A Comparison of the Formation Rates and Composition of Tree-Related Microhabitats in Beech-Dominated Primeval Carpathian and Hyrcanian Forests
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Regions and Design
2.2. Statistical Analyses
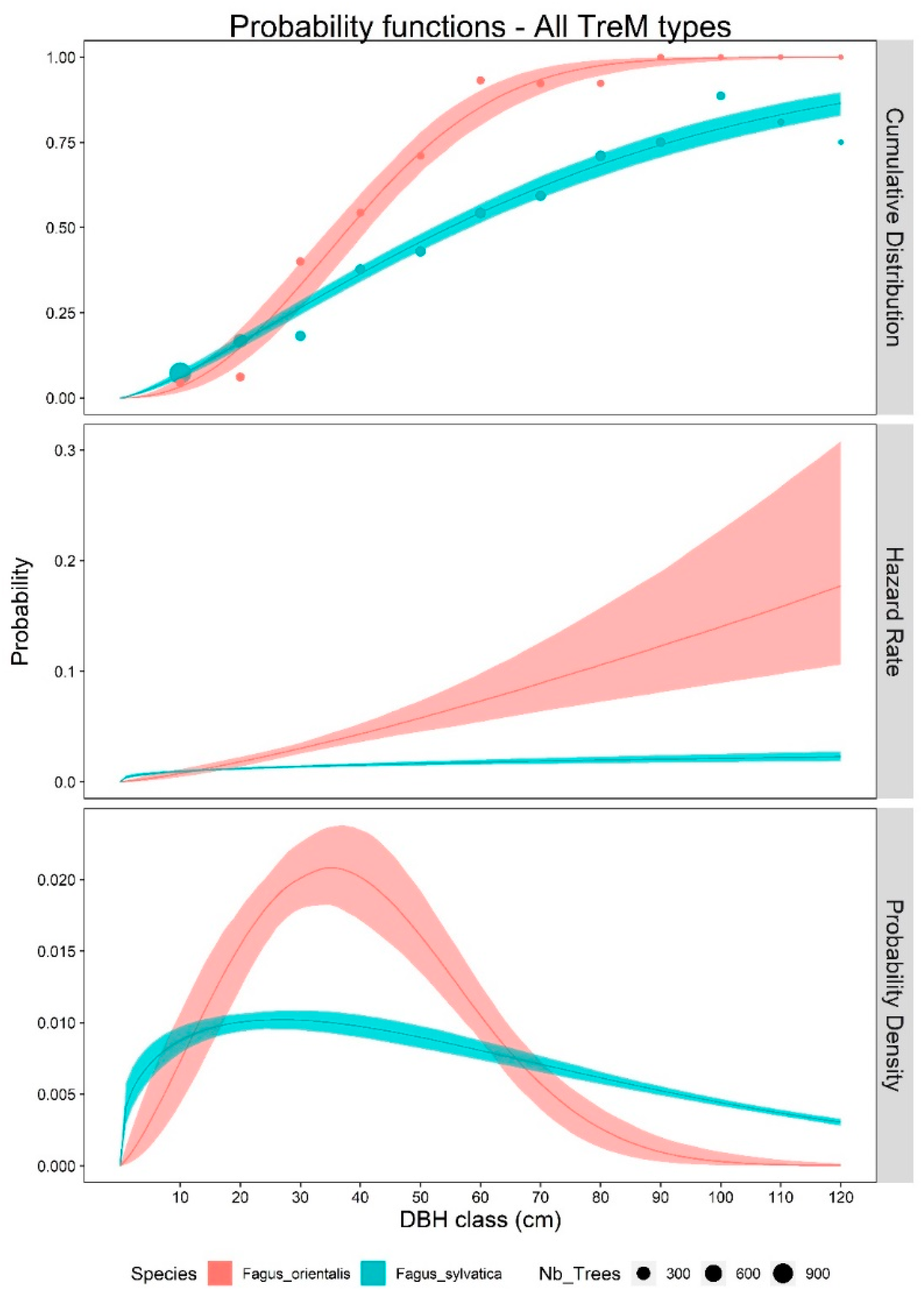
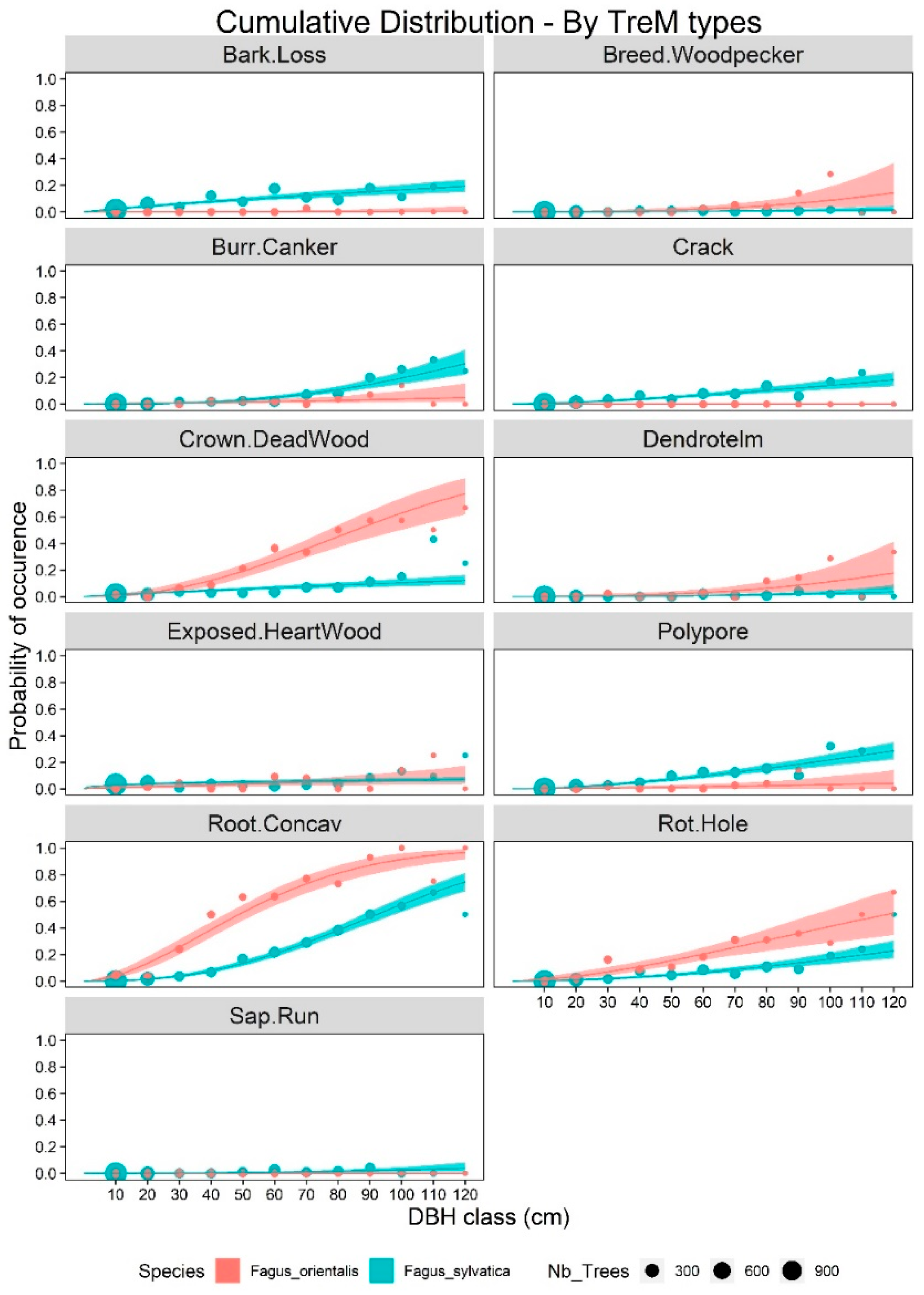
3. Results
4. Discussion
4.1. TreM Variation Due to Tree Species
4.2. Trem Variation Due to the Environment
4.3. Are Tree-Related Microhabitats Critical for Endemic Saproxylics?
4.4. Implications for Nature Conservation in Broadleaf Forests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Speight, M.C.D. Saproxylic invertebrates and their conservation. Counc. Eur. Nat. Environ. Ser. 1989, 42, 1–79. [Google Scholar]
- Seibold, S.; Bässler, C.; Brandl, R.; Gossner, M.M.; Thorn, S.; Ulyshen, M.D.; Müller, J. Experimental studies of dead-wood biodiversity—A review identifying global gaps in knowledge. Biol. Conserv. 2015, 191, 139–149. [Google Scholar] [CrossRef]
- Grove, S. The influence of forest management history on the integrity of the saproxylic beetle fauna in an australian lowland tropical rainforest. Biol. Conserv. 2002, 104, 149–171. [Google Scholar] [CrossRef]
- Sandström, J.; Bernes, C.; Junninen, K.; Lõhmus, A.; Macdonald, E.; Müller, J.; Jonsson, B.G. Impacts of dead-wood manipulation on the biodiversity of temperate and boreal forests - A systematic review. J. Appl. Ecol. 2019, 56, 1770–1781. [Google Scholar] [CrossRef] [Green Version]
- Dörfler, I.; Müller, J.; Gossner, M.M.; Hofner, B.; Weisser, W.W. Success of deadwood enrichment strategy in production forests depends on stand type and management intensity. For. Ecol. Manag. 2017, 400, 607–620. [Google Scholar] [CrossRef]
- Lachat, T.; Nagel, P.; Cakpo, Y.; Attignon, S.; Goergen, G.; Sinsin, B. Dead wood and saproxylic beetle assemblages in a semi-deciduous forest in Southern Benin. Ecol. Manag. 2006, 225, 27–38. [Google Scholar] [CrossRef]
- Remm, J.; Lohmus, A. Tree cavities in forests: The broad distribution of a keystone structure for biodiversity. For. Ecol. Manag. 2012, 262, 579–585. [Google Scholar] [CrossRef]
- Ranius, T. Osmoderma eremita as an indicator of species richness of beetles in tree hollows. Biodivers Conserv. 2002, 11, 931–941. [Google Scholar] [CrossRef]
