Design of Self-Supported Flexible Nanostars MFe-LDH@ Carbon Xerogel-Modified Electrode for Methanol Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Carbon Xerogels (CX)
2.3. Preparation of Ni-Fe LDH Nanosheet Array on NF Substrate
2.4. Preparation of Ni-Fe LDH Nanosheet Array/CX on NF Substrate
2.5. Characterization
2.6. Electrochemical Measurements
3. Results and Discussions
3.1. Physicochemical Properties
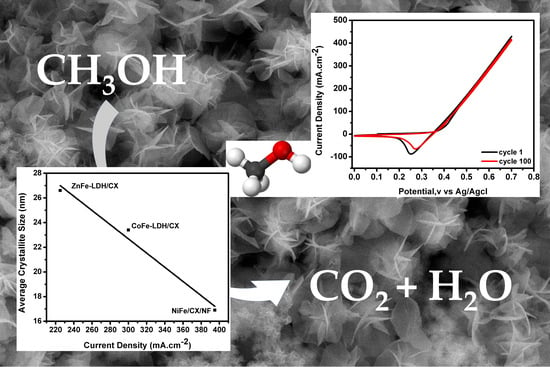
3.2. Electrochemical Activity for Methanol Oxidation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, Z.; Wang, Y.; Qi, T. Hierarchical Ni–Fe layered double hydroxide/MnO2 sphere architecture as an efficient noble metal-free electrocatalyst for ethanol electro-oxidation in alkaline solution. RSC Adv. 2015, 5, 83314–83319. [Google Scholar] [CrossRef]
- Carrette, L.; Friedrich, K.A.; Stimming, U. Fuel cells: Principles, types, fuels, and applications. ChemPhysChem 2000, 1, 162–193. [Google Scholar] [CrossRef]
- Dong, B.; Li, W.; Huang, X.; Ali, Z.; Zhang, T.; Yang, Z.; Hou, Y. Fabrication of hierarchical hollow Mn doped Ni(OH)2 nanostructures with enhanced catalytic activity towards electrochemical oxidation of methanol. Nano Energy 2019, 55, 37–41. [Google Scholar] [CrossRef]
- Tsang, A.C.H.; Hui, K.S.; Ren, L. Deposition of Pd/graphene aerogel on nickel foam as a binder-free electrode for direct electro-oxidation of methanol and ethanol. J. Mater. Chem. A 2014, 2, 17986–17993. [Google Scholar] [CrossRef]
- Sheikh-Mohseni, M.A.; Hassanzadeh, V.; Habibi, B. Reduced graphene oxide supported bimetallic Ni–Co nanoparticles composite as an electrocatalyst for oxidation of methanol. Solid State Sci. 2019, 98, 106022. [Google Scholar] [CrossRef]
- Tu, W.; Sun, Y.; Wu, D.; Wang, H.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Cobalt oxyhydroxide and carbon dots modified by platinum as superior electrocatalyst for methanol oxidation. Mater. Chem. Phys. 2019, 225, 64–71. [Google Scholar] [CrossRef]
- Askari, M.B.; Beheshti-Marnani, A.; Seifi, M.; Rozati, S.M.; Salarizadeh, P. Fe3O4@MoS2/RGO as an effective nano-electrocatalyst toward electrochemical hydrogen evolution reaction and methanol oxidation in two settings for fuel cell application. J. Colloid Interface Sci. 2019, 537, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lin, Y.; Fan, J.; Lv, D.; Min, Y.; Wu, T.; Xu, Q. Well-dispersed palladium nanoparticles on three-dimensional hollow N-doped graphene frameworks for enhancement of methanol electro-oxidation. Electrochem. Commun. 2016, 73, 75–79. [Google Scholar] [CrossRef]
- Chu, D.; Jiang, R. Novel electrocatalysts for direct methanol fuel cells. Solid State Ionics 2002, 148, 591–599. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Xu, K.Q.; Xia, J.; Liu, Q.; Wang, Z. Highly dispersed ultrafine Pt nanoparticles on nickel-cobalt layered double hydroxide nanoarray for enhanced electrocatalytic methanol oxidation. Int. J. Hydrog. Energy 2018, 43, 16302–16310. [Google Scholar] [CrossRef]
