Polyphenolic Profile and Antioxidant Activity of Juglans regia L. Leaves and Husk Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Preparation of the Extracts
2.3. Measurement Methods
2.3.1. Thermal Decomposition
2.3.2. FTIR and UV-VIS Spectra
2.3.3. HPLC-PDA Analysis of Phenolic Compounds
2.3.4. Cyclic and Differential Pulse Voltammetry
2.3.5. Antioxidant Activity Properties Measured by ABTS and DPPH Methods
2.3.6. Determination of Ion Reduction—Iron by FRAP Method and Copper by CUPRAC Method
3. Results and Discussion
3.1. Characteristics of Thermal Decomposition of Plant Materials
3.2. Analysis of Polyphenolic Profile of Extracts
3.3. Analysis of Properties of Extracts
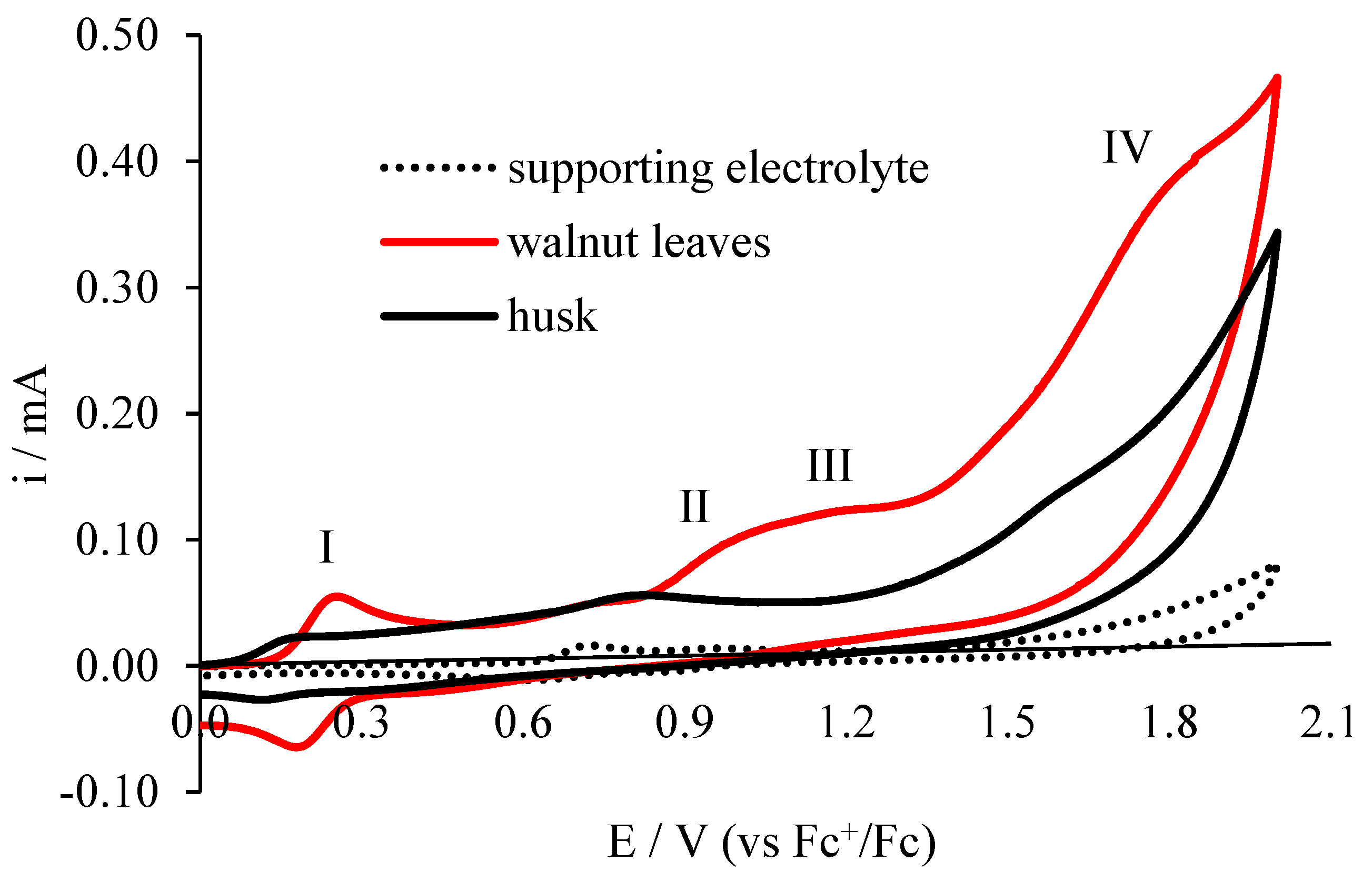
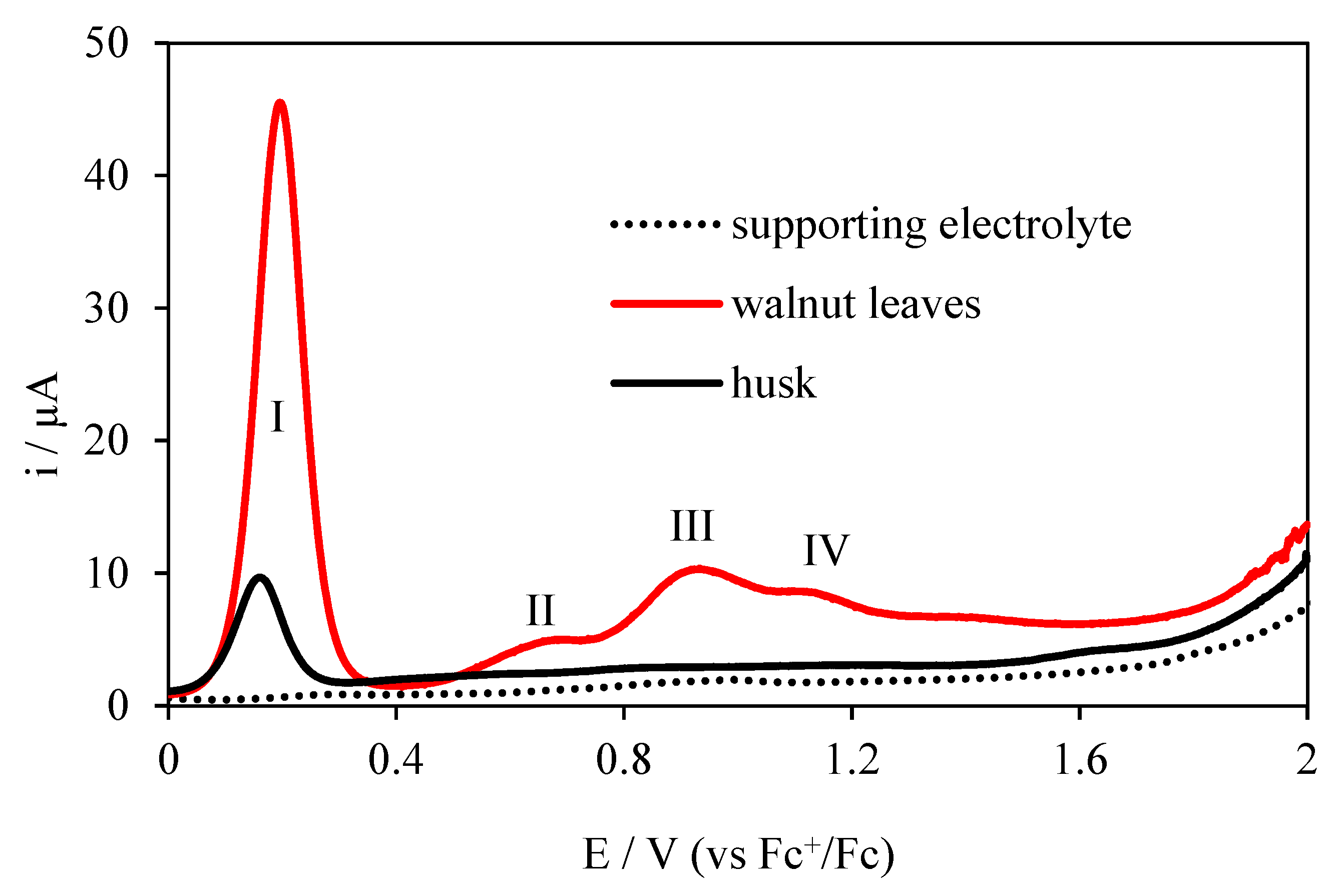
The Electrochemical Behavior of Walnut Leaves and Husk at the Pt Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Di Mauro, M.D.; Giardina, R.C.; Fava, G.; Mirabella, E.F.; Acquaviva, R.; Renis, M.; D’Antona, N. Polyphenolic profile and antioxidant activity of olive mill wastewater from two Sicilian olive cultivars: Cerasuola and Nocellara etnea. Eur. Food Res. Technol. 2017, 243, 1895–1903. [Google Scholar] [CrossRef]
- Mauro, M.D.D.; Fava, G.; Spampinato, M.; Aleo, D.; Melilli, B.; Saita, M.G.; Centonze, G.; Maggiore, R.; Antona, D.; Di Mauro, M.D.; et al. Polyphenolic Fraction from Olive Mill Wastewater: Scale-Up and in Vitro Studies for Ophthalmic Nutraceutical Applications. Antioxidants 2019, 8, 462. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acquaviva, R.; D’Angeli, F.; Malfa, G.A.; Ronsisvalle, S.; Garozzo, A.; Stivala, A.; Ragusa, S.; Nicolosi, D.; Salmeri, M.; Genovese, C. Antibacterial and anti-biofilm activities of walnut pellicle extract (Juglans regia L.) against coagulase-negative staphylococci. Nat. Prod. Res. 2019, 2019, 1–6. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos-Brozio, M.; Zaborski, M. Characteristics of juglone (5-hydroxy-1,4,-naphthoquinone) using voltammetry and spectrophotometric methods. Food Chem. 2019, 301, 125279. [Google Scholar] [CrossRef]
