Foliar Water Uptake Capacity in Six Mangrove Species
Abstract
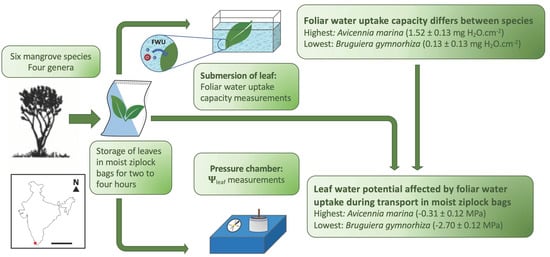
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Set-Up
2.3. Foliar Water Uptake Capacity
2.4. Water Potential
2.5. Leaf Anatomy
2.6. Statistical Analysis
3. Results
3.1. Species Level
3.2. Leaf Anatomy
3.3. Salt Excretors versus Non-Excretors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spalding, M.; Kainuma, M.; Collins, L. World Atlas of Mangroves; Earthscan: London, UK, 2010. [Google Scholar]
- Bunting, P.; Rosenqvist, A.; Lucas, R.M.; Rebelo, L.M.; Hilarides, L.; Thomas, N.; Hardy, A.; Itoh, T.; Shimada, M.; Finlayson, C.M. The Global Mangrove Watch—A New 2010 Global Baseline of Mangrove Extent. Remote Sens. 2018, 10, 1669. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Special Report: Global Warming of 1.5 °C; IPCC: Geneva, Switzerland, 2018.
- Gilman, E.L.; Ellison, J.; Duke, N.C.; Field, C. Threats to Mangroves from Climate Change and Adaptation Options: A Review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Duke, N.C.; Field, C.; Mackenzie, J.R.; Meynecke, J.-O.; Wood, A.L.; Duke, N.C.; Field, C.; Mackenzie, J.R.; Meynecke, J.-O.; Wood, A.L. Rainfall and Its Possible Hysteresis Effect on the Proportional Cover of Tropical Tidal-Wetland Mangroves and Saltmarsh–Saltpans. Mar. Freshw. Res. 2019, 70, 1047–1055. [Google Scholar] [CrossRef]
- Ward, R.D.; Friess, D.A.; Day, R.H.; Mackenzie, R.A. Impacts of Climate Change on Mangrove Ecosystems: A Region by Region Overview. Ecosyst. Health Sustain. 2016, 2, e01211. [Google Scholar] [CrossRef] [Green Version]
- Field, C.D. Impact of Expected Climate Change on Mangroves. In Asia-Pacific Symposium on Mangrove Ecosystems; Springer: Dordrecht, The Netherlands, 1995; pp. 75–81. [Google Scholar] [CrossRef]
- Duke, N.C.; Ball, M.C.; Ellison, J.C. Factors Influencing Biodiversity and Distributional Gradients in Mangroves. Glob. Ecol. Biogeogr. Lett. 1998, 7, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Dixon, H.H.; Joly, J. On the Ascent of Sap. Philos. Trans. R. Soc. Lond. 1895, 186, 563–576. [Google Scholar] [CrossRef]
- Nobel, P.S. Physicochemical and Environmental Plant Physiology, 4th ed.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Goldsmith, G.R. Changing Directions: The Atmosphere-Plant-Soil Continuum. New Phytol. 2013, 199, 4–6. [Google Scholar] [CrossRef]
- Schreel, J.D.M.; Steppe, K. Foliar Water Uptake in Trees: Negligible or Necessary? Trends Plant Sci. 2020, 25, 590–603. [Google Scholar] [CrossRef]
- Limm, E.B.; Simonin, K.A.; Bothman, A.G.; Dawson, T.E. Foliar Water Uptake: A Common Water Acquisition Strategy for Plants of the Redwood Forest. Oecologia 2009, 161, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Burgess, S.; Dawson, T. The Contribution of Fog to the Water Relations of Sequoia sempervirens (D. Don): Foliar Uptake and Prevention of Dehydration. Plant. Cell Environ. 2004, 27, 1023–1034. [Google Scholar] [CrossRef]
- Nadezhdina, N.; David, T.; David, J.; Ferreira, M.; Dohnal, M.; Tesar, M.; Gartner, K.; Leitgeb, E.; Nadezhdin, V.; Cermák, J.; et al. Trees Never Rest: The Multiple Facets of Hydraulic Redistribution. Ecohydrology 2010, 3, 431–444. [Google Scholar] [CrossRef]
- Schreel, J.D.M.; Van de Wal, B.A.E.; Hervé-Fernandez, P.; Boeckx, P.; Steppe, K. Hydraulic Redistribution of Foliar Absorbed Water Causes Turgor-Driven Growth in Mangrove Seedlings. Plant Cell Environ. 2019, 42, 2437–2447. [Google Scholar] [CrossRef]