- Cao, J.; Chen, H.; Zhang, X.; Zhang, Y.; Liu, X. Graphene-supported platinum/nickel phosphide electrocatalyst with improved activity and stability for methanol oxidation. RSC Adv. 2018, 8, 8228–8232. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Liang, T.; Zhang, D.; Zhang, M.; Lin, Y.; Lai, C. Facile fabrication of 3D porous nickel networks for electro-oxidation of methanol and ethanol in alkaline medium. Mater. Chem. Phys. 2019, 221, 390–396. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Y.; Qi, T. Pd nanoparticles supported on Mg–Al–CO3 layered double hydroxide as an effective catalyst for methanol electro-oxidation. RSC Adv. 2015, 5, 62142–62148. [Google Scholar] [CrossRef]
- Sreekanth, T.; Ramaraghavulu, R.; Vattikuti, S.P.; Shim, J.; Yoo, K. Microwave synthesis: ZnCo2O4 NPs as an efficient electrocatalyst in the methanol oxidation reaction. Mater. Lett. 2019, 253, 450–453. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Y.; Wang, X.; Zeng, L.; Luo, F.; Liu, A. Amorphous nickel coating on carbon nanotubes supported Pt nanoparticles as a highly durable and active electrocatalyst for methanol oxidation reaction. J. Electroanal. Chem. 2020, 856, 113739. [Google Scholar] [CrossRef]
- Sun, H.; Liu, J.; Zhang, C.; Yuan, Q.; Ye, Y.; Yan, W.; Tian, Z.; Liang, C. S,N dual-doped carbon nanotubes as substrate to enhance the methanol oxidation performance of NiO nanoparticles. Carbon 2019, 152, 114–119. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Wang, W.; Cao, D. Recent Progress in MOF-Derived, Heteroatom-Doped Porous Carbons as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction in Fuel Cells. Adv. Funct. Mater. 2018, 28, 1704537. [Google Scholar] [CrossRef]
- Vlamidis, Y.; Fiorilli, S.L.; Giorgetti, M.; Gualandi, I.; Scavetta, E.; Tonelli, D. Role of Fe in the oxidation of methanol electrocatalyzed by Ni based layered double hydroxides: X-ray spectroscopic and electrochemical studies. RSC Adv. 2016, 6, 110976–110985. [Google Scholar] [CrossRef]
- Long, X.; Wang, Z.; Xiao, S.; An, Y.; Yang, S. Transition metal based layered double hydroxides tailored for energy conversion and storage. Mater. Today 2016, 19, 213–226. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, A.; Li, L.; Ai, L. Nickel–cobalt layered double hydroxide nanosheets as high-performance electrocatalyst for oxygen evolution reaction. J. Power Sources 2015, 278, 445–451. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, H.; Peng, W.; Liu, L.; Gao, F.; Li, M. Gold nanoparticle-coated Ni/Al layered double hydroxides on glassy carbon electrode for enhanced methanol electro-oxidation. Int. J. Hydrog. Energy 2012, 37, 9324–9329. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Xie, W.; Han, X.; Feng, Q.; Jiang, R.; Shang, H.; Zhang, F.; Gao, L.; Wang, Z. Construction of Highly Active Pt/Ni-Fe Layered Double Hydroxide Electrocatalyst towards Methanol Oxidation in Alkaline Medium. Int. J. Electrochem. Sci. 2019, 14, 7961–7972. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Peng, W.; Liu, L.; Li, M. Electrocatalytic oxidation of methanol at Ni–Al layered double hydroxide film modified electrode in alkaline medium. Electrochim. Acta 2011, 56, 5754–5758. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Tang, M.; Xu, S.; Li, M. Electrocatalysis of gold nanoparticles/layered double hydroxides nanocomposites toward methanol electro-oxidation in alkaline medium. Electrochim. Acta 2010, 55, 4045–4049. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Kaneti, Y.V.; Guo, Y.; Yuliarto, B.; Jiang, X.; Ide, Y.; Nugraha, N.; Dipojono, H.K.; Yu, A.; Sugahara, Y.; et al. Holey Assembly of Two-Dimensional Iron-Doped Nickel-Cobalt Layered Double Hydroxide Nanosheets for Energy Conversion Application. ChemSusChem 2020, 13, 1645–1655. [Google Scholar] [CrossRef]