- Salejda, A.M.; Janiewicz, U.; Korzeniowska, M.; Kolniak-Ostek, J.; Krasnowska, G. Effect of walnut green husk addition on some quality properties of cooked sausages. LWT 2016, 65, 751–757. [Google Scholar] [CrossRef]
- Amaral, J.S.; Valentão, P.; Andrade, P.B.; Martins, R.C.; Seabra, R.M. Do Cultivar, Geographical Location and Crop Season Influence Phenolic Profile of Walnut Leaves? Molecules 2008, 13, 1321–1332. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yang, B.; Jiang, Y.; Liu, Z.; Liu, Y.; Wang, X.; Kuang, H. Studies on Cytotoxic Activity against HepG-2 Cells of Naphthoquinones from Green Walnut Husks of Juglans mandshurica Maxim. Molecules 2015, 20, 15572–15588. [Google Scholar] [CrossRef] [Green Version]
- Rusu, M.E.; Gheldiu, A.-M.; Mocan, A.; Moldovan, C.; Popa, D.-S.; Tomuta, I.; Vlase, L. Process Optimization for Improved Phenolic Compounds Recovery from Walnut (Juglans regia L.) Septum: Phytochemical Profile and Biological Activities. Molecules 2018, 23, 2814. [Google Scholar] [CrossRef]
- Liu, R.; Wu, L.; Du, Q.; Ren, J.-W.; Chen, Q.-H.; Li, D.; Mao, R.-X.; Liu, X.-R.; Li, Y. Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) and Their Anti-Fatigue Effects in Mice. Molecules 2019, 24, 45. [Google Scholar] [CrossRef]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.; Bento, A.A.; Estevinho, L.; Pereira, J.A.; Estevinho, M.L.M.F. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Ros, E.; Izquierdo-Pulido, M.; Sala-Vila, A. Beneficial effects of walnut consumption on human health role of micronutrients. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Diferent Parts of Walnut (Juglans regia L.) Fruit and Tree. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.J.; Teuber, S.S.; Gobeille, A.; Cremin, P.; Waterhouse, A.L.; Steinberg, F.M. Walnut Polyphenolics Inhibit In Vitro Human Plasma and LDL Oxidation. J. Nutr. 2001, 131, 2837–2842. [Google Scholar] [CrossRef] [PubMed]
