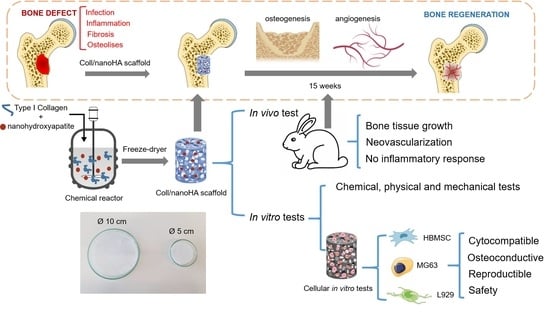
1. Introduction
The extracellular matrix (ECM) is very important for cells’ microenvironments and survival. It not only gives structural support to cells and tissues but also provides signaling cues that regulate cells’ behaviour in multicellular organisms, such as cell growth, differentiation, shape and viability [
1]. It is composed of structural proteins (i.e., collagen), polysaccharides/glycosaminoglycans (GAGs), and adhesion proteins, such as integrins, which make the connection between the ECM and the cells. These constituents are constantly being synthesized, secreted and exchanged between the cells and the ECM. In bone, the extracellular matrix is a key part of the tissues’ structure and function. It is more rigid than the ECM that is present in other tissues because of tissue mineralization, which is the deposition of calcium phosphate crystals, corresponding to the inorganic phase of bone [
2]. Bone loss due to congenital defects, trauma, accident or infections, or after tumour resection are major clinical problems that need to be addressed [
3,
4]. The use of bone grafts is the most common procedure to treat these clinical problems, though there is the possibility of infection and implant rejection, as well as donor site morbidity. In addition, they are associated with high costs [
3,
5]. In order to try to avoid these major disadvantages, alternative materials need to be found that are able to mimic the mechanical properties, structure and functions of the bone tissue [
6]. To this end, 3D scaffolds are widely used in bone regeneration to promote tissue growth by mimicking the ECM of the native tissues [
7,
8,
9]. Ideal scaffolds should be biocompatible, biodegradable and non-immunogenic, should promote cell and material surface interactions to allow adhesion, should provide the diffusion of nutrients and other molecules through their structures, and should have suitable mechanical properties, depending on their final application. Thus, an adequate biomaterial for bone regeneration applications needs to be osteoinductive, osteoconductive and osteogenic [
10]. To this end, 3D scaffolds can have different shapes and sizes, different fabrication techniques and different composition. Nowadays, some of the most common scaffolds are electrospinning fibers, decellularized tissues, microspheres, ceramics, hydrogels and cryogels. This last form of scaffold, with a composition of collagen and nanohydroxyapatite similar to the bone, was the one chosen in our work to be applied as a medical device in bone regeneration. The objective of this work was to perform and evaluate a scaled-up production process of a porous scaffold based on collagen and nanohydroxyapatite, which promotes bone regeneration and works as a barrier for both fibrosis and the proliferation of scar tissue. The scaffold was characterized in terms of its chemical, physical and mechanical properties, and showed similar characteristics to the native bone ECM. We also observed the in vitro and in vivo cell/tissue behavior within the different samples, and they showed biocompatibility, high cellular viability, proliferation and osteogenic differentiation, which is an advantage for application as a medical device in bone regeneration.
2. Materials and Methods
2.1. Materials
Type I collagen from bovine Achilles tendon (Sigma-Aldrich, St. Louis, MO, USA), Viscolma collagen suspension dissolved at 12% (Viscofan BioEngineering, Weinheim, Germany), nanohydroxyapatite (nanoXIM) aggregates (Fluidinova S.A., Maia, Portugal), 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) (Fluka, Buchs, Switzerland), hydrochloric acid (HCl) (Merck KGaA, Darmstadt, Germany), Vancomycin (50 mg/mL, HIKMA Farmacêutica, S.A., Sintra, Portugal) and Gentamicin (40 mg/mL, Labesfal—Laboratórios Almiro, S.A., Portugal), were kindly provided by Artur Salgado S.A. (Maia, Portugal).