- Breazeale, E.L.; McGeorge, W.T.; Breazeale, J.F. Moisture Absorption from Plants from an Atmosphere of High Humidity. Plant Physiol. 1950, 25, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, Z.C.; Emery, N.C.; Gotsch, S.G.; Goldsmith, G.R. Foliar Water Uptake: Processes, Pathways, and Integration into Plant Water Budgets. Plant Cell Environ. 2019, 42, 410–423. [Google Scholar] [CrossRef] [Green Version]
- Steppe, K.; Vandegehuchte, M.W.; Van de Wal, B.A.E.; Hoste, P.; Guyot, A.; Lovelock, C.E.; Lockington, D.A. Direct Uptake of Canopy Rainwater Causes Turgor-Driven Growth Spurts in the Mangrove Avicennia marina. Tree Physiol. 2018, 38, 979–991. [Google Scholar] [CrossRef] [Green Version]
- Simonin, K.A.; Santiago, L.S.; Dawson, T.E. Fog Interception by Sequoia sempervirens (D. Don) Crowns Decouples Physiology from Soil Water Deficit. Plant Cell Environ. 2009, 32, 882–892. [Google Scholar] [CrossRef]
- Duke, N.C.; Kovacs, J.M.; Griffiths, A.D.; Preece, L.; Hill, D.J.E.; Van Oosterzee, P.; Mackenzie, J.; Morning, H.S.; Burrows, D. Large-Scale Dieback of Mangroves in Australia’s Gulf of Carpentaria: A Severe Ecosystem Response, Coincidental with an Unusually Extreme Weather Event. Mar. Freshw. Res. 2017, 68, 1816–1829. [Google Scholar] [CrossRef]
- Schreel, J.D.M.; Leroux, O.; Goossens, W.; Brodersen, C.; Rubinstein, A.; Steppe, K. Identifying the Pathways for Foliar Water Uptake in Beech (Fagus sylvatica L.): A Major Role for Trichomes. Plant J. 2020, 103, 769–780. [Google Scholar] [CrossRef]
- Guzmán-Delgado, P.; Laca, E.; Zwieniecki, M.A. Unravelling Foliar Water Uptake Pathways: The Contribution of Stomata and the Cuticle. Plant Cell Environ. 2021, 44, 1728–1740. [Google Scholar] [CrossRef]
- Bryant, C.; Fuenzalida, T.I.; Zavafer, A.; Nguyen, H.T.; Brothers, N.; Harris, R.J.; Beckett, H.A.A.; Holmlund, H.I.; Binks, O.; Ball, M.C. Foliar Water Uptake via Cork Warts in Mangroves of the Sonneratia Genus. Plant Cell Environ. 2021, 44, 2925–2937. [Google Scholar] [CrossRef]
- Schönherr, J.; Bukovac, M.J. Penetration of Stomata by Liquids: Dependence on Surface Tension, Wettability, and Stomatal Morphology. Plant Physiol. 1972, 49, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, J.; Basi, S.; Pariyar, S.; Hunsche, M. Stomatal Penetration by Aqueous Solutions—An Update Involving Leaf Surface Particles. New Phytol. 2012, 196, 774–787. [Google Scholar] [CrossRef]
- Coopman, R.E.; Nguyen, H.T.; Mencuccini, M.; Oliveira, R.S.; Sack, L.; Lovelock, C.E.; Ball, M.C. Harvesting Water from Unsaturated Atmospheres: Deliquescence of Salt Secreted onto Leaf Surfaces Drives Reverse Sap Flow in a Dominant Arid Climate Mangrove, Avicennia marina. New Phytol. 2021, 231, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.E.; von Willert, D.J. Leaf Epidermal Hydathodes and the Ecophysiological Consequences of Foliar Water Uptake in Species of Crassula from the Namib Desert in Southern Africa. Plant Biol. 2000, 2, 229–242. [Google Scholar] [CrossRef]
- Fernández, V.; Bahamonde, H.A.; Peguero-Pina, J.J.; Gil-Pelegrín, E.; Sancho-Knapik, D.; Gil, L.; Goldbach, H.E.; Eichert, T. Physico-Chemical Properties of Plant Cuticles and Their Functional and Ecological Significance. J. Exp. Bot. 2017, 68, 5293–5306. [Google Scholar] [CrossRef]
- Schreiber, L.; Hartmann, K.; Skrabs, M.; Zeier, J. Apoplastic Barriers in Roots: Chemical Composition of Endodermal and Hypodermal Cell Walls on JSTOR. J. Exp. Bot. 1999, 50, 1267–1280. [Google Scholar] [CrossRef] [Green Version]