- Lino, F.; De Sá, L.; Torres, I.; Rocha, M.; Dinis, T.; Ghedini, P.; Somerset, V.; Gil, E.; Rocha, M. Voltammetric and spectrometric determination of antioxidant capacity of selected wines. Electrochim. Acta 2014, 128, 25–31. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Escudero-Gilete, M.L.; Hernández-Hierro, J.M.; Heredia, F.J.; Hernanz, D. Cyclic voltammetry to evaluate the antioxidant potential in winemaking by-products. Talanta 2017, 165, 211–215. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos, M.; Zaborski, M. Influence of hydroxyl substitution on flavanone antioxidants properties. Food Chem. 2017, 215, 501–507. [Google Scholar] [CrossRef]
- Barros, L.; Cabrita, L.; Boas, M.V.; Carvalho, A.M.; Ferreira, I.C. Chemical, biochemical and electrochemical assays to evaluate phytochemicals and antioxidant activity of wild plants. Food Chem. 2011, 127, 1600–1608. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Kosmalska, A.; Zaborski, M. Antioxidant activity determination in Sencha and Gun Powder green tea extracts with the application of voltammetry and UV-VIS spectrophotometry. Comptes Rendus Chime 2012, 15, 424–427. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Kosmalska, A.; Zaborski, M. Characteristics of compounds in hops using cyclic voltammetry, UV–VIS, FTIR and GC–MS analysis. Food Chem. 2014, 156, 353–361. [Google Scholar] [CrossRef]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, F.C.; Raymundo-Pereira, P.A.; Janegitz, B.C.; Machado, S.A.; Fatibello-Filho, O. Nanostructured carbon black for simultaneous sensing in biological fluids. Sens. Actuators B Chem. 2016, 227, 610–618. [Google Scholar] [CrossRef]
- Rebelo, M.; Rego, R.; Ferreira, M.; Oliveira, M.C. Comparative study of the antioxidant capacity and polyphenol content of Douro wines by chemical and electrochemical methods. Food Chem. 2013, 141, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry—Principles, Methods and Applications; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Escarpa, A. Food electroanalysis: Sense and simplicity. Chem. Rec. 2012, 12, 72–91. [Google Scholar] [CrossRef]