Alamar blue dye (resazurin), magnesium chloride (MgCl2), 4′-6-diamidine-2-phenylindole (DAPI), formaldehyde 4% and Triton X100 were purchased from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO, USA); the dimethyl sulfoxide (DMSO) was obtained from Merck (Merck KGaA, Darmstadt, Germany). Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS), fungizone, penicillin-streptomycin and trypsin were purchased from Gibco (Thermo Fisher Scientific, Waltham, MA, USA). The DC™ protein assay was purchased from Bio-Rad. The Alexa fluorconjugated phalloidin 594 and the Quant-iT™ Picogreen® DNA assay kit were purchased from Invitrogen (Thermo Fisher Scientific, Waltham, MA, USA).
2.2. Preparation of the Collagen-NanoHA Scaffolds
For batch A, Achilles tendon bovine collagen type I was dissolved using a diluted solution of HCl (10 mM) and kept at 4 °C. For batch B, the 12% Viscolma collagen suspension was used. In order to remove all of the lumps from the solutions, they were homogenized at 20,000 rpm (Ultra Turrax T25, IKA) at 4 °C for about 1 h and 30 min. In order to produce the samples, the collagen solution was mixed using a peristaltic pump, first with a HCl and nanohydroxyapatite solution, and after homogenization with a HCl, EDC (40 mM) and NHS (20 mM) solution. The collagen and nanoHA were in 50:50 % w/w proportions. The resultant solution filled two sizes of glass molds (5 and 10 cm of diameter) and was kept in the freezer for 24 h. Finally, the samples were dried using a freeze-drier (Labconco) for 24 h (−80 °C). Some samples were submitted to sterilization through e-beam irradiation (15 kGy). Eight different materials were studied, before (A and B) and after (A’ and B’) their e-beam irradiation, with 5 and 10 cm diameters.
2.3. Characterization of the Cryogels
2.3.1. Scanning Electron Microscope Analysis
A scanning electron microscope (SEM, FEI Quanta 400FEG, Hillsboro, ON, USA) was used to analyse the morphology of the samples. The samples were first attached with Araldite™ to an aluminium sample holder and then sputter-coated with palladium-gold (Bal-Tec: SCD 050) to become electrically conductive and be analysed.
2.3.2. Swelling Capacity Test
The swelling capacity test was carried out at room temperature to evaluate the ability of the scaffolds to capture and retain a solution. Samples with a cubic shape were submerged both in distilled water and aqueous phosphate-buffered saline (PBS). The study was carried out over 1 h, and the samples were weighed at the beginning of it and after each time-point. The equilibrium of the absorption—the swelling equilibrium (
Cw)—was calculated using the formula
where
Ws corresponds to the weight of the swollen sample after the immersion, and
Wd corresponds to the dry weight (before the immersion in water or PBS). Three samples of each type of scaffold were used, and an average was calculated and used to obtain a variation.
2.3.3. Dynamical Mechanical Analysis
The dynamical mechanical analysis (DMA) assay was carried out in order to evaluate the mechanical properties of the samples under compression, and was conducted in a Tritec2000 dynamic mechanical analyser (Triton Technology Ltd., Nottinghamshire, UK). Samples of parallelepiped form with 5 mm thickness and around 10 mm length were cut and submerged in 10 mL water for 10 min. The scaffolds were subjected to cycles of compression with frequencies varying between 0.1 Hz and 15 Hz at room temperature. The sample modulus was calculated automatically by Triton software with the sample stiffness (N/m)/geometry factor (calculated from each sample dimensions) at each frequency. An average of 5 cycles of three independent materials (same batch) were considered for the graphic plot.
2.3.4. Fourier Transform Infrared Spectroscopy
The cryogels were analysed using Fourier transform infrared (FT-IR) spectrophotometer (Perkin Elmer) in order to study their chemical composition. The analysis was carried out in the ATR mode, by compressing the samples until a clear spectrum was shown in the screen and the transmittance peaks were then registered. Every sample was analysed with a resolution of 4 cm−1 and an OPD of 0.2. In total, 100 scans were performed to obtain each graphic.