- Shen, J.; Ye, S.; Xu, X.; Liang, J.; He, G.; Chen, H. Reduced graphene oxide based NiCo layered double hydroxide nanocomposites: An efficient catalyst for epoxidation of styrene. Inorg. Chem. Commun. 2019, 104, 219–222. [Google Scholar] [CrossRef] [Green Version]
- Gamil, S.; Antuch, M.; Zedan, I.T.; El Rouby, W.M.A. 3D NiCr-layered double hydroxide/reduced graphene oxide sand rose-like structure as bifunctional electrocatalyst for methanol oxidation. Colloids Surf. A Physicochem. Eng. Aspects 2020, 602, 125067. [Google Scholar] [CrossRef]
- Park, C.; Lee, J.-H.; Seo, J.; Ran, W.; Whang, D.; Hwang, S.; Kim, Y.-J. Graphene/PVDF Composites for Ni-rich Oxide Cathodes toward High-Energy Density Li-ion Batteries. Materials 2021, 14, 2271. [Google Scholar] [CrossRef]
- Kamedulski, P.; Lukaszewicz, J.; Witczak, L.; Szroeder, P.; Ziolkowski, P. The Importance of Structural Factors for the Electrochemical Performance of Graphene/Carbon Nanotube/Melamine Powders towards the Catalytic Activity of Oxygen Reduction Reaction. Materials 2021, 14, 2448. [Google Scholar] [CrossRef]
- Chae, G.; Youn, D.; Lee, J. Nanostructured Iron Sulfide/N, S Dual-Doped Carbon Nanotube-Graphene Composites as Efficient Electrocatalysts for Oxygen Reduction Reaction. Materials 2021, 14, 2146. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Z.; Wang, H.; Wang, Y. Toward efficient single-atom catalysts for renewable fuels and chemicals production from biomass and CO2. Appl. Catal. B Environ. 2021, 292, 120162. [Google Scholar] [CrossRef]
- Pérez-Cadenas, M.; Moreno-Castilla, C.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Surface chemistry, porous texture, and morphology of N-doped carbon xerogels. Langmuir 2008, 25, 466–470. [Google Scholar] [CrossRef]
- Pekala, R.W. Organic aerogels from the polycondensation of resorcinol with formaldehyde. J. Mater. Sci. 1989, 24, 3221–3227. [Google Scholar] [CrossRef]
- Abdelwahab, A.; Castelo-Quibén, J.; Pérez-Cadenas, M.; Elmouwahidi, A.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Cobalt-Doped Carbon Gels as Electro-Catalysts for the Reduction of CO2 to Hydrocarbons. Catalysts 2017, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- El-Deeb, M.M.; El Rouby, W.M.A.; Abdelwahab, A.; Farghali, A.A. Effect of pore geometry on the electrocatalytic performance of nickel cobaltite/carbon xerogel nanocomposite for methanol oxidation. Electrochim. Acta 2018, 259, 77–85. [Google Scholar] [CrossRef]
- Li, D.; Zang, J.; Zhang, J.; Ao, K.; Wang, Q.; Dong, Q.; Wei, Q.J.M. Sol-gel synthesis of carbon xerogel-ZnO composite for detection of catechol. Materials 2016, 9, 282. [Google Scholar] [CrossRef] [Green Version]
- Wasfey, M.A.; Abdelwahab, A.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Abdullah, H.H.; Yahia, I.S.; Farghali, A.A. Nickel Cobaltite Functionalized Silver Doped Carbon Xerogels as Efficient Electrode Materials for High Performance Symmetric Supercapacitor. Materials 2020, 13, 4906. [Google Scholar] [CrossRef]
- Du, Y.Y.; Jin, Q.; Feng, J.T.; Zhang, N.; He, Y.F.; Li, D.Q. Flower-like Au/Ni–Al hydrotalcite with hierarchical pore structure as a multifunctional catalyst for catalytic oxidation of alcohol. Catal. Sci. Technol. 2015, 5, 3216–3225. [Google Scholar] [CrossRef]