- Duygu, D.; Baykal, T.; Acikgoz, D.; Yildiz, K. Fourier Transform Infrared (FT-IR) Spectroscopy for Biological Studies. J. Sci. 2009, 22, 117–121. [Google Scholar]
- Ashokkumar, R.; Ramaswamy, M. Phytochemical screening by FTIR spectroscopic analysis of leaf extracts of selected Indian Medicinal plants. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 395–406. [Google Scholar]
- Dyrby, M.; Westergaard, N.; Stapelfeldt, H. Light and heat sensitivity of red cabbage extract in soft drink model systems. Food Chem. 2001, 72, 431–437. [Google Scholar] [CrossRef]
- Amaral, J.S.; Seabra, R.M.; Andrade, P.B.; Valentão, P.; Pereira, J.A.; Ferreres, F. Phenolic profile in the quality control of walnut (Juglans regia L.) leaves. Food Chem. 2004, 88, 373–379. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Cosmulescu, S. HPLC Determination of phenolic acids, flavonoids and juglone in walnut leaves. J. Chromatogr. Sci. 2013, 51, 883–890. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.; Ferreres, F.; Bento, A.A.; Seabra, R.; Estevinho, L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007, 45, 2287–2295. [Google Scholar] [CrossRef]
- Sipkina, N.Y.; Skorik, Y.A. Detection and determination of some phenolic and cinnamic acids in plant extracts. J. Anal. Chem. 2015, 70, 1406–1411. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Effect of Impregnation of Biodegradable Polyesters with Polyphenols from Cistus Linnaeus and Juglans regia Linnaeus Walnut Green Husk. Polymers 2019, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, R.C.; Amorim, A.D.G.N.; Thomasi, S.S.; Figueiredo, F.C.; Carneiro, C.S.; Da Silva, P.R.P.; Neto, W.R.D.V.; Ferreira, A.G.; Júnior, J.R.D.S.; Leite, J.R.D.S.D.A. Development of an electrolytic method to obtain antioxidant for biodiesel from cashew nut shell liquid. Fuel 2015, 144, 415–422. [Google Scholar] [CrossRef]
- Foo, L.Y. Proanthocyanidins: Gross Chemical Red Spectra Structures by Infra-Red Spectra. Phytochemistry 1981, 20, 1397–1402. [Google Scholar] [CrossRef]