- Kortmann, M.; Hurst, J.; Brinkmann, R.; Heurich, M.; Gonzalez, R.S.; Mueller, J.; Thorn, S. Beauty and the beast: How a bat utilizes forests shaped by outbreaks of an insect pest. Anim. Conserv. 2018, 21, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Fritz, Ö.; Heilmann-Clausen, J. Rot holes create key microhabitats for epiphytic lichens and bryophytes on beech (Fagus sylvatica). Biol. Conserv. 2010, 143, 1008–1016. [Google Scholar] [CrossRef]
- Lindo, Z.; Winchester, N.N. Scale dependent diversity patterns in arboreal and terrestrial oribatid mite (Acari: Oribatida) communities. Ecography 2008, 31, 53–60. [Google Scholar] [CrossRef]
- Vuidot, A.; Paillet, Y.; Archaux, F.; Gosselin, F. Influence of tree characteristics and forest management on tree microhabitats. Biol. Conserv. 2011, 144, 441–450. [Google Scholar] [CrossRef]
- Möller, G. Struktur-Und Substratbindung Holzbewohnender Insekten, Schwerpunkt Coleoptera-Käfer; Freien Universität Berlin: Berlin, Germany, 2009. [Google Scholar]
- Larrieu, L.; Cabanettes, A. Species, live status, and diameter are important tree features for diversity and abundance of tree microhabitats in subnatural montane beech-fir forests. Can. J. For. Res. 2012, 42, 1433–1445. [Google Scholar] [CrossRef]
- Winter, S.; Möller, G. Microhabitats in Lowland Beech Forests as monitoring tool for Nature Conservation. For. Ecol. Manag. 2008, 255, 1251–1261. [Google Scholar] [CrossRef]
- Larrieu, L.; Paillet, Y.; Winter, S.; Bütler, R.; Kraus, D.; Krumm, F.; Lachat, T.; Michel, A.K.; Regnery, B.; Vandekerkhove, K. Tree related microhabitats in temperate and Mediterranean European forests: A hierarchical typology for inventory standardization. Ecol. Indic. 2018, 84, 194–207. [Google Scholar] [CrossRef]
- Courbaud, B.; Pupin, C.; Letort, A.; Cabanettes, A.; Larrieu, L. Modelling the probability of microhabitat formation on trees using cross-sectional data. Methods Ecol. Evol. 2017, 8, 1347–1359. [Google Scholar] [CrossRef] [Green Version]
- Browne, J.E. Studies of decay in relation to tree species, age, and site and their application in forest inventories and establishment of priority cutting schedules in British Columbia. For. Chron. 1956, 32, 53–57. [Google Scholar] [CrossRef]
- Asbeck, T.; Pyttel, P.; Frey, J.; Bauhus, J. Predicting abundance and diversity of tree-related microhabitats in Central European montane forests from common forest attributes. For. Ecol. Manag. 2019, 432, 400–408. [Google Scholar] [CrossRef]
- Larrieu, L.; Cabanettes, A.; Delarue, A. Impact of silviculture on dead wood and on the distribution and frequency of tree microhabitats in montane beech-fir forests of the Pyrenees. Eur. J. For. Res. 2012, 131, 773–786. [Google Scholar] [CrossRef]