- Li, Z.; Shao, M.; An, H.; Wang, Z.; Xu, S.; Wei, M.; Evans, D.G.; Duan, X. Fast electrosynthesis of Fe-containing layered double hydroxide arrays toward highly efficient electrocatalytic oxidation reactions. Chem. Sci. 2015, 6, 6624–6631. [Google Scholar] [CrossRef] [Green Version]
- Gamil, S.; El Rouby, W.M.A.; Antuch, M.; Zedan, I.T. Nanohybrid layered double hydroxide materials as efficient catalysts for methanol electrooxidation. RSC Adv. 2019, 9, 13503–13514. [Google Scholar] [CrossRef] [Green Version]
- Verlato, E.; Cattarin, S.; Comisso, N.; Gambirasi, A.; Musiani, M.; Gomez, L.V. Preparation of Pd-Modified Ni Foam Electrodes and Their Use as Anodes for the Oxidation of Alcohols in Basic Media. Electrocatalysis 2012, 3, 48–58. [Google Scholar] [CrossRef]
- Jadhav, H.S.; Roy, A.; Chung, W.-J.; Gil Seo, J. Growth of urchin-like ZnCo2O4 microspheres on nickel foam as a binder-free electrode for high-performance supercapacitor and methanol electro-oxidation. Electrochim. Acta 2017, 246, 941–950. [Google Scholar] [CrossRef]
- Yu, M.; Chen, J.; Liu, J.; Li, S.; Ma, Y.; Zhang, J.; An, J. Mesoporous NiCo2O4 nanoneedles grown on 3D graphene-nickel foam for supercapacitor and methanol electro-oxidation. Electrochim. Acta 2015, 151, 99–108. [Google Scholar] [CrossRef]
- Roy, A.; Jadhav, H.S.; Thorat, G.M.; Gil Seo, J. Electrochemical growth of Co(OH)2 nanoflakes on Ni foam for methanol electro-oxidation. New J. Chem. 2017, 41, 9546–9553. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Z.-H.; Yang, Z.; Wang, Z.; Tang, X.; Wang, T.; Fan, L.; Ooi, K. Preparation of Ni2+−Fe3+ Layered Double Hydroxide Material with High Crystallinity and Well-Defined Hexagonal Shapes. Chem. Mater. 2008, 20, 360–363. [Google Scholar] [CrossRef]
- Miao, M.; Han, X.; Jia, R.; Ma, W.; Han, G. Synthesis and Electrocatalytic Properties of Ni–Fe-Layered Double Hydroxide Nanomaterials. In Energy Technology; Wang, T., Chen, X., Guillen, D.P., Zhang, L., Sun, Z., Wang, C., Haque, N., Howarter, J.A., Neelameggham, N.R., Ikhmayies, S., et al., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 293–301. [Google Scholar]
- El-Reesh, G.Y.A.; Farghali, A.A.; Taha, M.; Mahmoud, R.K. Novel synthesis of Ni/Fe layered double hydroxides using urea and glycerol and their enhanced adsorption behavior for Cr(VI) removal. Sci. Rep. 2020, 10, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahab, A.; Castelo-Quibén, J.; Pérez-Cadenas, M.; Maldonado-Hódar, F.J.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Insight of the effect of graphitic cluster in the performance of carbon aerogels doped with nickel as electrodes for supercapacitors. Carbon 2018, 139, 888–895. [Google Scholar] [CrossRef]
- Castelo-Quibén, J.; Abdelwahab, A.; Pérez-Cadenas, M.; Morales-Torres, S.; Hódar, F.J.M.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Carbon–Iron electro-catalysts for CO2 reduction. The role of the iron particle size. J. CO2 Util. 2018, 24, 240–249. [Google Scholar] [CrossRef]
