Evaluation of Anti-Tyrosinase and Antioxidant Properties of Four Fern Species for Potential Cosmetic Applications
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Extracts Preparation
2.4. Phytochemical Screening by Thin Layer Chromatography (TLC) and Total Phenolic Content (TPC)
2.5. Determination of Antioxidant Activities
2.5.1. DPPH Radical Scavenging Activity
2.5.2. ORAC Assay
2.5.3. Superoxide Radicals Generated by Xanthine/Xanthine Oxidase (X/XO)
2.6. Inhibition of Tyrosinase Activity
2.7. Statistical Analysis
3. Results
3.1. Plant Material and Yields
3.2. Polyphenol Content by Thin Layer Chromatography (TLC) and Folin Method
3.3. Antioxidant Activity
3.3.1. DPPH Radical Scavenging Activity
3.3.2. ORAC Assay
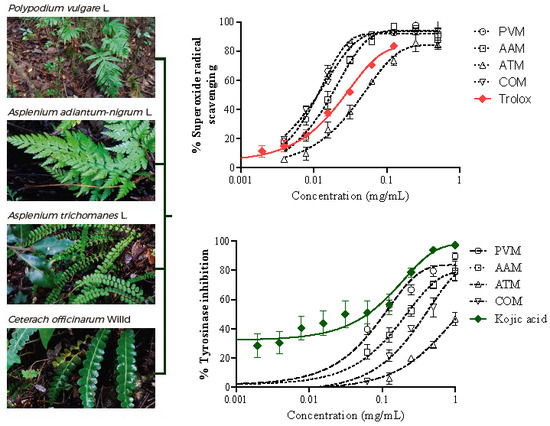
3.3.3. Superoxide Radicals Generated by Xanthine/Xanthine Oxidase (X/XO)
3.4. Tyrosinase Inhibition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Asplenium adiantum-nigum |
| AAPH | 2,2’-azobis(2-amidinopropane) dihydrochloride |
| AT | Asplenium trichomanes |
| CO | Ceterach officinarum |
| DMSO | dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| GAE | gallic acid equivalents |
| NBT | nitrotetrazolium blue chloride |
| ORAC | oxygen radical absorbance capacity |
| PV | Polypodium vulgare |
| TE | trolox equivalents |
| TLC | thin layer chromatography |
| TPC | total phenolic content |
| X/XO | xanthine/xanthine oxidase |
References
- Leiter, U.; Keim, U.; Eigentler, T.; Katalinic, A.; Holleczek, B.; Martus, P.; Garbe, C. Incidence, Mortality, and Trends of Nonmelanoma Skin Cancer in Germany. J. Investig. Dermatol. 2017, 137, 1860–1867. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.T.; Schulman, J.; Fisher, D.E. UV and pigmentation: Molecular mechanisms and social controversies. Pigment Cell Melanoma Res. 2008, 21, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Kimlin, M.G.; Guo, Y.M. Assessing the impacts of lifetime sun exposure on skin damage and skin aging using a non-invasive method. Sci. Total Environ. 2012, 425, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Wang, S.Q.; Burnett, M.; Osterwalder, U.; Lim, H.W. Photoprotection Part I. Photoprotection by naturally occurring, physical, and systemic agents. J. Am. Acad. Dermatol. 2013, 69, 12. [Google Scholar]
- Smit, N.P.M.; Kolb, R.M.; Lentjes, E.; Noz, K.C.; van der Meulen, H.; Koerten, H.K.; Vermeer, B.J.; Pavel, S. Variations in melanin formation by cultured melanocytes from different skin types. Arch. Dermatol. Res. 1998, 290, 342–349. [Google Scholar] [CrossRef]
- Gloster, H.M.; Neal, K. Skin cancer in skin of color. J. Am. Acad. Dermatol. 2006, 55, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Sklar, L.R.; Almutawa, F.; Lim, H.W.; Hamzavi, I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: A review. Photochem. Photobiol. Sci. 2013, 12, 54–64. [Google Scholar] [CrossRef]
- Ortonne, J.P.; Passeron, T. Melanin pigmentary disorders: Treatment update. Dermatol. Clin. 2005, 23, 209–226. [Google Scholar] [CrossRef]
- Gonzalez, S.; Fernandez-Lorente, M.; Gilaberte-Calzada, Y. The latest on skin photoprotection. Clin. Dermatol. 2008, 26, 614–626. [Google Scholar] [CrossRef]
- Jansen, R.; Osterwalder, U.; Wang, S.Q.; Burnett, M.; Lim, H.W. Photoprotection Part II. Sunscreen: Development, efficacy, and controversies. J. Am. Acad. Dermatol. 2013, 69, 14. [Google Scholar]