2.3.5. In Vitro Biodegradation Analysis
The biodegradation analysis evaluated the effect of exposing different samples to simulated body fluid (prepared according to Kokubo and Takadama, 2006) [
11] at 37 °C, with 200 rpm agitation for 7, 14 and 28 days. The samples were incubated in polyethylene tubes with SBF at a ratio of 1 g material per 10 mL solution, changing the solution twice a week. The biodegradation of the samples with and without sterilization was measured by weighing the sample before and after incubation in SBF.
2.3.6. Vancomycin and Gentamicin Loading and Release
Vancomycin and Gentamicin were adsorbed on different Coll/nanoHA samples (A, B, A’ and B’) for 2 h at 37 °C and 120 rpm in the orbital shaker (KS 4000 IC control, IKA®). The samples were incubated with vancomycin and gentamicin aqueous solution. Afterwards, both solutions were removed, and every 24 h for 10 days, 200 µL of the solution was removed to determine the concentration of vancomycin or gentamicin released from the different samples, which was then replaced with 200 µL fresh PBS. The removed supernatant solution was centrifuged at 14,000 rpm for 5 min (Heraeus Fresco 21 Centrifuge, Thermo Scientific™). The vancomycin and gentamicin concentration was determined by molecular absorption spectrophotometry at 280 nm using a UV–Vis Spectrophotometer (NanoDrop® ND–1000, Thermofisher Scientific, Waltham, MA, USA) and the obtained standard calibration (Vancomycin—50 to 0.005 mg/mL and gentamicin—40 to 0.004 mg/mL). All of the tests were performed in triplicate for each type of sample; 100% vancomycin release refers to the 50 mg/mL solution, and 100% gentamicin release refers to the 40 mg/mL solution.
2.4. In Vitro Biological Studies
2.4.1. Cell Culture
The L929 (ATCC), MG63 (ATCC) and HBMSC (human bone marrow stromal cells—Hospital São João, Portugal) cells were maintained in Dulbecco’s modified eagle medium (DMEM, Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS) (Gibco), 1% (v/v) fungizone and 1% (v/v) penicillin-streptomycin (Gibco). The cells were kept in a cell culture incubator (Binder, Tuttlingen, Germany) at 37 °C and 5% carbon dioxide (CO2), in a humidified atmosphere. After a cell confluence of 90% on a 75 cm2 T-flask (Nunc), which occurred every 3–4 days, the cells were detached using trypsin (0.5%, Gibco) as the dissociation reagent to be seeded on the cell culture plates or within the scaffolds.
2.4.2. Cellular Metabolic Activity
The resazurin (Alamar blue dye) assay was performed to study the direct cytotoxicity of the samples when they were in contact with MG63 and L929 cells, through its metabolic activity. The samples were sterilized as described before and incubated with complete DMEM for 30 min at room temperature in 24-well plates (no tissue culture). The medium was removed and 20 μL of the cells (3 × 105 cells) was added to each sample. The plates were incubated for 2 h at 37 °C and the samples were then covered with complete cell culture medium. The samples were incubated for 1, 7, 14 and 21 days, and the medium was changed every three days. After the removal of the medium, 1 mL of a solution of 10% resazurin (0.1 mg/mL, Sigma-Aldrich) was added and protected from the light, and the plates were incubated for 3 h at 37 °C. A solution of 100 μL of each well was transferred to a black 96-well plate (triplicates) and the fluorescent intensity was measured in a fluorometer (Synergy Mx, BioTek) at 530 nm for excitation and at 590 nm for emission. After that, the samples were washed twice with PBS and re-incubated with fresh complete cell culture medium. The seeding of L929 and MG63 cells in Tissue Culture Polystyrene (TCPS) with supplemented DMEM was also performed to analyse the cells’ metabolic activity in the absence of the collagen-nanoHA scaffolds.