- Boanares, D.; Ferreira, B.G.; Kozovits, A.R.; Sousa, H.C.; Isaias, R.M.S.; França, M.G.C. Pectin and Cellulose Cell Wall Composition Enables Different Strategies to Leaf Water Uptake in Plants from Tropical Fog Mountain. Plant Physiol. Biochem. 2018, 122, 57–64. [Google Scholar] [CrossRef]
- Kamtsikakis, A.; Baales, J.; Zeisler-Diehl, V.V.; Vanhecke, D.; Zoppe, J.O.; Schreiber, L.; Weder, C. Asymmetric Water Transport in Dense Leaf Cuticles and Cuticle-Inspired Compositionally Graded Membranes. Nat. Commun. 2021, 12, 1267. [Google Scholar] [CrossRef]
- Scholander, P.F. How Mangroves Desalinate Seawater. Physiol. Plant. 1968, 21, 251–261. [Google Scholar] [CrossRef]
- Tomlinson, P.B. The Botany of Mangroves; Cambridge University Press: Cambridge, UK, 1986. [Google Scholar]
- Parida, A.; Jha, B. Salt Tolerance Mechanisms in Mangroves: A Review. Trees–Struct. Funct. 2010, 24, 199–217. [Google Scholar] [CrossRef]
- Ball, M.C. Ecophysiology of Mangroves. Trees 1988, 2, 129–142. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Jyothi-Prakash, P.A.; Qin, L.; He, J.; Lin, Q.; Loh, C.S.; Kumar, P.P. Role of Root Hydrophobic Barriers in Salt Exclusion of a Mangrove Plant Avicennia Officinalis. Plant Cell Environ. 2014, 37, 1656–1671. [Google Scholar] [CrossRef]
- Antony, M.M.; Ignatius, J. A Hydrological Study of Ashtamudi Lake, Kerala, India with Special Reference to Its Ecological Difference. Int. J. Sci. Res. 2016, 5, 1841–1846. [Google Scholar]
- Osborn, T.J.; Jones, P.D. The CRUTEM4 Land-Surface Air Temperature Data Set: Construction, Previous Versions and Dissemination via Google Earth. Earth Syst. Sci. Data 2014, 6, 61–68. [Google Scholar] [CrossRef] [Green Version]
- India Meteorological Department. Customized Rainfall Information System (CRIS); India Meteorological Department: New Delhi, India, 2020.
- Devassy, V.P.; Gopinathan, C.K. Hydrobiological Features of the Kerala Backwaters during Premonsoon and Monsoon Months. Fish. Technol. 1970, 7, 190–194. [Google Scholar]
- Ball, M.C. Salinity Tolerance in the Mangroves Aegiceras Corniculatum and Avicennia marina. I. Water Use in Relation to Growth, Carbon Partitioning, and Salt Balance. Funct. Plant Biol. 1988, 15, 447–464. [Google Scholar] [CrossRef]
- Cram, J.W.; Torr, P.G.; Rose, D.A. Salt Allocation during Leaf Development and Leaf Fall in Mangroves. Trees 2002, 16, 112–119. [Google Scholar] [CrossRef]
- Yasumoto, E.; Adachi, K.; Kato, M.; Sano, H.; Sasamoto, H.; Baba, S.; Ashihara, H. Uptake of Inorganic Ions and Compatible Solutes in Cultured Mangrove Cells during Salt Stress; Society for In Vitro Biology: Raleigh, NC, USA, 1999. [Google Scholar]
- Kura-Hotta, M.; Mimura, M.; Tsujimura, T.; Washitani-Nemoto, S.; Mimura, T. High Salt-Treatment-Induced Na+ Extrusion and Low Salt-Treatment-Induced Na+ Accumulation in Suspension-Cultured Cells of the Mangrove Plant, Bruguiera sexangula. Plant Cell Environ. 2001, 24, 1105–1112. [Google Scholar] [CrossRef]
- Lawton, J.R.; Todd, A.; Naidoo, D.K. Preliminary Investigations into the Structure of the Roots of the Mangroves, Avicennia marina and Bruguiera Gymnorrhiza, in Relation to Ion Uptake; Wiley: New York, NY, USA, 1981. [Google Scholar]
- Reef, R.; Lovelock, C.E. Regulation of Water Balance in Mangroves. Ann. Bot. 2014, 115, 385–395. [Google Scholar] [CrossRef] [Green Version]
- Lovelock, C. Field Guide to the Mangroves of Queensland; Australian Institute of Marine Science: Townsville, Australia, 1993.