- Rennie, A.J.R.; Martins, V.L.; Smith, R.; Hall, P.J. Influence of Particle Size Distribution on the Performance of Ionic Liquid-based Electrochemical Double Layer Capacitors. Sci. Rep. 2016, 6, 22062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Guo, T.; Yin, D.; Liu, Q.; Zhang, X.; Zhang, X. A high-efficiency noble metal-free electrocatalyst of cobalt-iron layer double hydroxides nanorods coupled with graphene oxides grown on a nickel foam towards methanol electrooxidation. J. Taiwan Inst. Chem. Eng. 2020, 112, 212–221. [Google Scholar] [CrossRef]
- Li, P.; Du, C.; Gao, X.; Zhuang, Z.; Xiang, D.; Zhang, C.; Chen, W. Insights into the morphology and composition effects of one-dimensional CuPt nanostructures on the electrocatalytic activities and methanol oxidation mechanism by in situ FTIR. Nanoscale 2020, 12, 13688–13696. [Google Scholar] [CrossRef]
- Ma, K.; Cheng, J.; Liu, F.; Zhang, X. Co-Fe layered double hydroxides nanosheets vertically grown on carbon fiber cloth for electrochemical capacitors. J. Alloy. Compd. 2016, 679, 277–284. [Google Scholar] [CrossRef]
- Wang, F.; Wang, T.; Sun, S.; Xu, Y.; Yu, R.; Li, H. One-step synthesis of Nickle Iron-layered double hydroxide/reduced graphene oxide/carbon nanofibres composite as electrode materials for asymmetric supercapacitor. Sci. Rep. 2018, 8, 8908. [Google Scholar] [CrossRef] [Green Version]
- Zaher, A.; Taha, M.; Farghali, A.A.; Mahmoud, R.K. Zn/Fe LDH as a clay-like adsorbent for the removal of oxytetracycline from water: Combining experimental results and molecular simulations to understand the removal mechanism. Environ. Sci. Pollut. Res. 2020, 27, 12256–12269. [Google Scholar] [CrossRef]
- Zhang, X.; Ikram, M.; Liu, Z.; Teng, L.; Xue, J.; Wang, D.; Li, L.; Shi, K. Expanded graphite/NiAl layered double hydroxide nanowires for ultra-sensitive, ultra-low detection limits and selective NOx gas detection at room temperature. RSC Adv. 2019, 9, 8768–8777. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, With Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051. [Google Scholar] [CrossRef] [Green Version]
- Kruk, M.; Jaroniec, M. Gas Adsorption Characterization of Ordered Organic−Inorganic Nanocomposite Materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Rajeshkhanna, G.; Rao, G.R. Micro and nano-architectures of Co3O4 on Ni foam for electro-oxidation of methanol. Int. J. Hydrog. Energy 2018, 43, 4706–4715. [Google Scholar] [CrossRef]
- Zhou, X.-C.; Yang, X.-Y.; Fu, Z.-B.; Yang, Q.; Yang, X.; Tang, Y.-J.; Wang, C.-Y.; Yi, Y. Single-crystalline ultrathin nanofilms of Ni aerogel with Ni(OH)2 hybrid nanoparticles towards enhanced catalytic performance for ethanol electro-oxidation. Appl. Surf. Sci. 2019, 492, 756–764. [Google Scholar] [CrossRef]
- Sennu, P.; Aravindan, V.; Lee, Y.-S. High energy asymmetric supercapacitor with 1D@2D structured NiCo2O4@Co3O4 and jackfruit derived high surface area porous carbon. J. Power Sources 2016, 306, 248–257. [Google Scholar] [CrossRef]
- Das, S.; Dutta, K.; Kundu, P.P.; Bhattacharya, S.K. Nanostructured Polyaniline: An Efficient Support Matrix for Platinum-Ruthenium Anode Catalyst in Direct Methanol Fuel Cell. Fuel Cells 2018, 18, 369–378. [Google Scholar] [CrossRef]
- Sheikh, A.; Abd-Alftah, K.E.-A.; Malfatti, C.F. On reviewing the catalyst materials for direct alcohol fuel cells (DAFCs). Energy 2014, 1, 1–10. [Google Scholar]
- Huang, W.; Wang, H.; Zhou, J.; Wang, J.; Duchesne, P.N.; Muir, D.; Zhang, P.; Han, N.; Zhao, F.; Zeng, M.; et al. Highly active and durable methanol oxidation electrocatalyst based on the synergy of platinum–nickel hydroxide–graphene. Nat. Commun. 2015, 6, 10035. [Google Scholar] [CrossRef]