- Chen, A.C.; Halliday, G.M.; Damian, D.L. Non-melanoma skin cancer: Carcinogenesis and chemoprevention. Pathology 2013, 45, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, J.Q. Use of Polypodium leucotomas Extract in Clinical Practice: A Primer for the Clinician. J. Clin. Aestheti. Dermatol. 2016, 9, 37–42. [Google Scholar]
- Murbach, T.S.; Beres, E.; Vertesi, A.; Glavits, R.; Hirka, G.; Endres, J.R.; Clewell, A.E.; Szakonyine, I.P. A comprehensive toxicological safety assessment of an aqueous extract of Polypodium leucotomos, Fernblock, R)). Food Chem. Toxicol. 2015, 86, 328–341. [Google Scholar] [CrossRef]
- Palomino, O.M. Current knowledge in Polypodium leucotomos effect on skin protection. Arch. Dermatol. Res. 2015, 307, 199–209. [Google Scholar] [CrossRef]
- Wolf, P.G.; Pryer, K.M.; Smith, A.R.; Hasebe, M. Phylogenetic studies of extant pteridophytes. In Molecular Systematics of Plants, II: DNA Sequencing; Soltis, D.E., Soltis, P.S., Doyle, J.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998; pp. 541–556. [Google Scholar]
- Christenhusz, M.J.M.; Chase, M. Trends and concepts in fern classification. Ann. Bot. 2014, 113, 571–594. [Google Scholar] [CrossRef] [Green Version]
- Berman, B.; Ellis, C.; Elmets, C. Polypodium Leucotomos—An Overview of Basic Investigative Findings. J. Drugs Dermatol. 2016, 15, 224–228. [Google Scholar]
- Cao, H.; Chai, T.T.; Wang, X.; Morais-Braga, M.F.B.; Yang, J.H.; Wong, F.C.; Wang, R.B.; Yao, H.K.; Cao, J.G.; Cornara, L.; et al. Phytochemicals from fern species: Potential for medicine applications. Phytochem. Rev. 2017, 16, 379–440. [Google Scholar] [CrossRef]
- Amoroso, V.B.M.; Rainear, A.; Villalobos, A.P. Bringing back the lost value of Philippine edible ferns: Their antioxidant, proteins and utilization. Int. J. Adv. Res. 2017, 5, 757–770. [Google Scholar] [CrossRef]
- Tomsik, P. Ferns and Lycopods—A Potential Treasury of Anticancer Agents but Also a Carcinogenic Hazard. Phytother. Res. 2014, 28, 798–810. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Zhao, B.T.; Ali, M.Y.; Choi, J.S.; Rhyu, D.Y.; Min, B.S.; Woo, M.H. Insulin-Mimetic Selaginellins from Selaginella tamariscina with Protein Tyrosine Phosphatase 1B, PTP1B, Inhibitory Activity. J. Nat. Prod. 2015, 78, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.K.; Wang, W.W.; Zhang, L.; Su, C.F.; Wu, Y.Y.; Ke, Y.Y.; Hou, Q.W.; Liu, Z.Y.; Gao, A.S.; Feng, W.S. Antihyperlipidaemic and antioxidant effect of the total flavonoids in Selaginella tamariscina, Beauv., Spring in diabetic mice. J. Pharm. Pharmacol. 2013, 65, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Saiz, J.C.M.; Pataro, L.; Sotomayor, S.P. Atlas of the pteridophytes of the Iberian Peninsula and the Balearic Islands. Acta Bot. Malacit. 2015, 40, 5–55. [Google Scholar]
- Do, Q.T.; Bernard, P. Reverse pharmacognosy: A new concept for accelerating natural drug discovery. In Lead Molecules from Natural Products: Discovery and New Trends; Khan, M.T.H., Ather, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 1–20. [Google Scholar]
- Tanew, A.; Radakovic, S.; Gonzalez, S.; Venturini, M.; Calzavara-Pinton, P. Oral administration of a hydrophilic extract of Polypodium leucotomos for the prevention of polymorphic light eruption. J. Am. Acad. Dermatol. 2012, 66, 58–62. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. Available online: http://www.theplantlist.org/ (accessed on 16 November 2016).
