.; Gao, D.; Zhang, J. Enhancement of chromium uptake in tanning process of goat garment leather using nanocomposite. J. Clean. Prod. 2016, 133, 487–494. [Google Scholar] [CrossRef]
- Teng, B.; Jian, X.; Gao, Y.; Chen, W. Comparison of polyflavonoids in bayberry tanning effluent and commercial bayberry tannin: Prerequisite information for vegetable tanning effluent recycling. J. Clean. Prod. 2016, 112, 972–979. [Google Scholar] [CrossRef]
- Zhang, C.; Lin, J.; Jia, X.; Peng, B. A salt-free and chromium discharge minimizing tanning technology: The novel cleaner integrated chrome tanning process. J. Clean. Prod. 2016, 112, 1055–1063. [Google Scholar] [CrossRef]
- Silambarasan, S.; Aravindhan, R.; Raghava Rao, J.; Thanikaivelan, P. Waterless tanning: Chrome tanning in ethanol and its derivatives. RSC Adv. 2015, 5, 66815–66823. [Google Scholar] [CrossRef]
- Plavan, V. Chrome Tanning Improvement by Chitosan Application. J. Soc. Leather Technol. Chem. 2012, 96, 89–93. [Google Scholar]
- Mahmoud, A.E.D.; Fawzy, M.; Hosny, G.; Obaid, A. Equilibrium, kinetic, and diffusion models of chromium(VI) removal using Phragmites australis and Ziziphus spina-christi biomass. Int. J. Environ. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Marsal, A.; Cuadros, S.; Ollé, L.; Bacardit, A.; Manich, A.M.; Font, J. Formaldehyde scavengers for cleaner production: A case study focused on the leather industry. J. Clean. Prod. 2018, 186, 45–56. [Google Scholar] [CrossRef]
- Covington, A.D. Tanning Chemistry: The Science of Leather; RSC Publishing: Cambridge, UK, 2009. [Google Scholar]
- Beghetto, V.; Agostinis, L.; Gatto, V.; Samiolo, R.; Scrivanti, A. Sustainable use of 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride as metal free tanning agent. J. Clean. Prod. 2019, 220, 864–872. [Google Scholar] [CrossRef]
- Beghetto, V.; Gatto, V.; Conca, S.; Bardella, N.; Scrivanti, A. Polyamidoamine Dendrimers and Cross-Linking Agents for Stabilized Bioenzymatic Resistant Metal-Free Bovine Collagen. Molecules 2019, 24, 3611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sole, R.; Agostinis, L.; Conca, S.; Gatto, V.; Bardella, N.; Morandini, A.; Buranello, C.; Beghetto, V. Synthesis of Amidation Agents and Their Reactivity in Condensation Reactions. Synthesis 2021, 53, 1672–1682. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, C.; Sang, J.; Lin, W. A Novel Non-Pickling Combination Tanning for Chrome-free Leather Based on Reactive Benzensulphonate and Tannic acid. J. Am. Leather Chem. Assoc. 2020, 115, 1–36. [Google Scholar]
- Onem, E.; Yorgancioglu, A.; Karavana, H.A.; Yilmaz, O. Comparison of different tanning agents on the stabilization of collagen via differential scanning calorimetry. J. Therm. Anal. Calorim. 2017, 129, 615–622. [Google Scholar] [CrossRef]
- Di, Y.; Heat, R.J.; Long, A.; Hartnung, K. Comparison of the tanning abilities of some epoxides and aldehydic compounds. J. Soc. Leather Technol. Chem. 2006, 90, 93–101. [Google Scholar]
- Beghetto, V.; Scrivanti, A.; Bertoldini, M.; Aversa, M.; Zancanaro, A.; Matteoli, U. A Practical, Enantioselective Synthesis of Fragrances Canthoxal and Silvial®, and Evaluation of Their Olfactory Activity. Synthesis 2015, 47, 272–288. [Google Scholar] [CrossRef]