- Grove, S.J. Saproxylic insect ecology and the sustainable management of forests. Annu. Rev. Ecol. Syst. 2002, 33, 1–23. [Google Scholar] [CrossRef]
- Brunet, J.; Fritz, Ö.; Richnau, G. Biodiversity in European beech forests – a review with recommendations for sustainable forest management. Ecol. Bull. 2010, 53, 77–94. [Google Scholar]
- Leibundgut, H. Europäische Urwälder; Haupt: Bern, Switzerland; Stuttgart, Germany; Wien, Austria, 1993. [Google Scholar]
- Hobi, M.L.; Commarmot, B.; Bugmann, H. Pattern and process in the largest primeval beech forest of Europe (Ukrainian Carpathians). J. Veg. Sci. 2015, 26, 323–336. [Google Scholar] [CrossRef]
- Magri, D.; Vendramin, G.G.; Comps, B.; Dupanloup, I.; Geburek, T.; Gömory, D.; Latalowa, M.; Litt, T.; Paule, L.; Roure, J.M.; et al. A new scenario for the Quaternary history of European beech populations: Palaeobotanical evidence and genetic consequences. New Phytol. 2006, 171, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Drag, L.; Hauck, D.; Rican, O.; Schmitt, T.; Shovkoon, D.F.; Godunko, R.J.; Curletti, G.; Cizek, L. Phylogeography of the endangered saproxylic beetle Rosalia longicorn, Rosalia alpina (Coleoptera, Cerambycidae), corresponds with its main host, the European beech (Fagus sylvatica, Fagaceae). J. Biogeogr. 2018, 45, 2631–2644. [Google Scholar] [CrossRef]
- Jimenez-Alfaro, B.; Girardello, M.; Chytry, M.; Svenning, J.C.; Willner, W.; Gegout, J.C.; Agrillo, E.; Campos, J.A.; Jandt, U.; Kacki, Z.; et al. History and environment shape species pools and community diversity in European beech forests. Nat. Ecol. Evol. 2018, 2, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Renner, S.S.; Grimm, G.W.; Kapli, P.; Denk, T. Species relationships and divergence times in beeches: New insights from the inclusion of 53 young and old fossils in a birth-death clock model. Philos. Trans. R. Soc. B Biol. Sci. 2015, 371, 20150135. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.; Mohadjer, M.R.M.; Sefidi, K.; Zobiri, M.; Omidi, A. Over-mature beech trees (Fagus orientalis Lipsky) and close-to-nature forestry in northern Iran. J. For. Res. 2012, 23, 289–294. [Google Scholar] [CrossRef]
- Sefidi, K.; Mohadjer, M.R.M.; Mosandl, R.; Copenheaver, C.A. Canopy gaps and regeneration in old-growth Oriental beech (Fagus orientalis Lipsky) stands, northern Iran. For. Ecol. Manag. 2011, 262, 1094–1099. [Google Scholar] [CrossRef]