2.4.3. Alkaline Phosphatase (ALP) Activity
After the resazurin assay, samples with MG63 and HBMSC cells from day 14 were washed twice with PBS and incubated with milli-Q water for 1 h at 37 °C, and then freeze at −80 °C for 1 h. After that, the samples were cut into small pieces, homogenized in the vortex for 1 min and centrifuged (Centrifuge 2-16PK, Sigma) at 2000 rpm for 5 min. The supernatant (20 μL) was then transferred to a 96-well flat-bottom plate (triplicates), and 200 μL ALP substrate was added according to protocol published before [
12]. The absorbance was measured in a microplate reader (Synergy Mx, BioTeK) at the wavelength of 405 nm, and the p-nitrophenol was quantified. In order to correlate the amount of ALP to the total quantity of protein present in the samples, the total protein content was measured using Lowry’s method, according to the manufacture’s recommendations (DC™ protein assay, Bio-Rad). Finally, the amount of ALP was calculated using the equations of both the calibration curves referred to before, and it was expressed in nmol per minute per mg of protein.
2.4.4. DNA Quantification Assay
Samples from the HBMSC culture after 7, 14 and 241 days were processed as described in the previous assay, and the supernatant was used to quantify the amount of DNA present in the samples, according to the manufacture’s recommendations (Quant-iT™ Picogreen® DNA assay, Invitrogen, UK). Briefly, a suspension of each sample (10 μL) was pipetted into a black 96-well flat-bottom plate (triplicates), as well as a blank composed of milli-Q water, and 90 μL TE buffer (1×) was added in triplicate. High-range standard solutions (1 ng/mL to 1 μg/mL) were prepared from a cDNA stock solution (2 μg/mL). Each standard solution (100 μL) was pipetted in triplicates to the same plate of the samples. Afterwards, 100 μL PicoGreen reagent was added to every well (samples and standards) and the plate was incubated for 5 min whilst protecting it from the light. The fluorescent intensity was measured in a fluorimeter (Synergy Mx, BioTek) with an excitation wavelength of 480 nm and an emission of 520 nm.
2.4.5. Confocal Laser Scanning Microscopy
Confocal laser scanning microscopy (CLSM, Leica SP2 AOBS SE camera) was used to study the morphology of the MG63 cells after 14 days of being incubated with the samples before and after e-beam irradiation. The samples were incubated in paraformaldehyde 4% (Sigma-Aldrich) for 30 min at room temperature. After that, the materials were incubated with Triton X100 solution (0.1%, Sigma-Aldrich) for 30 min at room temperature and washed twice with PBS. The samples were then incubated for 30 min. The cells’ cytoplasm (phalloidin) was stained with Alexa fluor conjugated phalloidin 594 (1:400, Invitrogen) and the nuclei were stained with DAPI (4′-6-diamidine-2-phenylindole, 1 µg/mL, Sigma-Aldrich) for 5 min. The samples were washed twice in PBS evaluated by confocal laser microscopy. The images were captured using excitation lasers of 405 nm and 594 nm.
2.5. In Vivo Biological Studies
2.5.1. Animal Model Protocol
E-beam-sterilized collagen/nanohydroxyapatite (#A’) prototype was implanted in the tibia of 14 male, 13-week-old rabbits (UTAD, Portugal). The study was carried out in accordance with the Animal Studies Ethics Committee and fulfilled all of the legal requirements (approved by the Animal Welfare and Ethics Body, UTAD, Portugal and Direção Geral de Alimentação e Veterinaria (DGAV) (approval 010532/2018). The surgical procedures were performed under standard aseptic conditions. One bone defect was created with a 6 mm diameter for a study implant (Coll/ nanoHA membrane, #A’). The rabbits were sacrificed 5 and 15 weeks after the prototype implantation, and the proximal tibia was collected for analysis.