- Paganelli, S.; Alam, M.M.; Beghetto, V.; Scrivanti, A.; Amadio, E.; Bertoldini, M.; Matteoli, U.; Scrivanti, A. A pyridyl-triazole ligand for ruthenium and iridium catalyzed C=C and C=O hydrogenations in water/organic solvent biphasic systems. Appl. Catal. A Gen. 2015, 503, 20–25. [Google Scholar] [CrossRef]
- Petta, D.; Eglina, D.; Grijpma, D.W.; D’Este, M. Enhancing hyaluronan pseudoplasticity via 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholiniumchloride-mediated conjugation with short alkyl moieties. Carbohydr. Polym. 2016, 151, 576–583. [Google Scholar] [CrossRef]
- D’Este, M.; Eglin, D.; Alini, M. A systematic analysis of DMTMM vs EDC/NHS for ligation of amines to Hyaluronan in water. Carbohydr. Polym. 2014, 108, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Scrivanti, A.; Bortoluzzi, M.; Sole, R.; Beghetto, V. Synthesis and characterization of yttrium, europium, terbium and dysprosium complexes containing a novel type of triazolyl-oxazoline ligand. Chem. Pap. 2017, 72, 799–808. [Google Scholar] [CrossRef]
- Scrivanti, A.; Sole, R.; Bortoluzzi, M.; Beghetto, V.; Bardella, N.; Dolmella, A. Synthesis of new triazolyl-oxazoline chiral ligands and study of their coordination to Pd(II) metal centers. Inorg. Chim. Acta 2019, 498, 119129. [Google Scholar] [CrossRef]
- Hou, X.M.; Liang, T.M.; Guo, Z.Y.; Wang, C.Y.; Shao, C.L. Discovery, absolute assignments, and total synthesis of asperversiamides A-C and their potent activity against Mycobacterium marinum. Chem. Commun. 2019, 55, 1104. [Google Scholar] [CrossRef]
- Tsutsumi, L.S.; Tan, G.T.; Sun, D. Solid-phase synthesis of cyclic hexapeptides wollamides A, B and desotamide B. Tetrahedron Lett. 2017, 58, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Kunishima, M.; Kawachi, C.; Morita, J.; Terao, K.; Iwasaki, F.; Tani, S. 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride: An efficient condensing agent leading to the formation of amides and esters. Tetrahedron 1999, 55, 13159–13170. [Google Scholar] [CrossRef]
- Kunishima, M.; Ujigawa, T.; Nagaoka, Y.; Kawachi, C.; Hioki, K.; Shiro, M. Study on 1,3,5-Triazine chemistry in dehydrocondensation: Gauche effect on the generation of active triazinylammonium species. Chem. Eur. J. 2012, 18, 15856–15867. [Google Scholar] [CrossRef]
- Beghetto, V. Method for the Industrial Production of 2 halo-4,6-dialcoxy-1,3,5-triazine and their Use in the Presence of Amines. WO Patent WO2016/103185A3, 6 October 2016. [Google Scholar]
- Sole, R.; Gatto, V.; Conca, S.; Bardella, N.; Morandini, A. Sustainable Triazine-Based Dehydro-Condensation Agents for Amide Synthesis. Molecules 2021, 26, 191. [Google Scholar] [CrossRef]
- Raw, S.A. An improved process for the synthesis of DMTMM-based coupling reagents. Tetrahedron Lett. 2009, 50, 946–948. [Google Scholar] [CrossRef]
- Kitamura, M.; Komine, S.; Yamada, K.; Kunishima, M. Triazine-based dehydrative condensation reagents bearing carbon-substituents. Tetrahedron 2020, 76, 130900. [Google Scholar] [CrossRef]
- El-Faham, A.; Albericio, F. Peptide Coupling Reagents, More than a Letter Soup. Chem. Rev. 2011, 111, 6557–6602. [Google Scholar] [CrossRef]