- Banco de Datos de Biodiversidad de Cataluña, Generalidad de Cataluña. Available online: http://biodiver.bio.ub.es/biocat/index.jsp (accessed on 16 November 2016).
- Bonnier, G.; de Layens, G. Claves Para la determinación de Plantas Vasculares, 1st ed.; Ediciones Omega, S.L.: Barcelona, Spain, 1988. [Google Scholar]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin, Germany, 1996. [Google Scholar]
- Singleton, V.L. Citation classic–Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Curr. Contents Agric. Biol. Environ. Sci. 1985, 48, 18. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Bioactive and functional properties of sour cherry juice (Prunus cerasus). Food Funct. 2016, 7, 4675–4682. [Google Scholar] [CrossRef]
- Davalos, A.; Gomez-Cordoves, C.; Bartolome, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein, assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Fridovich, I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J. Biol. Chem. 1970, 245, 4053–4057. [Google Scholar]
- Thayer, W.S. Superoxide-Dependent and Superoxide-Independent Pathways for Reduction of Nitroblue Tetrazolium in Isolated Rat Cardiac Myocytes. Arch. Biochem. Biophys. 1990, 276, 139–145. [Google Scholar] [CrossRef]
- Senol, F.S.; Orhan, I.E.; Ozgen, U.; Renda, G.; Bulut, G.; Guven, L.; Karaoglan, E.S.; Sevindik, H.G.; Skalicka-Wozniak, K.; Caliskan, U.K.; et al. Memory-vitalizing effect of twenty-five medicinal and edible plants and their isolated compounds. S. Afr. J. Bot. 2016, 102, 102–109. [Google Scholar] [CrossRef]
- Ho, R.; Teai, T.; Bianchini, J.P.; Lafont, R.; Raharivelomanana, P. Ferns: From Traditional Uses to Pharmaceutical Development, Chemical Identification of Active Principles, Working with Ferns: Issues and Applications; Springer: New York, NY, USA, 2010; pp. 321–346. [Google Scholar]
- European Medicines Agency (EMA). Assessment Report on Polypodiumvulgare L.; Rizoma European Medicines Agency: London, UK, 2008; p. 22.
- Garcia, F.; Pivel, J.P.; Guerrero, A.; Brieva, A.; Martinez-Alcazar, M.; Caamano-Somoza, M.; Gonzalez, S. Phenolic components and antioxidant activity of Fernblock (R), an aqueous extract of the aerial parts of the fern Polypodium leucotomos. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Iwashina, T.; Matsumoto, S. Flavonoid Properties of Six Asplenium Species in Vanuatu and New Caledonia, and Distribution of Flavonoid and Related Compounds in Asplenium, Bulletin of the National Museum of Nature and Science; Series B; Botany/National Museum of Nature and Science: Tokyo, Japan, 2011; p. 13. [Google Scholar]
- Zhang, M.; Cao, J.G.; Dai, X.L.; Chen, X.F.; Wang, Q.X. Flavonoid Contents and Free Radical Scavenging Activity of Extracts from Leaves, Stems, Rachis and Roots of Dryopteris erythrosora. Iran. J. Pharm. Res. 2012, 11, 991–997. [Google Scholar]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Roginsky, V.; Lissi, E.A. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Kamisan, F.H.; Yahya, F.; Mamat, S.S.; Kamarolzaman, M.F.F.; Mohtarrudin, N.; Kek, T.L.; Salleh, M.Z.; Hussain, M.K.; Zakaria, Z.A. Effect of methanol extract of Dicranopteris linearis against carbon tetrachloride-induced acute liver injury in rats. BMC Complement. Altern. Med. 2014, 14, 10. [Google Scholar] [CrossRef]