- Yeung, E.C.T.; Stasolla, C.; Sumner, M.J.; Huang, B.Q. Plant Microtechniques and Protocols; Springer International Publishing: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill: New York, NY, USA, 1940. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org (accessed on 1 May 2022).
- RStudio. RStudio: Integrated Development Environment for R; RStudio: Boston, MA, USA, 2021; Available online: https//www.rstudio.com (accessed on 1 May 2022).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2019; Available online: https://socialsciences.mcmaster.ca/jfox/Books/Companion/ (accessed on 1 May 2022).
- Burns, J.H.; Strauss, S.Y. More Closely Related Species Are More Ecologically Similar in an Experimental Test. Proc. Natl. Acad. Sci. USA 2011, 108, 5302–5307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerly, D. Conservatism and Diversification of Plant Functional Traits: Evolutionary Rates versus Phylogenetic Signal. Proc. Natl. Acad. Sci. USA 2009, 106 (Suppl. S2), 19699–19706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, L.S.; Testo, Z.M.; Cerutti, J.A. Characterization of Internal Airflow within Tissues of Mangrove Species from Australia: Leaf Pressurization Processes. J. Torrey Bot. Soc. 2009, 136, 70–83. [Google Scholar] [CrossRef]
- Hayes, M.A.; Chapman, S.; Jesse, A.; O’Brien, E.; Langley, J.A.; Bardou, R.; Devaney, J.; Parker, J.D.; Cavanaugh, K.C. Foliar Water Uptake by Coastal Wetland Plants: A Novel Water Acquisition Mechanism in Arid and Humid Subtropical Mangroves. J. Ecol. 2020, 108, 2625–2637. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Meir, P.; Wolfe, J.; Mencuccini, M.; Ball, M.C. Plumbing the Depths: Extracellular Water Storage in Specialized Leaf Structures and Its Functional Expression in a Three-Domain Pressure–Volume Relationship. Plant Cell Environ. 2017, 40, 1021–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constable, L. Nocturnal Top-down Rehydration: Contribution of Atmospheric Moisture to Water Status of the Mangrove, Avicennia marina. Doctoral Dissertation, The Australian National University, Canberra, Australia, 2014. [Google Scholar]
- Nguyen, H.T.; Meir, P.; Sack, L.; Evans, J.R.; Oliveira, R.S.; Ball, M.C. Leaf Water Storage Increases with Salinity and Aridity in the Mangrove Avicennia marina: Integration of Leaf Structure, Osmotic Adjustment and Access to Multiple Water Sources. Plant Cell Environ. 2017, 40, 1576–1591. [Google Scholar] [CrossRef] [Green Version]
- Tan, W.K.; Lin, Q.; Lim, T.M.; Kumar, P.; Loh, C.S. Dynamic Secretion Changes in the Salt Glands of the Mangrove Tree Species Avicennia Officinalis in Response to a Changing Saline Environment. Plant Cell Environ. 2013, 36, 1410–1422. [Google Scholar] [CrossRef]
- Shi, G.; Cai, Q. Leaf plasticity in peanut (Arachis hypogaea L.) in response to heavy metal stress. Env. and Exp. Bot. 2009, 67, 112–117. [Google Scholar] [CrossRef]
- Khan, M.N.I.; Khatun, S.; Azad, M.S.; Mollick, A.S. Leaf morphological and anatomical plasticity in Sundri (Heritiera fomes Buch.-Ham.) along different canopy light and salinity zones in the Sundarbans mangrove forest, Bangladesh. Glob. Ecol. and Cons. 2020, 23, e01127. [Google Scholar] [CrossRef]
- Wahid, S.M.; Babel, M.S.; Bhuiyan, A.R. Hydrologic monitoring and analysis in the Sundarbans mangrove ecosystem, Bangladesh. J. of Hydr. 2007, 332, 381–395. [Google Scholar] [CrossRef]
- Hoppe-Speer, S.C.; Adams, J.B.; Rajkaran, A.; Bailey, D. The response of the red mangrove Rhizophora mucronata Lam. to salinity and inundation in South Africa. Aquat. Bot. 2011, 95, 71–76. [Google Scholar] [CrossRef]
- Vovides, A.G.; Vogt, J.; Kollert, A.; Berger, U.; Grueters, U.; Peters, R.; Lara-Domínguez, A.L.; López-Portillo, J. Morphological plasticity in mangrove trees: Salinity-related changes in the allometry of Avicennia germinans. Trees 2014, 28, 1413–1425. [Google Scholar] [CrossRef]