- Kacuráková, M. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- McGhie, T.K.; Rowan, D.R.; Edwards, P.J. Structural Identification of Two Major Anthocyanin Components of Boysenberry by NMR Spectroscopy. J. Agric. Food Chem. 2006, 54, 8756–8761. [Google Scholar] [CrossRef]
- Agustin-Salazar, S.; Gamez-Meza, N.; Medina-Juárez, L.Á.; Malinconico, M.; Cerruti, P. Stabilization of Polylactic Acid and Polyethylene with Nutshell Extract: Efficiency Assessment and Economic Evaluation. ACS Sustain. Chem. Eng. 2017, 5, 4607–4618. [Google Scholar] [CrossRef]
- Joshi, D.D. UV–Vis. Spectroscopy: Herbal Drugs and Fingerprints. In Herbal Drugs and Fingerprints: Evidence Based Herbal Drugs; Springer: Delhi, India, 2012; pp. 101–120. [Google Scholar] [CrossRef]
- Engida, A.M.; Faika, S.; Nguyen-Thi, B.T.; Ju, Y.-H. Analysis of major antioxidants from extracts of Myrmecodia pendans by UV/visible spectrophotometer, liquid chromatography/tandem mass spectrometry, and high-performance liquid chromatography/UV techniques. J. Food Drug Anal. 2015, 23, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Chen, C.; Zhao, S.; Ge, F.; Liu, D.; Shi, D.; Zhang, T. Interaction of gallic acid with trypsin analyzed by spectroscopy. J. Food Drug Anal. 2015, 23, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Rojas, J.; Londono, C.; Ciro, Y. The health benefits of natural skin UVA photoprotective compounds found in botanical sources. Int. J. Pharm. Pharm. Sci. 2016, 8, 13–23. [Google Scholar]
- Ebrahimia, I.; Gashtib, M.P. Extraction of juglone from Pterocarya fraxinifolia leaves for dyeing, anti-fungal finishing, and solar UV protection of wool. Colora Technol. 2015, 131, 451–457. [Google Scholar] [CrossRef]
- Butnariu, M.; Coradini, C.Z. Evaluation of Biologically Active Compounds from Calendula officinalis Flowers using Spectrophotometry. Chem. Central J. 2012, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Hadjmohammadi, M.R.; Kamel, K. Determination of juglone (5-hydroxy 1,4,-naphthoquinone) in Pterocarya flaxinifolia by RP-HPLC. Iran. J. Chem. Chem. Eng. 2006, 25, 73–76. [Google Scholar]