- Sagheb-Talebi, K.; Schütz, J.P. The structure of natural oriental beech (Fagus orientalis) forests in the Caspian region of Iran and potential for the application of the group selection system. Forestry 2002, 75, 465–472. [Google Scholar] [CrossRef] [Green Version]
- Sagheb-Talebi, K.; Sajedi, T.; Pourhashemi, M. (Eds.) Forests of Iran—A Treasure from the Past, a Hope for the Future; Springer: Dordrecht, The Netherlands, 2014; Volume 10, p. 152. [Google Scholar]
- Jalili, A.; Jamzad, Z. Red data book of Iran. A preliminary survey of endemic, rare and endangered plant species in Iran. Res. Inst. For. Rangel. Tehran Tech. 1999, 215, 748. [Google Scholar]
- Müller, J.; Varandi, H.B.; Babaii, M.R.; Farashiani, M.E.; Sageb-Talebi, K.; Lange, F.; Gossner, M.M.; Jarzabek-Müller, A.; Roth, N.; Thorn, S.; et al. The diversity of saproxylic insects (Coleoptera, Heteroptera) on four tree species of the Hyrcanian Forest in Iran. J. Insect Conserv. 2018, 22, 607–625. [Google Scholar] [CrossRef]
- Mikoláš, M.; Svoboda, M.; Pouska, V.; Morrissey, R.C.; Donato, D.C.; Keeton, W.S.; Nagel, T.A.; Popescu, V.D.; Müller, J.; Bässler, C.; et al. Comment on “Opinion paper: Forest management and biodiversity”: The role of protected areas is greater than the sum of its number of species. Web Ecol. 2014, 14, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Müller, J.; Sagheb-Talebi, K.; Thorn, S. Protect Iran’s ancient forest from logging. Science 2017, 355, 919. [Google Scholar] [CrossRef]
- Müller, J.; Thorn, S.; Baier, R.; Sagheb-Talebi, K.; Barimani, H.; Seibold, S.; Ulyshen, M.D.; Gossner, M.M. Protecting the forests while allowing removal of damaged trees may imperil saproxylic insect biodiversity in the Hyrcanian beech forests of Iran. Conserv. Lett. 2016, 9, 106–113. [Google Scholar]
- Bauhus, J.; Puettmann, K.; Messier, C. Silviculture for old-growth attributes. For. Ecol. Manag. 2009, 258, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Christensen, M.; Hahn, K.; Mountford, E.P.; Ódor, P.; Standóvar, T.; Rozenbergar, D.; Diaci, J.; Wijdeven, S.; Meyer, P.; Winter, S.; et al. Dead wood in European beech (Fagus sylvatica) forest reserves. For. Ecol. Manag. 2005, 210, 267–282. [Google Scholar] [CrossRef]
- Ódor, P.; Heilmann-Clausen, J.; Christensen, M.; Aude, E.; Dort, v.K.W.; Piltaver, A.; Siller, I.; Veerkamp, M.T.; Standovár, T.; Hees, V.A.F.M.; et al. Diversity of dead wood inhabiting fungi and bryophytes in semi-natural beech forests in Europe. Biol. Conserv. 2006, 131, 58–71. [Google Scholar] [CrossRef]