2.5.2. Histological Analysis
All of the samples were removed and fixed in 10% neutralized buffered formalin for 3 days, decalcified after microCT analysis with 10% formic acid, and then processed for histology. The processed samples were embedded in paraffin and sectioned longitudinally. The slides were stained with hematoxylin-eosin (H&E).
2.6. Statistical Analysis
The data from the in vitro assays with cell cultures was presented as the mean ± standard deviation, and was analysed using a two-way ANOVA test. The differences between the samples were considered statistically significant when p < 0.05.
4. Discussion
The main tasks of the work included the physical, chemical, mechanical and biological characterization of the scaffolds produced on a large scale as industry prototypes. In the SEM analysis (
Figure 1) showed ceramic nanoparticles aggregates within the scaffolds, probably due to problems in the homogenization step of the ceramic powder during the production of the cryogels. These aggregates are similar to the ones observed in the nanoHA granules studied by Laranjeira et al. [
14]. The pores observed in all of the samples were very heterogeneous, with sizes varying from 50 µm to 900 µm, as measured by imaging software (Image J), which gave the materials’ both microporosity and macroporosity. This was an important characteristic, as pores with sizes larger than 100 µm should be essential for cell seeding and tissue ingrowth, whereas pores with diameters higher than 140 µm are important to promote angiogenesis [
15,
16,
17,
18,
19,
20]. From the results of both tables, it is possible to observe that the non-irradiated scaffolds presented larger pore sizes. E-beam irradiation have an important impact in synthetic polymer degradation, inducing chain scission, but in this work, it does not have the same effect on the natural polymer crosslinking, so it should not have an effect on the pore sizes [
21]. Regarding the comparison between the scaffolds with Ø 10 cm and Ø 5 cm, no correlation could be established, as the irradiated samples showed larger pores and, in contrast, the non-irradiated samples showed smaller pores. In previous studies, scaffolds of collagen/nanoHA 50:50%
w/w produced by Rodrigues and collaborators also showed a heteroporous morphology, with pores of an average size of 74.39 ± 49.05 µm, a maximum size of 322.09 µm and a minimum of 12.16 µm [
12]. Collagen-nanoHA (50:50%
w/w) scaffolds produced by Sionkowska et al. also showed a heteroporous morphology, with pore sizes varying from 50 to 150 µm [
22].
Because the values of the
Cw were maintained from minute 2 until minute 60, it is possible to conclude that the equilibrium was reached at 2 min, as a similar result was observed in Jain et al. studies [
23]. It is possible to see that the samples that showed lower values of the swelling coefficient were the ones from batch B, especially the samples with Ø 5 cm. The fastest water uptake, shown after the first 2 min of our study, is closely related to a higher interconnectivity of the pores and the hydrophilicity of the material, which is the objective of the scaffold for clinical applications, as it allows a higher diffusion of molecules, nutrients, gases and fluids throughout the material [
23]. In comparison, the hydrogels’ water uptake is a slower process, as it is dependent on the water diffusion [
23]. Furthermore, scaffolds that retain a higher amount of water are associated with the presence of larger pores [
24,
25]. This higher retention of water and PBS will result in the swelling capacity of the scaffolds, which will expand the material structure that is available for cell migration and adhesion, because of a closer contact with the surrounding tissue. However, the higher retention of the solvent can also decrease the mechanical properties of the scaffold by decreasing its elastic strength [
26]. Therefore, the objective for the final clinical application is a structure with interconnected pores that will allow an equilibrium between the swelling capacity and the mechanical properties, without compromising any of them. In this study, these properties correspond to the materials with lower
Cw values. The samples from batch B showed better swelling capacities with both water and PBS, before and after irradiation, closer to the results observed in previous studies, which were 18.54 (water) and 17.56 (PBS) after 15 min for collagen-nanoHA (30:70%
w/w) scaffolds. As for the collagen-nanoHA (50:50%
w/w) scaffolds, the values were higher, at 28.63 (water) and 30.04 (PBS) [
12]. The collagen used for batch B was from a different source in comparison to the other cryogel (A). In this batch, the purchased collagen was already dissolved, so the collagen stability (degradation) could have been compromised because the solution pH was very acidic (pH < 2). The differences between batches A and B could also be related to the difficulties in performing an adequate homogenization of the collagen with nanoHA in the reactor during the materials’ production. Thus, samples with a higher concentration of nanoHA are connected to a lower swelling ratio. This could be explained by the lower hydrophilicity of hydroxyapatite, and by the fact that, when combined with collagen, the calcium and phosphate of hydroxyapatite will bind to the hydrophilic groups of collagen (COOH and NH
2), resulting in a decrease in the overall hydrophilicity of the sample [
22]. Although the results were similar to previous studies [
12], the protocol could be revised and optimized in order to obtain scaffolds with lower values of
Cw and higher mechanical properties.