- Wright, D.; Mitchelmore, C.; Dawson, R.; Cutler, H. The Influence of Water Quality on the Toxicity and Degradation of Juglone (5-Hydroxy 1,4-Naphthoquinone). Environ. Technol. 2007, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Solar, A.; Colaric, M.; Usenik, V.; Stampar, F. Seasonal variations of selected flavonoids, phenolic acids and quinines in annual ahoots of common walnut (Juglans regia L.). Plant Sci. J. 2006, 170, 453–461. [Google Scholar] [CrossRef]
- De Macêdo, I.Y.L.; Garcia, L.F.; Neto, J.R.O.; de Siqueira Leite, K.C.; Ferreira, V.S.; Ghedini, P.C.; de Souza Gil, E. Electroanalytical tools for antioxidant evaluation of red fruits dry extracts. Food Chem. 2017, 217, 326–331. [Google Scholar] [CrossRef]
- Masek, A. Antioxidant and Antiradical Properties of Green Tea Extract Compounds. Int. J. Electrochem. Sci. 2017, 12, 6600–6610. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. A decade of nutraceutical patents: Where are we now in 2018? Expert Opin. Ther. Patents 2018, 28, 875–882. [Google Scholar] [CrossRef]
- Santini, A.; Novellino, E. Nutraceuticals-shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2019, 12, 1–7. [Google Scholar] [CrossRef]

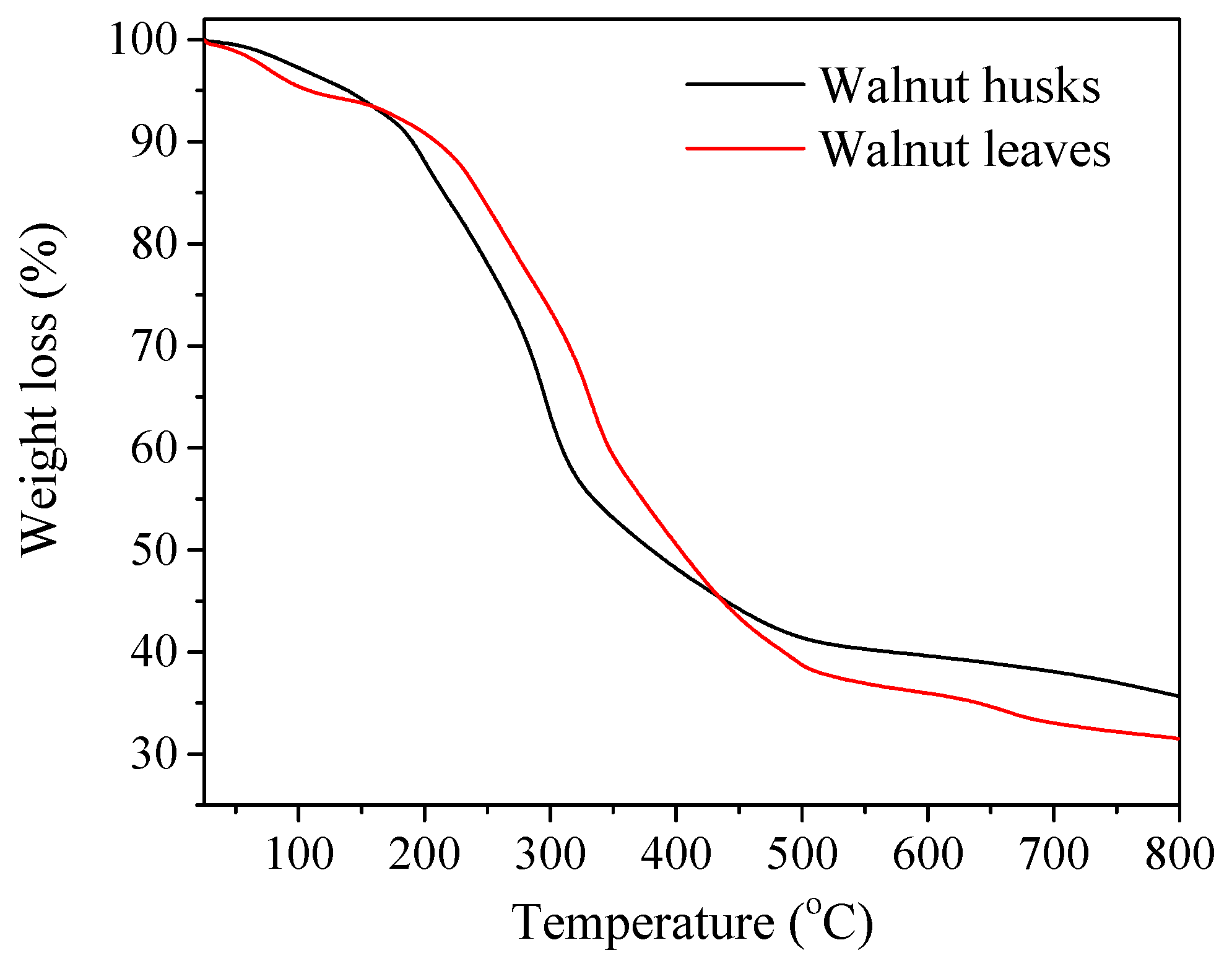


| Sample | T20 | T50 | T65 |
|---|---|---|---|
| Walnut husks | 240 | 380 | 799 |
| Walnut leaves | 267 | 403 | 640 |
| Compounds | Content (mg/g of Extract) | |
|---|---|---|
| Leaf Extract | Husk Extract | |
| Gallic acid | 0.59 ± 0.03 | 6.06 ± 0.18 |
| Syringic acid | - | 1.75 ± 0.16 |
| Other hydroxybenzoic acids 1 | 9.11 ± 1.64 | 4.68 ± 0.06 |
| Ellagic acid | - | 0.44 ± 0.01 |
| Neochlorogenic acid | 11.20 ± 0.25 | 3.82 ± 0.14 |
| Chlorogenic acid | 3.56 ± 0.17 | - |
| Caffeic acid | 0.65 ± 0.03 | - |
| Sinapic acid | 0.12 ± 0.01 | 0.73 ± 0.04 |
| Ferulic acid | 0.09 ± 0.00 | - |
| p-Coumaric acid | 0.07 ± 0.01 | - |
| Other hydroxycinnamic acids 2 | 50.25 ± 6.15 | 17.68 ± 1.47 |
| Myricetin | 0.45 ± 0.04 | - |
| Quercetin | 1.85 ± 0.31 | - |