- Winter, S.; Flade, M.; Schumacher, H.; Kerstan, E.; Möller, G. The importance of near natural stand structures for the biocoenosis of lowland beech forests. Snow Lansc. Res. 2005, 79, 127–144. [Google Scholar]
- Dörfler, I.; Mueller, J.; Weisser, W.; Gossner, M.M.; Seibold, S. Deadwood enrichment combining integrative and segregative conservation elements enhances biodiversity of multiple taxa in managed forests. Biol. Conserv. 2018, 228, 70–78. [Google Scholar] [CrossRef]
- Gossner, M.M.; Lachat, T.; Brunet, J.; Isacsson, G.; Bouget, C.; Brustel, H.; Brandl, R.; Weisser, W.W.; Müller, J. Current “near-to-nature” forest management effects on functional trait composition of saproxylic beetles in beech forests. Conserv. Biol. 2013, 27, 605–614. [Google Scholar] [CrossRef]
- Lachat, T.; Chumak, M.; Chumak, V.; Jakoby, O.; Müller, J.; Tanadini, M.; Wermelinger, B. Influence of canopy gaps on saproxylic beetles in primeval beech forests: A case study from the Uholka-Shyrokyi Luh forest, Ukraine. Insect Conserv. Divers. 2016, 9, 559–573. [Google Scholar] [CrossRef]
- Hagge, J.; Abrego, N.; Bässler, C.; Bouget, C.; Brin, A.; Brustel, H.; Christensen, M.; Gossner, M.M.; Heilmann-Clausen, J.; Horak, J.; et al. Congruent patterns of functional diversity in saproxylic beetles and fungi across European beech forests. J. Biogeogr. 2019, 46, 1054–1065. [Google Scholar] [CrossRef]
- Commarmot, B.; Brändli, U.B.; Hamor, F.; Lavnyy, V. (Eds.) Inventory of the Largest Primeval Beech Forest in Europe: A Swiss-Ukrainian Scientific Adventure; Swiss Federal Research Institute WSL: Birmensdorf, Switzerland, 2013. [Google Scholar]
- Commarmot, B.; Bachofen, H.; Bundziak, Y.; Buergi, A.; Ramp, B.; Shparyk, Y.; Sukhariuk, D.; Viter, R.; Zingg, A. Structures of virgin and managed beech forests in Uholka (Ukraine) and Sihlwald (Switzerland): A comparative study. For. Snow Landsc. Res. 2005, 79, 45–56. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Deslauriers, A.; Fonti, P.; Rossi, S.; Rathgeber, C.B.K.; Gricar, J. Ecophysiology and Plasticity of Wood and Phloem Formation. In Dendroecology: Tree-Ring Analyses Applied to Ecological Studies; Amoroso, M.M., Daniels, L.D., Baker, P.J., Camarero, J.J., Eds.; Springer International Publishing Ag: Cham, Germany, 2017; Volume 231, pp. 13–33. [Google Scholar]