In the scaffolds A, B, A’ and B’, as in the materials produced by Rodrigues and co-workers, the peak of amide I appears at 1648 cm
−1 and not at 1658 cm
−1 when the collagen is not combined, probably due to the interaction of nanoHA and collagen through carbonyl groups [
27,
28].
The scaffolds before and after the sterilization process were evaluated under a dynamic compressive stress state. The DMA analysis showed the variation of the storage modulus (E’) for the samples, which refers to the elastic component of a material [
29], with respect to the frequency. The higher the storage modulus, the higher the stiffness of the material. By comparing the results from the materials before and after irradiation, it is possible to observe that samples A and B reached higher values of E’ and lower values of Tan delta in higher frequencies. These discrepancies between all of the material batches may be a result of a heterogeneity of the nanoHA solution dispersion into the collagen structure. Higher concentrations of nanohydroxyapatite will lead to a decrease in the loss factor and an enhancement of the storage modulus [
12,
30], showing the higher stiffness of the material. Nevertheless, in the same batch, the results from both of the evaluated parameters (E’ and Tan delta) were also different before and after the e-beam irradiation, which could have been affected by some alteration in the collagen chains induced by energy generated by the irradiation. According to previous works, e-beam radiation induces chain polydispersity of synthetic polymer, affecting the mechanical and degradation properties, but maintaining the crosslink. This will lead to a decrease in the solubility of the polymer and its fragmentation, which can explain the differences observed in the mechanical properties of samples A, B, A’ and B’ [
31]. The increase of the E’ is related to material’s higher stiffness and mechanical resistance. Rodrigues and co-workers (2013) observed a faster increase in the E’ as the frequency increases, followed by a stabilization of the modulus value. However, their study showed higher E’ results when compared with the similar materials evaluated in this work, as shown in
Figure 2 [
12]. Studies performed by Salgado and collaborators (2016) showed an increase of the storage modulus with the enhancement of the frequency applied to the materials. Moreover, the studies also presented by Rodrigues et al. (2013) showed an enhancement of the loss factor as the frequency increased, with values varying between 0.2 and 0.8 [
12,
30]. The enhancement of the tan delta shows a more viscous and less elastic behaviour of the material; as such, the ideal material’s response would be a lower value of the loss factor. Taking this into consideration, it was shown that sample A’, with Ø 10 cm, presented higher values of E’ and lower values of tan delta. Through the analysis of the results, it was also possible to observe that the larger scaffolds (Ø 10 cm) presented higher values of E’ and lower values of tan delta, and therefore the material could be used for the proposed clinical application.
The resazurin assay is a test used to infer the cell viability through its metabolic activity. Resazurin is a non-fluorescent dye that is reduced in living cells by mitochondrial enzymes to resorufin, a fluorescent dye [
32]. Studies carried out by Laranjeira et al. (2010), showed a cell viability of 100% for MG63 cells cultured for 3 days on nanoHA aggregates, which were higher cell viability values compared to the results shown in this work. After 6 days of incubation, there was as increase in cell viability [
14]. The higher results should be related to the material’s composition, because in these studies, unlike ours, the nanoHA was sintered and not dispersed, which is more stable and has lower degradation rate. The fibroblasts (L929) showed higher metabolic activity in all of the tested materials during the evaluated period of time, and showed similar values for both the irradiated and non-irradiated samples. However, the osteoblast-like cells (MG63) viability was mainly enhanced in the samples that undergo e-beam irradiation. This should be associated to the irradiation process, in which the energy produced by the sterilization method could induce some chemical alterations in the polymer chain’s surface, and could affect the cellular response.