- Robak, J.; Gryglewski, R.J. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988, 37, 837–841. [Google Scholar] [CrossRef]
- Gomes, A.J.; Lunardi, C.N.; Gonzalez, S.; Tedesco, A.C. The antioxidant action of Polypodium leucotomos extract and kojic acid: Reactions with reactive oxygen species. Braz. J. Med Biol. Res. 2001, 34, 1487–1494. [Google Scholar] [CrossRef]
- Thiem, B.; Kikowska, M.; Malinski, M.P.; Kruszka, D.; Napierala, M.; Florek, E. Ecdysteroids: Production in plant in vitro cultures. Phytochem. Rev. 2017, 16, 603–622. [Google Scholar] [CrossRef]
- Arai, Y.; Shiojima, K.; Ageta, H. Fern constituents: Cyclopodmenyl acetate, a cycloartanoid having a new 33-carbon skeleton, isolated from Polypodium vulgare. Chem. Pharm. Bull. 1989, 37, 560–562. [Google Scholar] [CrossRef]
- Lafont, R.; Dinan, L. Practical uses for ecdysteroids in mammals including humans: And update. J. Insect Sci. 2003, 3, 30. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z. Phytoecdysteroids from Silene plants: Distribution, diversity and biological (antitumour, antibacterial and antioxidant, activities. Bol. Latinoam. Y Del Caribe De Plantas Med. Y Aromat. 2012, 11, 474–497. [Google Scholar]
- Graßmann, J. Terpenoids as Plant Antioxidants; Gerald, L., Ed.; Vitamins & Hormones, Academic Press: Cambridge, MA, USA, 2005; pp. 505–535. [Google Scholar]
- Takaichi, S. Tetraterpenes: Carotenoids. In Natural Products; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin, Germany, 2013; pp. 3251–3283. [Google Scholar]
- Nguyen, P.H.; Ji, D.J.; Han, Y.R.; Choi, J.S.; Rhyu, D.Y.; Min, B.S.; Woo, M.H. Selaginellin and biflavonoids as protein tyrosine phosphatase 1B inhibitors from Selaginella tamariscina and their glucose uptake stimulatory effects. Bioorg. Med. Chem. 2015, 23, 3730–3737. [Google Scholar] [CrossRef]
- Wu, S.Q.; Li, J.; Wang, Q.X.; Cao, J.G.; Yu, H.; Cao, H.; Xiao, J.B. Chemical composition, antioxidant and anti-tyrosinase activities of fractions from Stenoloma chusanum. Ind. Crop. Prod. 2017, 107, 539–545. [Google Scholar] [CrossRef]
- Su, W.; Li, P.Y.; Huo, L.N.; Wu, C.Y.; Guo, N.N.; Liu, L.Q. Phenolic content and antioxidant activity of Phymatopteris hastate. J. Serb. Chem. Soc. 2011, 76, 1485–1496. [Google Scholar] [CrossRef]
- Chai, T.T.; Quah, Y.; Ooh, K.F.; Ismail, N.I.M.; Ang, Y.V.; Elamparuthi, S.; Yeoh, L.Y.; Ong, H.C.; Wong, F.C. Anti-Proliferative, Antioxidant and Iron-Chelating Properties of the Tropical Highland Fern, Phymatopteris triloba (Houtt, Pichi Serm (Family Polypodiaceae). Trop. J. Pharm. Res. 2013, 12, 747–753. [Google Scholar] [CrossRef]
- Ageta, H.; Arai, Y. Chemotaxonomy of ferns 3. triterpenoids from Polypodium–polypodioides. J. Nat. Prod. 1990, 53, 325–332. [Google Scholar] [CrossRef]