- Krah, F.S.; Seibold, S.; Brandl, R.; Baldrian, P.; Muller, J.; Bassler, C. Independent effects of host and environment on the diversity of wood-inhabiting fungi. J. Ecol. 2018, 106, 1428–1442. [Google Scholar] [CrossRef]
- Kahl, T.; Arnstadt, T.; Baber, K.; Bassler, C.; Bauhus, J.; Borken, W.; Buscot, F.; Floren, A.; Heibl, C.; Hessenmoller, D.; et al. Wood decay rates of 13 temperate tree species in relation to wood properties, enzyme activities and organismic diversities. For. Ecol. Manag. 2017, 391, 86–95. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Maruyama, P.K.; Bruun, H.H.; Dimitrov, D.; Laeessoe, T.; Froslev, T.G.; Dalsgaard, B. Citizen science data reveal ecological, historical and evolutionary factors shaping interactions between woody hosts and wood-inhabiting fungi. New Phytol. 2016, 212, 1072–1082. [Google Scholar] [CrossRef]
- Zahner, V.; Sikora, L.; Pasinelli, G. Heart rot as a key factor for cavity tree selection in the black woodpecker. For. Ecol. Manag. 2012, 271, 98–103. [Google Scholar] [CrossRef]
- Winter, S.; Hofler, J.; Michel, A.K.; Bock, A.; Ankerst, D.P. Association of tree and plot characteristics with microhabitat formation in European beech and Douglas-fir forests. Eur. J. For. Res. 2015, 134, 335–347. [Google Scholar] [CrossRef]
- Gouix, N.; Mertlik, J.; Jarzabek-Müller, A.; Németh, T.; Brustel, H. Known status of the endangered western Palaearctic violet click beetle (Limoniscus violaceus) (Coleoptera). J. Nat. Hist. 2012, 46, 769–802. [Google Scholar] [CrossRef]
- Gustafsson, L.; Bauhus, J.; Asbeck, T.; Lessa, A.; Augustynczik, D.; Basile, M.; Frey, J.; Gutzat, F.; Hanewinkel, M.; Helbach, J.; et al. Retention as an integrated biodiversity conservation approach for continuous-cover forestry in Europe. Ambio 2019, 85, 97. [Google Scholar] [CrossRef] [Green Version]
- Eckelt, A.; Müller, J.; Bense, U.; Brustel, H.; Bußler, H.; Chittaro, Y.; Cizek, L.; Frei, A.; Holzer, E.; Kadej, M.; et al. “Primeval forest relict beetles” of Central Europe: A set of 168 umbrella species for the protection of primeval forest remnants. J. Insect Conserv. 2018, 22, 15–28. [Google Scholar] [CrossRef]
- Müller, J.; Brunet, J.; Brin, A.; Bouget, C.; Brustel, H.; Bussler, H.; Förster, B.; Iscacsson, g.; Köhler, F.; Lachat, T.; et al. Implications from large-scale spatial diversity patterns of saproxylic beetles for the conservation of European Beech forests. Insect Conserv. Divers. 2013, 6, 162–169. [Google Scholar] [CrossRef]