The different cell behaviour (MG63) found between the different samples (A and B) should be due to a different topography of the cryogels with higher nanoHA aggregates on the surface (as observed in the SEM images—
Figure 1). Rougher surfaces promote a higher proliferation rate of osteoblast-like cells when compared to smoother ones [
33,
34,
35,
36]. In the studies performed by Rodrigues and co-workers, cryogels of collagen/nanoHA 50:50 %
w/
w cultured with MG63 cells showed a similar cell density over the material’s surface (Confocal microscopy images–
Figure 7) [
12]. Therefore, considering the similarity of the published results and the figures shown in this work, we can observe a high influence of the material characteristics on MG63 cell behaviour, favoring the cellular proliferation rate. The ALP activity results for the MG63 culture (
Figure 5) showed higher enzyme activity when compared to the values in studies conducted with similar biomaterials [
12,
14]. This higher activity could be induced by a higher nanoHA content of the B’ samples, because this ceramic had been demonstrated to have an influence on the enhancement of ALP activity of MG63 cells [
37,
38]. The ALP had a lower activity in sample A, probably due to the fact that the cells reached a maximum confluence [
39,
40], which might have occurred at day 14. In the results observed in similar studies, the values for the cells’ ALP activity cultured in scaffolds of collagen/nanoHA 50:50 %
w/
w were 0.35 nmol/min/µg for a cell culture time of 14 days [
12]. Previous studies by Laranjeira et al. (2010) showed an increase in the ALP activity from day 3 to day 6 for the cells seeded in nanoHA granules [
14].
The HBMSC proliferation rate was estimated by the quantification of DNA, and it was possible to observe that all of the evaluated scaffolds promoted a high proliferation rate until day 21. In addition, the total DNA content at 14 days of culture in samples A’ and B’ was significantly higher when compared to day 7, and remained with a slightly higher concentration after 21 days. Therefore, in these in vitro tests with HBMSC, scaffolds A, B and B’ exhibited increasing levels of differentiation by higher ALP activity and bone morphogenetic protein 2 (BMP-2) gene expression levels. Indeed, we observed an increasing of cellular proliferation (
Figure 9A) over the studied period; this behaviour was a positive result that should end up to a normal tissue growth for the proposed clinical application.
After 5 weeks, Coll/nanoHA scaffolds was removed from the rabbit tibia, the histological evaluation was performed and showed that the materials’ structure was partially present. The maintenance of the composite structure should be related to the chemical bonding of the collagen fibrils allowed by the crosslinking step (EDC/NHS). The histological findings indicate that Coll/nanoHA supported tissue ingrowth and new micro-vascularization. Importantly, in the context of bone ingrowth, the presence of connective tissue that replaces the hematoma during the early stages of tissue repair will favor the intramembranous ossification enhancing the bone repair. Previous studies showed that the biocomposite scaffold cell-binding domains could promote the in vivo migration and proliferation [
12,
30] of animal fibroblasts, an important cell population that will promote host cell migration and proliferation. Increased tissue growth was observed for the bone implants, similarly to
the in vitro analysis of the MG63 and HBMSC cells (
Figure 6A,B and
Figure 9A). The total bone ingrowth area was calculated by the total volume fraction (microCT) after 5 and 15 weeks (
Figure 10G). The results show that the A’ scaffolds induced the continuous growth of connective tissue within the porous structure after 5 weeks, along with new bone growth at the defect border. However, after 15 weeks, the defect area was almost filled with disorganized new-bone tissue, replacing the total scaffold area (material’s biodegradation) (
Figure 10E,F).