- Princz, M.A.; Sheardown, H. Modified Dendrimer Cross-Linked Collagen-Based Matrices. J. Biomater. Sci. Polym. Ed. 2012, 23, 2207–2222. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Qiang, X. Clean Production for Chrome Free Leather by Using a Novel Triazine Compound. J. Renew. Mater. 2019, 7, 57–71. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.R.; Covington, A.D.; Hancock, R.A. Use of DSC To Detect the Heterogeneity of Hydrothermal Stability in the Polyphenol-Treated Collagen Matrix. J. Agric. Food Chem. 2003, 51, 6652–6656. [Google Scholar] [CrossRef]
- Zhang, Y.; Snow, T.; Smith, A.J.; Holmes, G.; Prabakar, S. A guide to high-efficiency chromium (III)-collagen cross-linking: Synchrotron SAXS and DSC study. Int. J. Biol. Macromol. 2019, 126, 123–129. [Google Scholar] [CrossRef]
- Örk, N.; Özgünay, H.; Mutlu, M.M.; Öndoğan, Z. Comparative determination of physical and fastness properties of garment leathers tanned with various tanning materials for leather skirt production. Tekstil Ve Konfeksiyon 2014, 24, 413–418. [Google Scholar]



| Entry | Solvent | Coupling Agent (a) | Yield (%) (b) (15 min/60 min) |
|---|---|---|---|
| 1 | methanol | CDET/NMM | 88/91 |
| 2 | ethanol | CDET/NMM | 86/89 |
| 3 | propan-2-ol | CDET/NMM | 79/86 |
| 4 | H2O | CDET/NMM | 42/50 (c) |
| 5 | H2O/Acetone (90/10) | CDET/NMM | 88/90 |
| Entry | Coupling Agent (a) | Counter Anion | Yield (%) (b) (15 min/60 min) |
|---|---|---|---|
| 1 | CDET/NMM | Cl− | 88/91 |
| 2 | CDET/NMM | ClO4− | 57/69 |
| 3 | CDET/NMM | BF4− | 76/78 |
| 4 | CDET/NMP | Cl− | 76/85 |
| 5 | CDET/NMP | ClO4− | 57/59 |
| 6 | CDET/NMP | BF4− | 62/66 |
| 7 | CDET/MPD | Cl− | 79/90 |
| 8 | CDET/MPD | ClO4− | 66/68 |
| 9 | CDET/TMA | Cl− | 83/84 |
| 10 | CDET/TMA | ClO4− | 72/87 |
| 11 | CDET/NNDP | Cl− | 64/69 |
| 12 | CDET/NNDP | ClO4− | 51/70 |
| 13 | DETMM | ClO4− | 71/80 |
| 14 | DETMM | BF4− | 71/73 |
| Entry | Coupling Agent (a) | Ts (°C) (a) |
|---|---|---|
| 1 | CDET/NMM | 85 |
| 2 | CDET/MPD | 83 |
| 3 | CDET/TMA | 90 |
| Entry | Hide (kg) | CDET/NMM (a) | Water (kg) | t (h) | Ts (°C) (b) |
|---|---|---|---|---|---|
| 1 | 7.5 | 3.1/1.9 | 7.5 | 4.0 | 77 |
| 2 | 13 | 3.1/1.9 | 10 | 18.0 | 80 |
| 3 | 10 | 4.5/2.7 | 10 | 2.0 | 82 |
| 4 | 12 | 4.0/2.4 | 12 | 2.5 | 80 |
| Test | CDET/NMM (a) | Chrome (b) |
|---|---|---|
| Tensile strength (N/mm2) (c,d) | 20.0 ± 0.5 | 25.0 ± 0.8 |
| Tear strength (N/mm) (c,e) | 36.5 ± 0.3 | 40.0 ± 0.5 |
| Elongation (%) (c,d) | 44.5 ± 1.0 | 46.0 ± 0.8 |
| Resistance to grain cracking (N/mm2) (c,f) | 9.3 ± 0.2 | 10.0 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatto, V.; Conca, S.; Bardella, N.; Beghetto, V. Efficient Triazine Derivatives for Collagenous Materials Stabilization. Materials 2021, 14, 3069. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14113069
Gatto V, Conca S, Bardella N, Beghetto V. Efficient Triazine Derivatives for Collagenous Materials Stabilization. Materials. 2021; 14(11):3069. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14113069
Chicago/Turabian StyleGatto, Vanessa, Silvia Conca, Noemi Bardella, and Valentina Beghetto. 2021. "Efficient Triazine Derivatives for Collagenous Materials Stabilization" Materials 14, no. 11: 3069. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14113069