| Kaempferol | 1.03 ± 0.08 | - |
| Quercetin 3-rhamnoside | 8.04 ± 0.52 | 0.94 ± 0.04 |
| Quercetin 3-galactoside | 18.91 ± 0.03 | 0.43 ± 0.04 |
| Rutin | 0.23 ± 0.03 | - |
| Other flavonols 3 | 79.89 ± 1.12 | 2.47 ± 0.10 |
| Total | 186.04 ± 5.30 | 39.00 ± 0.65 |
| Method | Peak I | Peak II | Peak III | Peak IV | EItotal | ||||
|---|---|---|---|---|---|---|---|---|---|
| Ep (V) | ip (µA) | Ep (V) | ip (µA) | Ep (V) | ip (µA) | Ep (V) | ip (µA) | ||
| CV for husk | 0.15 | 21.00 | 0.80 | 56.00 | 1.58 | 134.00 | |||
| CV for walnut leaves | 0.23 | 51.00 | 0.94 | 87.00 | 1.17 | 122.00 | 1.75 | 357.00 | |
| DPV for husk | 10.15 | 9.07 | |||||||
| DPV for walnut leaves | 1.90 | 45.00 | 0.65 | 4.77 | 0.89 | 9.96 | 1.11 | 8.64 | |
| EI for husk (CV) | 138.16 | 70.00 | 84.81 | 292.97 | |||||
| EI for walnut leaves (CV) | 221.74 | 92.55 | 104.27 | 418.57 | |||||
| EI for husk (DPV) | 0.89 | 0.89 | |||||||
| EI for walnut leaves (DPV) | 23.68 | 7.34 | 11.19 | 7.79 | 50.00 | ||||
| Method | Walnut Leaves | |
|---|---|---|
| Antioxidant capacity | ||
| ABTS | inhibition [%] | 6.94 ± 0.35 |
| DPPH | inhibition [%] | 6.72 ± 0.34 |
| Reduction of iron and cooper ions | ||
| FRAP | Fe3+→Fe2+ (∆A, a.u.) | 0.82 ± 0.04 |
| CUPRAC | Cu2+→Cu1+ (∆A, a.u.) | 0.38 ± 0.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masek, A.; Latos-Brozio, M.; Chrzescijanska, E.; Podsedek, A. Polyphenolic Profile and Antioxidant Activity of Juglans regia L. Leaves and Husk Extracts. Forests 2019, 10, 988. https://0-doi-org.brum.beds.ac.uk/10.3390/f10110988
Masek A, Latos-Brozio M, Chrzescijanska E, Podsedek A. Polyphenolic Profile and Antioxidant Activity of Juglans regia L. Leaves and Husk Extracts. Forests. 2019; 10(11):988. https://0-doi-org.brum.beds.ac.uk/10.3390/f10110988
Chicago/Turabian StyleMasek, Anna, Malgorzata Latos-Brozio, Ewa Chrzescijanska, and Anna Podsedek. 2019. "Polyphenolic Profile and Antioxidant Activity of Juglans regia L. Leaves and Husk Extracts" Forests 10, no. 11: 988. https://0-doi-org.brum.beds.ac.uk/10.3390/f10110988