- Soofi, M.; Ghoddousi, A.; Zeppenfeld, T.; Shokri, S.; Soufi, M.; Jafari, A.; Ahmadpour, M.; Qashqaei, A.T.; Egli, L.; Ghadirian, T.; et al. Livestock grazing in protected areas and its effects on large mammals in the Hyrcanian forest, Iran. Biol. Conserv. 2018, 217, 377–382. [Google Scholar] [CrossRef]

| Fo Iran | Fs Ukraine | ||
|---|---|---|---|
| Total investigated area | 2.5 ha | 9.0 ha | |
| Investigated admixed tree species (n) | 19 | 114 | |
| Investigated Fagus trees ≥7 cm | 405 | 2251 | |
| Mean (min, max) dbh of Fagus trees | 44.3 (7–139) | 32.3 (7–129) | |
| Percentage of TreM-bearing trees ≥7 cm | 60% | 31% | |
| Percentage of TreM-bearing trees ≥20 cm | 78% | 60% | |
| TreM richness per TreM-bearing tree for 11 TreM subgroups (all 47 TreM types) | 2.15 (1.63) | 2.04 (1.51) | |
| TreM density/ha | p-value | ||
| ● Woodpecker cavity | 3.20 | 0.89 | p < 0.001 |
| ● Rot hole | 22.80 | 8.89 | p < 0.001 |
| ● Dendrotelm | 4.00 | 1.11 | p < 0.001 |
| ● Root concavity | 66.8 | 28.1 | p < 0.001 |
| ● Bark loss | 0.40 | 16.1 | p = 0.024 |
| ● Exposed heartwood | 4.80 | 10.1 | n.s. |
| ● Crack | 0.00 | 9.33 | n.s. |
| ● Crown deadwood | 30.0 | 9.33 | p < 0.001 |
| ● Burr and canker | 2.40 | 8.11 | n.s. |
| ● Polypore | 2.00 | 12.9 | n.s. |
| ● Sap run | 0.00 | 1.33 | n.s. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahed, R.R.; Kavousi, M.R.; Farashiani, M.E.; Sagheb-Talebi, K.; Babanezhad, M.; Courbaud, B.; Wirtz, R.; Müller, J.; Larrieu, L. A Comparison of the Formation Rates and Composition of Tree-Related Microhabitats in Beech-Dominated Primeval Carpathian and Hyrcanian Forests. Forests 2020, 11, 144. https://0-doi-org.brum.beds.ac.uk/10.3390/f11020144
Jahed RR, Kavousi MR, Farashiani ME, Sagheb-Talebi K, Babanezhad M, Courbaud B, Wirtz R, Müller J, Larrieu L. A Comparison of the Formation Rates and Composition of Tree-Related Microhabitats in Beech-Dominated Primeval Carpathian and Hyrcanian Forests. Forests. 2020; 11(2):144. https://0-doi-org.brum.beds.ac.uk/10.3390/f11020144
Chicago/Turabian StyleJahed, Razieh Rafiei, Mohammad Reza Kavousi, Mohammad Ebrahim Farashiani, Khosro Sagheb-Talebi, Manoochehr Babanezhad, Benoit Courbaud, Roland Wirtz, Jörg Müller, and Laurent Larrieu. 2020. "A Comparison of the Formation Rates and Composition of Tree-Related Microhabitats in Beech-Dominated Primeval Carpathian and Hyrcanian Forests" Forests 11, no. 2: 144. https://0-doi-org.brum.beds.ac.uk/10.3390/f11020144