- Qian, L.; Luo, S.; Wu, L.; Hu, X.; Chen, W.; Wang, X. In situ growth of metal organic frameworks derived hierarchical hollow porous Co3O4/NiCo2O4 nanocomposites on nickel foam as self-supported flexible electrode for methanol electrocatalytic oxidation. Appl. Surf. Sci. 2020, 503, 144306. [Google Scholar] [CrossRef]
- Habibi, B.; Ghaderi, S. Electrosynthesized Ni-Al Layered Double Hydroxide-Pt Nanoparticles as an Inorganic Nanocomposite and Potentate Anodic Material for Methanol Electrooxidation in Alkaline Media. Bull. Chem. React. Eng. Catal. 2017, 12, 1. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Geng, L.; Zhang, Y.; Chang, G.; Zhang, Z.; Liu, X.; Lei, M.; He, Y. Graphene-templated synthesis of palladium nanoplates as novel electrocatalyst for direct methanol fuel cell. Appl. Surf. Sci. 2019, 466, 385–392. [Google Scholar] [CrossRef]
- Liu, H.; Song, C.; Zhang, L.; Zhang, J.; Wang, H.; Wilkinson, D.P. A review of anode catalysis in the direct methanol fuel cell. J. Power Sources 2006, 155, 95–110. [Google Scholar] [CrossRef]









| Sample Name | The Average Crystallite Size (nm) |
|---|---|
| NiFe-LDH | 15.9 |
| CoFe-LDH | 17.3 |
| ZnFe-LDH | 18.7 |
| NiFe-LDH/CX | 16.9 |
| CoFe-LDH/CX | 23.4 |
| ZnFe-LDH/CX | 26.6 |
| Sample | SBET | Pore Volume | Mean Pore Diameter |
|---|---|---|---|
| m2/g | cm3/g | nm | |
| NiFe-LDH | 98.6 | 0.07 | 2.24 |
| CoFe-LDH | 101.2 | 0.09 | 3.60 |
| ZnFe-LDH | 99.1 | 0.11 | 4.78 |
| NiFe-LDH/CX | 47.1 | 0.28 | 23.37 |
| CoFe-LDH/CX | 64.4 | 0.34 | 18.16 |
| ZnFe-LDH/CX | 68.6 | 0.32 | 15.93 |
| Electrocatalyst | Electrolyte | Applied Voltage | Current Density | Ref. |
|---|---|---|---|---|
| NiFe-LDH/CX/NF | 1.0 M KOH/0.5 M CH3OH | 0–0.7 V vs. Ag/AgCl | 400 mA·cm−2 | This work |
| 3D NiCr-LDH/rGO | 1.0 M KOH/3.0 M CH3OH | 0–0.6 V vs. Ag/AgCl | 2.2 mA·cm−2 | [27] |
| Microsphere Co3O4/NF | 1.0 M KOH/0.5 M CH3OH | 0–0.6 V vs. Hg/HgO | 36 A·g−1 | [59] |
| Co(OH)2/NF | 1.0 M KOH/0.5 M CH3OH | 0–0.5 V vs. SCE | 150 A·g−1 | [44] |
| Mn doped Ni(OH)2 | 1.0 M KOH/0.5 M CH3OH | 1–2 V vs. RHE | 20 A·g−1 | [3] |
| 3D nickel networks | 1.0 M NaOH/1.0 M CH3OH | 0–0.8 V vs. Hg/HgO | 175 mA·cm−2 | [12] |
| NiCo2O4/CX | 1.0 M KOH/0.5 M CH3OH | 0–0.6 V vs. Ag/AgCl | 98 mA·cm−2 | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelrazek, G.M.; EL-Deeb, M.M.; Farghali, A.A.; Pérez-Cadenas, A.F.; Abdelwahab, A. Design of Self-Supported Flexible Nanostars MFe-LDH@ Carbon Xerogel-Modified Electrode for Methanol Oxidation. Materials 2021, 14, 5271. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14185271
Abdelrazek GM, EL-Deeb MM, Farghali AA, Pérez-Cadenas AF, Abdelwahab A. Design of Self-Supported Flexible Nanostars MFe-LDH@ Carbon Xerogel-Modified Electrode for Methanol Oxidation. Materials. 2021; 14(18):5271. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14185271
Chicago/Turabian StyleAbdelrazek, Ghada M., Mohamed M. EL-Deeb, Ahmed A. Farghali, Agustín F. Pérez-Cadenas, and Abdalla Abdelwahab. 2021. "Design of Self-Supported Flexible Nanostars MFe-LDH@ Carbon Xerogel-Modified Electrode for Methanol Oxidation" Materials 14, no. 18: 5271. https://0-doi-org.brum.beds.ac.uk/10.3390/ma14185271