- Jiang, G.-F.; Goodale, U.M.; Liu, Y.-Y.; Hao, G.-Y.; Cao, K.-F. Salt Management Strategy Defines the Stem and Leaf Hydraulic Characteristics of Six Mangrove Tree Species. Tree Physiol. 2017, 37, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, R.; Liu, T.; Zheng, H.-L. Solute Accumulation and Osmotic Adjustment Characteristics of the Mangrove Avicennia marina under NaCl-Induced Salinity Stress. Bot. Mar. 2011, 54, 335–341. [Google Scholar] [CrossRef]
- Rodriguez-Dominguez, C.M.; Forner, A.; Martorell, S.; Choat, B.; Lopez, R.; Peters, J.M.R.; Pfautsch, S.; Mayr, S.; Carins-Murphy, M.R.; McAdam, S.A.M.; et al. Leaf Water Potential Measurements Using the Pressure Chamber: Synthetic Testing of Assumptions towards Best Practices for Precision and Accuracy. Plant Cell Environ. 2022. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Feller, I.C.; Reef, R.; Hickey, S.; Ball, M.C. Mangrove Dieback during Fluctuating Sea Levels. Sci. Rep. 2017, 7, 1680. [Google Scholar] [CrossRef]
- McIvor, A.L.; Spencer, T.; Möller, I.; Spalding, M. The Response of Mangrove Soil Surface Elevation to Sea Level Rise. Natural Coastal Protection Series: Report 3, Cambridge Coastal Research Unit Working Paper 42; The Nature Conservancy and Wetlands International,. 2013. Available online: https://www.conservationgateway.org/ConservationPractices/Marine/crr/library/Documents/mangrove-surface-elevation-and-sea-level-rise.pdf (accessed on 1 May 2022).
- Ellison, J.C. Long-term retrospection on mangrove development using sediment cores and pollen analysis: A review. Aquat. Bot. 2008, 89, 93–104. [Google Scholar] [CrossRef]
- Shearman, P.; Bryan, J.; Walsh, J.P. Trends in deltaic change over three decades in the Asia-pacific region. J. Coast Res. 2013, 29, 1169–1183. [Google Scholar] [CrossRef]
- Godoy, M.D.; Lacerda, L.D.D. Mangroves response to climate change: A review of recent findings on mangrove extension and distribution. An. Acad. Bras. Ciênc. 2015, 87, 651–667. [Google Scholar] [CrossRef] [Green Version]
- Alongi, D.M. The impact of climate change on mangrove forests. Curr. Clim. Chang. Rep. 2015, 1, 30–39. [Google Scholar] [CrossRef]
- Record, S.; Charney, N.D.; Zakaria, R.M.; Ellison, A.M. Projecting global mangrove species and community distributions under climate change. Ecosphere 2013, 4, 1–23. [Google Scholar] [CrossRef]
- Clarke, P.J.; Kerrigan, R.A. The effects of seed predators on the recruitment of mangroves. J. Ecol. 2002, 90, 728–736. [Google Scholar] [CrossRef] [Green Version]
- Ellison, A.M. Managing mangroves with benthic biodiversity in mind: Moving beyond roving banditry. J. Sea Res. 2008, 59, 2–15. [Google Scholar] [CrossRef]
- Binks, O.; Mencuccini, M.; Rowland, L.; da Costa, A.C.L.; de Carvalho, C.J.R.; Bittencourt, P.; Eller, C.; Teodoro, G.S.; Carvalho, E.J.M.; Soza, A.; et al. Foliar Water Uptake in Amazonian Trees: Evidence and Consequences. Glob. Chang. Biol. 2019, 25, 2678–2690. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaepdryver, K.H.D.; Goossens, W.; Naseef, A.; Kalpuzha Ashtamoorthy, S.; Steppe, K. Foliar Water Uptake Capacity in Six Mangrove Species. Forests 2022, 13, 951. https://0-doi-org.brum.beds.ac.uk/10.3390/f13060951
Schaepdryver KHD, Goossens W, Naseef A, Kalpuzha Ashtamoorthy S, Steppe K. Foliar Water Uptake Capacity in Six Mangrove Species. Forests. 2022; 13(6):951. https://0-doi-org.brum.beds.ac.uk/10.3390/f13060951
Chicago/Turabian StyleSchaepdryver, Katrien H. D., Willem Goossens, Abdulla Naseef, Sreejith Kalpuzha Ashtamoorthy, and Kathy Steppe. 2022. "Foliar Water Uptake Capacity in Six Mangrove Species" Forests 13, no. 6: 951. https://0-doi-org.brum.beds.ac.uk/10.3390/f13060951