- Arai, Y.; Yamaide, M.; Yamazaki, S.; Ageta, H. Fern constituents–Triterpenoids isolated from Polypodium-vulgare, Polypodium-fauriei and Polypodium-virginianum. Phytochemistry 1991, 30, 3369–3377. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Khan, S.B.; Ather, A. Tyrosinase inhibitory cycloartane type triterpenoids from the methanol extract of the whole plant of Amberboa ramosa Jafri and their structure-activity relationship. Bioorg. Med. Chem. 2006, 14, 938–943. [Google Scholar] [CrossRef] [PubMed]




| Species | Spanish Common Name | Methanol Extract | Hexane Extract | ||||
|---|---|---|---|---|---|---|---|
| Code | Yield (%) a | TPC (µGAE/mg) | Code | Yield (%) a | TPC (µGAE/mg) | ||
| Polypodium vulgare L. (Polypodiaceae) | “Polipodio” | PVM | 23.53 | 172.8 ± 3.8 | PVH | 1.49 | 74.7 ± 5.8 |
| Asplenium adiantum-nigrum L. (Aspleniaceae) | “Culantrillo negro” | AAM | 16.27 | 113.5 ± 5.8 | AAH | 1.57 | 96.0 ± 3.8 |
| Asplenium trichomanes L. (Aspleniaceae) | “Culantrillo rojo” | ATM | 29.55 | 100.4 ± 0.7 | ATH | 2.01 | 70.3 ± 6.2 |
| Ceterach officinarum Willd (Aspleniaceae) | “Doradilla” | COM | 28.04 | 193.2 ± 3.8 | COH | 2.34 | 70.3 ± 7.6 |
| Ferns | ORAC (µmol Trolox Equivalents/mg Sample) | |
|---|---|---|
| Methanolic Extract | Hexane Extract | |
| Polypodium vulgare (PV) | 2.34 ± 0.04 | 0.38 ± 0.02 |
| Asplenium adiantum-nigrum (AA) | 2.25 ± 0.03 | 0.34 ± 0.11 |
| Asplenium trichomanes (AT) | 2.25 ± 0.14 | 0.44 ± 0.01 |
| Ceterach officinarum (CO) | 2.93 ± 0.23 * | 0.84 ± 0.06 # |
| Species | IC50 (mg/mL) a | |||||
|---|---|---|---|---|---|---|
| DPPH Radical | O2− Radical | Tyrosinase Inhibition | ||||
| Methanol Extract | Hexane Extract | Methanol Extract | Hexane Extract | Methanol Extract | Hexane Extract | |
| Polypodium vulgare (PV) | 0.007 | 0.233 | 0.011 | 0.201 | 0.107 | 0.233 |
| Asplenium adiantum-nigrum (AA) | 0.008 | 0.044 | 0.011 | 0.128 | 0.216 | ND |
| Asplenium trichomanes (AT) | 0.036 | 0.129 | 0.047 | 0.090 | 1.175 | ND |
| Ceterach officinarum (CO) | 0.007 | 0.072 | 0.012 | 0.073 | 0.392 | ND |
| Kojic acid | - | - | 0.063 | |||
| Trolox | 0.002 | 0.026 | - | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farràs, A.; Cásedas, G.; Les, F.; Terrado, E.M.; Mitjans, M.; López, V. Evaluation of Anti-Tyrosinase and Antioxidant Properties of Four Fern Species for Potential Cosmetic Applications. Forests 2019, 10, 179. https://0-doi-org.brum.beds.ac.uk/10.3390/f10020179
Farràs A, Cásedas G, Les F, Terrado EM, Mitjans M, López V. Evaluation of Anti-Tyrosinase and Antioxidant Properties of Four Fern Species for Potential Cosmetic Applications. Forests. 2019; 10(2):179. https://0-doi-org.brum.beds.ac.uk/10.3390/f10020179
Chicago/Turabian StyleFarràs, Adrià, Guillermo Cásedas, Francisco Les, Eva María Terrado, Montserrat Mitjans, and Víctor López. 2019. "Evaluation of Anti-Tyrosinase and Antioxidant Properties of Four Fern Species for Potential Cosmetic Applications" Forests 10, no. 2: 179. https://0-doi-org.brum.beds.ac.uk/10.3390/f10020179